Study on the Coupling Relationship of Low Temperature Fluidity and Oxidation Stability of Biodiesel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Materials
2.1.1. Low Temperature Fluidity Experiment Materials
2.1.2. Oxidation Stability Experiment Materials
2.2. Experimental Methods
2.2.1. Low Temperature Fluidity Experiment Methods
2.2.2. Oxidation Stability Experiment Methods
3. Experiment Results and Discussion
3.1. Low Temperature Fluidity Experiment Results
