Recent Developments of Solar Cells from PbS Colloidal Quantum Dots
Abstract
:1. Introduction
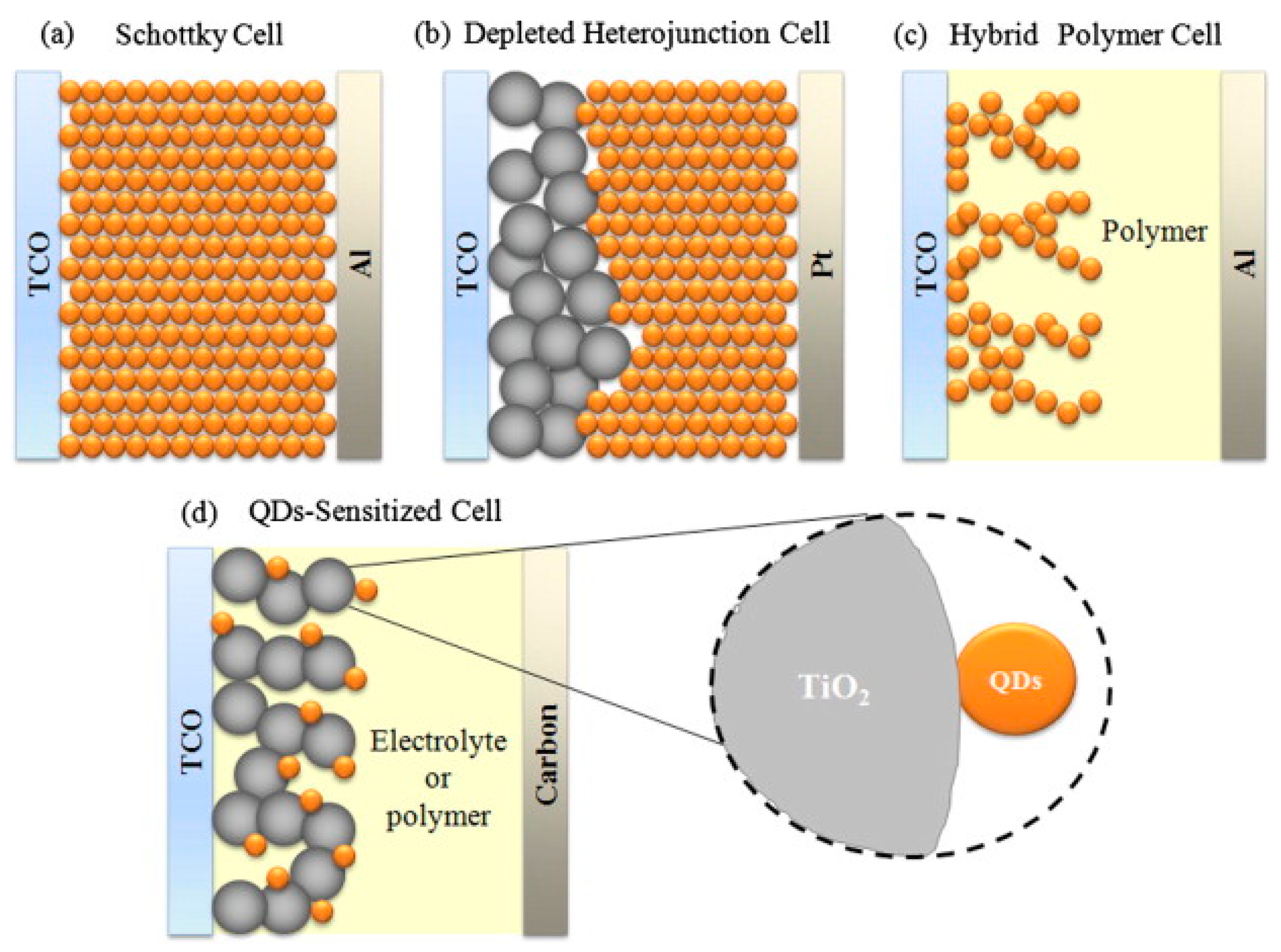
2. Solar Cells with PbS Quantum Dots—The Principle Function
3. PbS Quantum Dot Schottky Solar Cells
4. PbS Quantum Dot Sensitized Solar Cells
5. PbS Quantum Dot Heterojunction Solar Cells
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Storhoff, J.J.; Lazarides, A.A.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L.; Schatz, G.C. What controls the optical properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2000, 122, 4640–4650. [Google Scholar] [CrossRef]
- Lopez, R.; Feldman, L.C.; Haglund, R.F., Jr. Size-dependent optical properties of VO2 nanoparticle arrays. Phys. Rev. Lett. 2004, 93, 177403. [Google Scholar] [CrossRef] [PubMed]
- Byers, C.P.; Zhang, H.; Swearer, D.F.; Yorulmaz, M.; Hoener, B.S.; Huang, D.; Hoggard, A.; Chang, W.S.; Mulvaney, P.; Ringe, E.; et al. From tunable core-shell nanoparticles to plasmonic drawbridges: Active control of nanoparticle optical properties. Sci. Adv. 2015, 1, e1500988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeiffer, C.; Rehbock, C.; Hühn, D.; Carillo-Carrion, C.; Jimenez De Aberasturi, D.; Merk, V.M.; Barcikowski, S.; Parak, W.J. Interaction of colloidal nanoparticles with their local environment: The (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931. [Google Scholar] [CrossRef] [Green Version]
- Horie, M.; Fujita, K.; Kato, H.; Endoh, S.; Nishio, K.; Komaba, L.K.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Hagihara, Y.; et al. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: Metal ion release, adsorption ability and specific surface area. Metallomics 2012, 4, 350–360. [Google Scholar] [CrossRef]
- Wang, X.; Hanson, J.C.; Liu, G.; Rodriguez, J.A.; Iglesias-Juez, A.; Fernández-García, M. The behavior of mixed-metal oxides: Physical and chemical properties of bulk Ce1−xTbxO2 and nanoparticles of Ce1−xTbxOy. J. Chem. Phys. 2004, 121, 5434–5444. [Google Scholar] [CrossRef] [Green Version]
- Sudsom, D.; Juhász Junger, I.; Döpke, C.; Blachowicz, T.; Hahn, L.; Ehrmann, A. Micromagnetic simulation of vortex development in magnetic bi-material bow-tie structures. Condens. Matter 2020, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Sturm, S.; Siglreitmeier, M.; Wolf, D.; Vogel, K.; Gratz, M.; Faivre, D.; Lubk, A.; Büchner, B.; Sturm (née Rosseeva), E.V.; Cölfen, H. Magnetic nanoparticle chains in gelatin ferrogels: Bioinspiration from magnetotactic bacteria. Adv. Funct. Mater. 2019, 29, 1905996. [Google Scholar] [CrossRef]
- Blachowicz, T.; Kosmalska, D.; Döpke, C.; Leiste, H.; Hahn, L.; Ehrmann, A. Varying steps in hysteresis loops of Co square nano-frames. J. Magn. Magn. Mater. 2019, 491, 165619. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Debellis, D.; Suranna, G.P.; Gigli, G.; Giansante, C. The dynamic organic/inorganic interface of colloidal PbS quantum dots. Angew. Chem. Int. Ed. Engl. 2016, 55, 6628. [Google Scholar] [CrossRef] [PubMed]
- Giansante, C.; Infante, I.; Fabiano, E.; Grisorio, R.; Suranna, G.P.; Gigli, G. “Darker-than-Black” PbS Quantum Dots: Enhancing Optical Absorption of Colloidal Semiconductor Nanocrystals via Short Conjugated Ligands. J. Am. Chem. Soc. 2015, 137, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bian, K.; Hanrath, T.; Bassett, W.A.; Wang, Z. Decoding the Superlattice and Interface Structure of Truncate PbS Nanocrystal-Assembled Supercrystal and Associated Interaction Forces. J. Am. Chem. Soc. 2014, 136, 12047–12055. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 3rd ed.; John Wiley: New York, NY, USA, 1966. [Google Scholar]
- Dalven, R. A Review of the semiconductor properties of PbTe, PbSe, PbS and PbO. Infrared Phys. 1969, 9, 141–184. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.X.; Qian, X.F.; Zhang, X.M.; Xie, Y.; Qian, Y.T. A room temperature chemical route to nanocrystalline PbS semiconductor. Mater. Lett. 1999, 40, 255–258. [Google Scholar] [CrossRef]
- Nanda, K.K.; Sahu, S.N. One-dimensional quantum confinement in electrodeposited PbS nanocrystalline semiconductors. Adv. Mater. 2001, 13, 280–283. [Google Scholar] [CrossRef]
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 2002, 14, 815–818. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Huang, Y.; Wei, J.; Liu, F.; Shao, Z.; Hu, L.; Chen, S.; Yang, S.; Tang, J.; et al. Efficient inorganic solid solar cell composed of perovskite and PbS quantum dots. Nanoscale 2015, 7, 9902–9907. [Google Scholar] [CrossRef]
- Gao, J.; Luther, J.M.; Semonin, O.E.; Ellingson, R.J.; Nozik, A.J.; Beard, M.C. Quantum dot size dependent J-V characteristics in heterojunction ZnO/PbS quantum dot solar cells. Nano Lett. 2011, 11, 1002–1008. [Google Scholar] [CrossRef]
- Talapin, D.V.; Murray, C.B. PbSe Nanocrystal solids for N- and P-channel thin film field-effect Transistors. Science 2005, 310, 86–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.J.; Liu, M.X.; Sargent, E.H. Colloidal quantum dot solids for solution-processed solar cells. Nat. Energy 2016, 1, 16016. [Google Scholar] [CrossRef]
- Brown, P.R.; Kim, D.H.; Lunt, R.R.; Zhao, N.; Bawendi, M.G.; Grossman, J.C.; Bulovic, V. Energy level modification in lead sulfide quantum dot thin films through ligand exchange. ACS Nano 2014, 8, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Rumbles, G. Excitons in nanoscale systems. Nat. Mater. 2006, 5, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Sambur, J.B.; Novet, T.; Parkinson, B.A. Multiple exciton collection in a sensitized photovoltaic system. Science 2010, 330, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, M.C.; Ellingson, R.J.; Beard, M.; Yu, P.; Micic, O.I.; Nozik, A.J. Quantum dot solar cells: High efficiency through multiple exciton generation. In Proceedings of the 2004 DOE Solar Energy Technologies, Denver, CO, USA, 25–28 October 2004. [Google Scholar]
- Zhao, N.; Osedach, T.P.; Chang, L.Y.; Geyer, S.M.; Wanger, D.; Binda, M.T.; Arango, A.C.; Bawendi, M.G.; Bulovic, V. Colloidal PbS quantum dot solar cells with high fill factor. ACS Nano 2010, 4, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.H.; Thon, S.M.; Hoogland, S.; Voznyy, O.; Zhitomirsky, D.; Debnath, R.; Levina, L.; Rollny, L.R.; Carey, G.H.; Fischer, A.; et al. Hybrid passivated colloidal quantum dot solids. Nat. Nanotechnol. 2012, 7, 577–582. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Kohn, S.; Wehlage, D.; Juhász Junger, I.; Ehrmann, A. Electrospinning a dye-sensitized solar cell. Catalysts 2019, 9, 975. [Google Scholar] [CrossRef] [Green Version]
- Juhász Junger, I.; Udomrungkhajornchai, S.; Grimmelsmann, N.; Blachowicz, T.; Ehrmann, A. Effect of caffeine copigmentation of anthocyanin dyes on the DSSC efficiency. Materials 2019, 12, 2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Grötsch, G.; Cornelißen, C.; Streitenberger, A.; Ehrmann, A. Electrospun nanofiber mats with embedded non-sintered TiO2 for dye-sensitized solar cells (DSSCs). Fibers 2019, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Ehrmann, A.; Blachowicz, T. Recent coating materials for textile-based solar cells. AIMS Mater. Sci. 2019, 6, 234–251. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Sikhovatkin, S.; Hinds, S.; Brzozowski, L.; Sargent, E.H. Colloidal quantum-dot photodetectors exploiting multiexciton generation. Science 2009, 324, 1542–1544. [Google Scholar] [CrossRef] [Green Version]
- Pijpers, J.J.H.; Ulbricht, R.; Tielrooij, K.J.; Osherov, A.; Golan, Y.; Delerue, C.; Allan, G.; Bonn, M. Assessment of carrier-multiplication efficiency in bulk PbSe and PbS. Nat. Phys. 2009, 5, 811–814. [Google Scholar] [CrossRef] [Green Version]
- Beard, M.C.; Knutsen, K.P.; Yu, P.; Luther, J.M.; Song, Q.; Metzger, W.K.; Ellingson, R.J.; Nozik, A.J. Multiple exciton generation in colloidal silicon nanocrystals. Nano Lett. 2007, 7, 2506–2512. [Google Scholar] [CrossRef]
- Emin, S.; Singh, S.; Han, L.; Satoh, N.; Islam, A. Colloidal quantum dot solar cells. Sol. Energy 2011, 85, 1264–1282. [Google Scholar] [CrossRef]
- Clifford, J.P.; Johnston, K.W.; Levina, L.; Sargent, E.H. Schottky barriers to colloidal quantum dot films. Appl. Phys. Lett. 2007, 91, 253117. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, X.; Brzozowski, L.; Barkhouse, A.; Debnath, R.; Levina, L.; Sargent, E.H. Schottky quantum dot solar cells stable in air under solar illumination. Adv. Mater. 2010, 22, 1398–1402. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-heterojunction colloidal quantum dot solar cells. ACS Nano 2010, 4, 3374–3380. [Google Scholar] [CrossRef] [PubMed]
- Debnath, R.; Greiner, M.T.; Kramer, J.J.; Fuscher, A.; Tang, J.; Barkhouse, D.A.R.; Wang, X.; Levina, L.; Lu, Z.-H.; Sargent, E.H. Depleted-heterojunction colloidal quantum dot photovoltaics employing low-cost electrical contacts. Appl. Phys. Lett. 2010, 97, 023109. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.A.; Rhee, J.H.; Im, S.H.; Lee, Y.H.; Kim, H.J.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. High-performance nanostructured inorganic–organic heterojunction solar cells. Nano Lett. 2010, 10, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, A.; Word, R.C.; VanSant, K.; Könenkamp, R. Nanowire-quantum-dot-polymer solar cells. Phys. Stat. Sol. 2008, 245, 1834–1837. [Google Scholar] [CrossRef]
- Kniprath, R.; Rabe, J.P.; McLeskey, J.T.; Wang, D.; Kirstein, S. Hybrid photovoltaic cells with II–VI quantum dot sensitizers fabricated by layer-by-layer deposition of water-soluble components. Thin Solid Films 2009, 518, 295–298. [Google Scholar] [CrossRef]
- Plass, R.; Serge, P.; Krüger, J.; Grätzel, M.; Bach, U. Quantum dot sensitization of organic−inorganic hybrid solar cells. J. Phys. Chem. B 2002, 106, 7578–7580. [Google Scholar] [CrossRef]
- Acharya, K.P.; Hewa-Kaskarage, N.N.; Alabi, T.R.; Nemitz, I.; Khon, E.; Ullrich, B.; Anzenbacher, P.; Zamkov, M. Synthesis of PbS/TiO2 colloidal heterostructures for photovoltaic applications. J. Phys. Chem. C 2010, 114, 12496–12504. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Arrocena, M.C.; Mendoza, N.; Cortina-Marrero, H.J.; Nicho, M.E.; Hu, H. Influence of poly3-octylthiophene (P3OT) film thickness and preparation method on photovoltaic performance of hybrid ITO/CdS/P3OT/Au solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 29–33. [Google Scholar] [CrossRef]
- Dayal, S.; Reese, M.O.; Ferguson, A.J.; Ginley, D.S.; Rumbles, G.; Kopidakis, N. The effect of nanoparticle shape on the photocarrier dynamics and photovoltaic device performance of ploy(3-hexylthiophene): CdSe nanoparticl bulk heterojunction solar cells. Adv. Funct. Mater. 2010, 20, 2629–2635. [Google Scholar] [CrossRef]
- Vogel, R.; Pohl, K.; Weller, H. Sensitization of highly porous, polycrystalline TiO2 electrodes by quantum sized CdS. Chem. Phys. Lett. 1990, 174, 241–245. [Google Scholar] [CrossRef]
- Shalom, M.; Dor, S.; Rühle, S.; Grinis, L.; Zaban, A. Core/CdS quantum dot/shell mesoporous solar cells with improved stability and efficiency using an amorphous TiO2 coating. J. Phys. Chem. C 2009, 113, 3895–3898. [Google Scholar] [CrossRef]
- Peumans, P.; Yakimov, A.; Forrest, S.R. Small molecular weight organic thin-film photodetectors and solar cells. J. Appl. Phys. 2003, 93, 3693. [Google Scholar] [CrossRef]
- Maria, A.; Cyr, P.W.; Klem, E.J.D.; Levina, L.; Sargent, E.H. Solution-processed infrared photovoltaic devices with > 10% monochromatic internal quantum efficiency. Appl. Phys. Lett. 2005, 87, 213112. [Google Scholar] [CrossRef] [Green Version]
- Chuang, C.H.; Brown, P.R.; Bulovic, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, X.-D.; An, H.J.; Song, J.H.; Jang, J.; Kim, S.; Jeong, S. Inverted Schottky quantum dot solar cells with enhanced carrier extraction and air-stability. J. Mater. Chem. A 2014, 2, 20799–20805. [Google Scholar] [CrossRef]
- Mai, V.-T.; Duong, N.-H.; Mai, X.-D. Boosting the current density in inverted Schottky PbS quantum dot solar cells with conjugated electrolyte. Mater. Lett. 2019, 249, 37–40. [Google Scholar] [CrossRef]
- Fukuda, T.; Takahashi, A.; Takahira, K.; Wang, H.B.; Kubo, T.; Segawa, H. Limiting factor of performance for solution-phase ligand-exchanged PbS quantum dot solar cell. Sol. Energy Mater. Solar Cells 2019, 195, 220–227. [Google Scholar] [CrossRef]
- Kagan, C.R.; Murray, C.B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotechnol. 2015, 10, 1013–1026. [Google Scholar] [CrossRef]
- Li, M.; Yuan, X.; Ruan, H.; Wang, X.; Liu, Y.; Lu, Z.; Hai, J. Synthesis of PbS-CH3NH3PbI3 core-shell nanoparticles with enhanced photoelectric properties. J. Alloys Compd. 2017, 706, 395–400. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, B.; Gao, Y.; Liu, H.; Tian, Y.; Qin, D.; Wu, H.; Huang, W.; Hou, L. Novel Hybrid Ligands for Passivating PbS Colloidal Quantum Dots to Enhance the Performance of Solar Cells. Nanomicro Lett. 2015, 7, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speirs, M.J.; Balazs, D.M.; Dirin, D.N.; Kovalenko, M.V.; Loi, M.A. Increased efficiency in pn-junction PbS QD solar cells via NaHS treatment of the p-type layer. Appl. Phys. Lett. 2017, 110, 103904. [Google Scholar] [CrossRef] [Green Version]
- Speirs, M.J.; Dirin, D.N.; Abdu-Aguye, M.; Balazs, D.M.; Kovalenko, M.V.; Loi, M.A. Temperature dependent behaviour of lead sulfidequantum dot solar cells and films. Energy Environ. Sci. 2016, 9, 2916. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Mandelis, A.; Lan, X.; Melnikov, A.; Hoogland, S.; Sargent, E.H. Imbalanced charge carrier mobility and Schottky junction induced anomalous current-voltage characteristics of excitonic PbS colloidal quantum dot solar cells. Sol. Energy Mater. Sol. Cells 2016, 155, 155–165. [Google Scholar] [CrossRef]
- Jang, J.; Song, J.H.; Choi, H.; Baik, S.J.; Jeong, S. Photovoltaic light absorber with spatial energy band gradient using PbS quantum dot layers. Sol. Energy Mater. Sol. Cells 2015, 141, 270–274. [Google Scholar] [CrossRef]
- Ali, I.; Muhyuddin, M.; Mullani, N.; Kim, D.W.; Kim, D.H.; Basit, M.A.; Park, T.J. Modernized H2S-treatment of TiO2 nanoparticles: Improving quantum-dot deposition for enhanced photocatalytic performance. Curr. Appl. Phys. 2020, 20, 384–390. [Google Scholar] [CrossRef]
- Ul Hassan, F.; Ahmed, U.; Muhyuddin, M.; Yasir, M.; Ashiq, M.N.; Basit, M.A. Tactical modification of pseudo-SILAR process for enhanced quantum-dot deposition on TiO2 and ZnO nanoparticles for solar energy applications. Mater. Res. Bull. 2019, 120, 110588. [Google Scholar] [CrossRef]
- Mao, X.; Yu, J.; Xu, J.; Zhou, J.; Luo, C.; Wang, L.; Niu, H.; Xu, J.; Zhou, R. Enhanced performance of all solid-state quantum dot-sensitized solar cells via synchronous deposition of PbS and CdS quantum dots. New J. Chem. 2020, 44, 505–512. [Google Scholar] [CrossRef]
- Kraus, S.; Bonn, M.; Canovas, E. Room-temperature solution-phase epitaxial nucleation of PbS quantum dots on rutile TiO2 (100). Nanoscale Adv. 2020, 2, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yang, Q.; Kubie, L.; Stecher, J.T.; Galazka, Z.; Uecker, R.; Parkinson, B.A. Sensitization of SnO2 Single Crystals with Multidentate-Ligand-Capped PbS Colloid Quantum Dots to Enhance the Photocurrent Stability. ChemNanoMat 2020. [Google Scholar] [CrossRef]
- Kamruzzan, M. The effect of ZnO/ZnSe core/shell nanorod arrays photoelectrodes on PbS quantum dot sensitized solar cell performance. Nanoscale Adv. 2020, 2, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Purcell-Milton, F.; Curutchet, A.; Gun’ko, Y. Electrophoretic Deposition of Quantum Dots and Characterisation of Composites. Materials 2019, 12, 4089. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, S.; Pal, A.; Chatterjee, K.; Rana, T.; Bhattacharya, G.; Roy, S.S.; Chowdhury, P.; Sharma, G.D.; Biswas, S. Enhanced efficiency of PbS quantum dot-sensitized solar cells using plasmonic photoanode. J. Nanoparticle Res. 2018, 20, 198. [Google Scholar] [CrossRef]
- Huang, M.; Lu, Z.; Wen, L.; Zhang, X.; Huang, T.; Meng, Y.; Tang, J.; Zhou, L. Core/shell-structured TiO2/PbS photoanodes for high-performance CsPbBr3 perovskite solar cells. Mater. Lett. 2019, 256, 126619. [Google Scholar] [CrossRef]
- Hansen, K.R.; Peterson, J.R.; Perego, A.; Shelley, M.; Olsen, C.R.; Perez, L.D.; Hogg, H.L.; Watt, R.K.; Colton, J.S. Lead sulfide quantum dots inside ferritin: Synthesis and application to photovoltaics. Appl. Nanosci. 2018, 8, 1687–1699. [Google Scholar] [CrossRef]
- Zhang, H.; Selopal, G.S.; Zhou, Y.; Tong, X.; Benetti, D.; Jin, L.; Navarro-Pardo, F.; Wang, Z.; Sun, S.H.; Zhao, H.; et al. Controlled synthesis of near-infrared quantum dots for optoelectronic devices. Nanoscale 2017, 9, 16843–16851. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Wang, J.; Shen, Q.; Li, Y.; Zhong, X. Surface engineering of PbS quantum dot sensitized solar cells with a conversion efficiency exceeding 7%. J. Mater. Chem. A 2016, 4, 7214–7221. [Google Scholar] [CrossRef]
- Park, J.P.; Heo, J.H.; Im, S.H.; Kim, S.W. Highly efficient solid-state mesoscopic PbS with embedded CuS quantum dot-sensitized solar cells. J. Mater. Chem. A 2016, 4, 785–790. [Google Scholar] [CrossRef]
- Wang, H.; Gonzaez-Pedro, V.; Kubo, T.; Fabregat-Santiago, F.; Bisquert, J.; Sanehira, Y.; Nakazaki, J.; Segawa, H. Enhanced Carrier Transport Distance in Colloidal PbS Quantum-Dot-Based Solar Cells Using ZnO Nanowires. J. Phys. Chem. C 2015, 119, 27265–27274. [Google Scholar] [CrossRef]
- Hong, J.; Kim, B.S.; Hou, B.; Cho, Y.; Lee, S.H.; Pak, S.; Morris, S.M.; Sohn, J.I.; Cha, S. Plasmonic Effects of Dual-Metal Nanoparticle Layers for High-Performance Quantum Dot Solar Cells. Plasmonics 2020. [Google Scholar] [CrossRef]
- Dastjerdi, H.T.; Prochowic, D.; Yadav, P.; Tavakoli, M.M. Luminescence down-shifting enables UV-stable and efficient ZnO nanowire-based PbS quantum dot solar cells with J(SC) exceeding 33 mA cm−2. Sustain. Energy Fuels 2019, 3, 3128–3134. [Google Scholar] [CrossRef]
- Xie, Q.; Ming, S.; Chen, L.; Wu, Y.; Zhang, W.; Liu, X.; Cao, M.; Wang, H.; Fang, J. Parameters in planar quantum dot-polymer solar cell: Tuned by QD Eg, ligand exchange and fabrication process. Org. Electron. 2019, 69, 1–6. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.H.; Liu, F.; Nakazawa, N.; Huang, Q.X.; Hayase, S.; Ogomi, Y.; Toyoda, T.; Wang, R.X.; Shen, Q. Recombination Suppression in PbS Quantum Dot Heterojunction Solar Cells by Energy-Level Alignment in the Quantum Dot Active Layers. ACS Appl. Mater. Interfaces 2018, 10, 26142–26152. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kaewprajak, A.; Ling, X.; Hayakawa, A.; Zhou, S.; Song, B.; Kang, Y.; Hayashi, T.; Altun, M.E.; Nakaya, M.; et al. Finely Interpenetrating Bulk Heterojunction Structure for Lead Sulfide Colloidal Quantum Dot Solar Cells by Convective Assembly. ACS Energy Lett. 2019, 4, 960–967. [Google Scholar] [CrossRef]
- Majumder, S.; Mendhe, A.C.; Sankapal, B.R. Nanoheterojunction through PbS nanoparticles anchored CdS nanowires towards solar cell application. Int. J. Hydrogen Energy 2019, 44, 7095–7107. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.; Wang, Y.; Zang, S.; Wei, S.; Li, J.; Zhang, X. Defect Passivation of Low-Temperature Processed ZnO Electron Transport Layer with Polyethylenimine for PbS Quantum Dot Photovoltaics. ACS Appl. Energy Mater. 2019, 2, 1695–1701. [Google Scholar] [CrossRef]
- Zhao, G.; Cai, Q.; Liu, X.; Li, P.; Zhang, Y.; Shao, G.; Liang, C. PbS QDs as Electron Blocking Layer Toward Efficient and Stable Perovskite Solar Cells. IEEE J. Photovolt. 2019, 9, 194–199. [Google Scholar] [CrossRef]
- Zeng, T.; Su, X.; Feng, S.; Xie, Y.; Chen, Y.; Shen, Z.; Shi, W. Thermally-stable hydroxyl radicals implanted on TiO2 electron transport layer for efficient carrier extraction in PbS quantum dot photovoltaics. Sol. Energy Mater. Sol. Cells 2018, 188, 263–272. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Wang, Y.; Yang, S.; Ni, G.; Zou, B. Efficiency enhancement for solution-processed PbS quantum dots solar cells by inserting graphene oxide as hole-transporting and interface modifying layer. Org. Electron. 2018, 58, 270–275. [Google Scholar] [CrossRef]
- Imran, M.; Ikram, M.; Anjum, S.; Ali, S.; Huang, Y. Highly Efficient Hybrid Bulk Heterojunction Organic Solar Cells Integrating PbS Nanoparticles. Nanosci. Nanotechnol. Lett. 2018, 10, 1644–1650. [Google Scholar] [CrossRef]
- Xu, W.Z.; Tan, F.R.; Liu, Q.; Liu, X.S.; Jiang, Q.W.; Wei, L.; Zhang, W.F.; Wang, Z.J.; Qu, S.C.; Wang, Z.G. Efficient PbS QD solar cell with an inverted structure. Sol. Energy Mater. Sol. Cells 2017, 159, 503–509. [Google Scholar] [CrossRef]
- Luan, W.; Zhang, C.; Luo, L.; Yuan, B.; Jin, L.; Kim, Y.-S. Enhancement of the photoelectric performance in inverted bulk heterojunction solid solar cell with inorganic nanocrystals. Appl. Energy 2017, 185, 2217–2223. [Google Scholar] [CrossRef]
- Wang, R.L.; Wu, X.; Xu, K.; Zhou, W.; Shang, Y.; Tang, H.; Chen, H.; Ning, Z. Highly Efficient Inverted Structural Quantum Dot Solar Cells. Adv. Mater. 2018, 30, 1704882. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Simchi, A.; Tavakoli, R.; Fan, Z. Organic Halides and Nanocone Plastic Structures Enhance the Energy Conversion Efficiency and Self-Cleaning Ability of Colloidal Quantum Dot Photovoltaic Devices. J. Phys. Chem. A 2017, 121, 9757–9765. [Google Scholar] [CrossRef]
- Chang, J.; Ogomi, Y.H.; Ding, C.; Znhang, Y.H.; Toyoda, T.; Hayase, S.; Katayama, K.; Shen, Q. Ligand-dependent exciton dynamics and photovoltaic properties of PbS quantum dot heterojunction solar cells. Phys. Chem. Chem. Phys. 2017, 19, 6358–6367. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhai, G.; Zhang, J.; Yang, Y.Z.; Liu, X.G.; Li, X.; Xu, B.-S. PbS Quantum Dots: Size, Ligand Dependent Energy Level Structures and Their Effects on the Performance of Heterojunction Solar Cells. J. Inorg. Mater. 2016, 31, 915–922. [Google Scholar]
- Zhang, N.; Neo, D.C.J.; Tazawa, Y.; Li, X.; Assender, H.E.; Compton, R.G.; Watt, A.A.R. Narrow Band Gap Lead Sulfide Hole Transport Layers for Quantum Dot Photovoltaics. ACS Appl. Mater. Interfaces 2016, 8, 21417–21422. [Google Scholar] [CrossRef]
- Zang, S.; Wang, Y.; Li, M.; Su, W.; An, M.; Zhang, X.; Liu, Y. Performance enhancement of ZnO nanowires/PbS quantum dot depleted bulk heterojunction solar cells with an ultrathin Al2O3 interlayer. Chin. Phys. B 2018, 27, 018503. [Google Scholar] [CrossRef]
- Pradhan, S.; Stavrinadis, A.; Gupta, S.; Konstantatos, G. Reducing Interface Recombination through Mixed Nanocrystal Interlayers in PbS Quantum Dot Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 27390–27395. [Google Scholar] [CrossRef]
- Zhao, T.; Goodwin, E.D.; Guo, J.; Wang, H.; Diroll, B.T.; Murray, C.B.; Kagan, C.R. Advanced Architecture for Colloidal PbS Quantum Dot Solar Cells Exploiting a CdSe Quantum Dot Buffer Layer. ACS Nano 2016, 10, 9267–9273. [Google Scholar] [CrossRef]
- Zang, S.; Wang, Y.; Su, W.; Zhu, H.; Li, G.; Zhang, X.; Liu, Y. Increased open-circuit voltage of ZnO nanowire/PbS quantum dot bulk heterojunction solar cells with solution-deposited Mg(OH)2 interlayer. Phys. Stat. Sol. Rapid Res. Lett. 2016, 10, 745–748. [Google Scholar] [CrossRef]
- Hu, L.; Patterson, R.J.; Hu, Y.; Chen, W.J.; Zhang, Z.L.; Yuan, L.; Chen, Z.H.; Conibeer, G.J.; Wang, G.; Huang, S. High Performance PbS Colloidal Quantum Dot Solar Cells by Employing Solution-Processed CdS Thin Films from a Single-Source Precursor as the Electron Transport Layer. Adv. Funct. Mater. 2017, 27, 1703687. [Google Scholar] [CrossRef]
- Khan, J.; Yang, X.; Qiao, K.; Deng, H.; Zhang, J.; Liu, Z.; Ahmad, W.; Zhang, J.H.; Li, D.B.; Liu, H.; et al. Low-temperature-processed SnO2-Cl for efficient PbS quantum-dot solar cells via defect passivation. J. Mater. Chem. A 2017, 5, 17240–17247. [Google Scholar] [CrossRef]
- Wu, R.; Yang, Y.; Li, M.; Qin, D.; Zhang, Y.; Hou, L. Solvent Engineering for High-Performance PbS Quantum Dots Solar Cells. Nanomaterials 2017, 7, 201. [Google Scholar] [CrossRef] [Green Version]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachowicz, T.; Ehrmann, A. Recent Developments of Solar Cells from PbS Colloidal Quantum Dots. Appl. Sci. 2020, 10, 1743. https://doi.org/10.3390/app10051743
Blachowicz T, Ehrmann A. Recent Developments of Solar Cells from PbS Colloidal Quantum Dots. Applied Sciences. 2020; 10(5):1743. https://doi.org/10.3390/app10051743
Chicago/Turabian StyleBlachowicz, Tomasz, and Andrea Ehrmann. 2020. "Recent Developments of Solar Cells from PbS Colloidal Quantum Dots" Applied Sciences 10, no. 5: 1743. https://doi.org/10.3390/app10051743
APA StyleBlachowicz, T., & Ehrmann, A. (2020). Recent Developments of Solar Cells from PbS Colloidal Quantum Dots. Applied Sciences, 10(5), 1743. https://doi.org/10.3390/app10051743





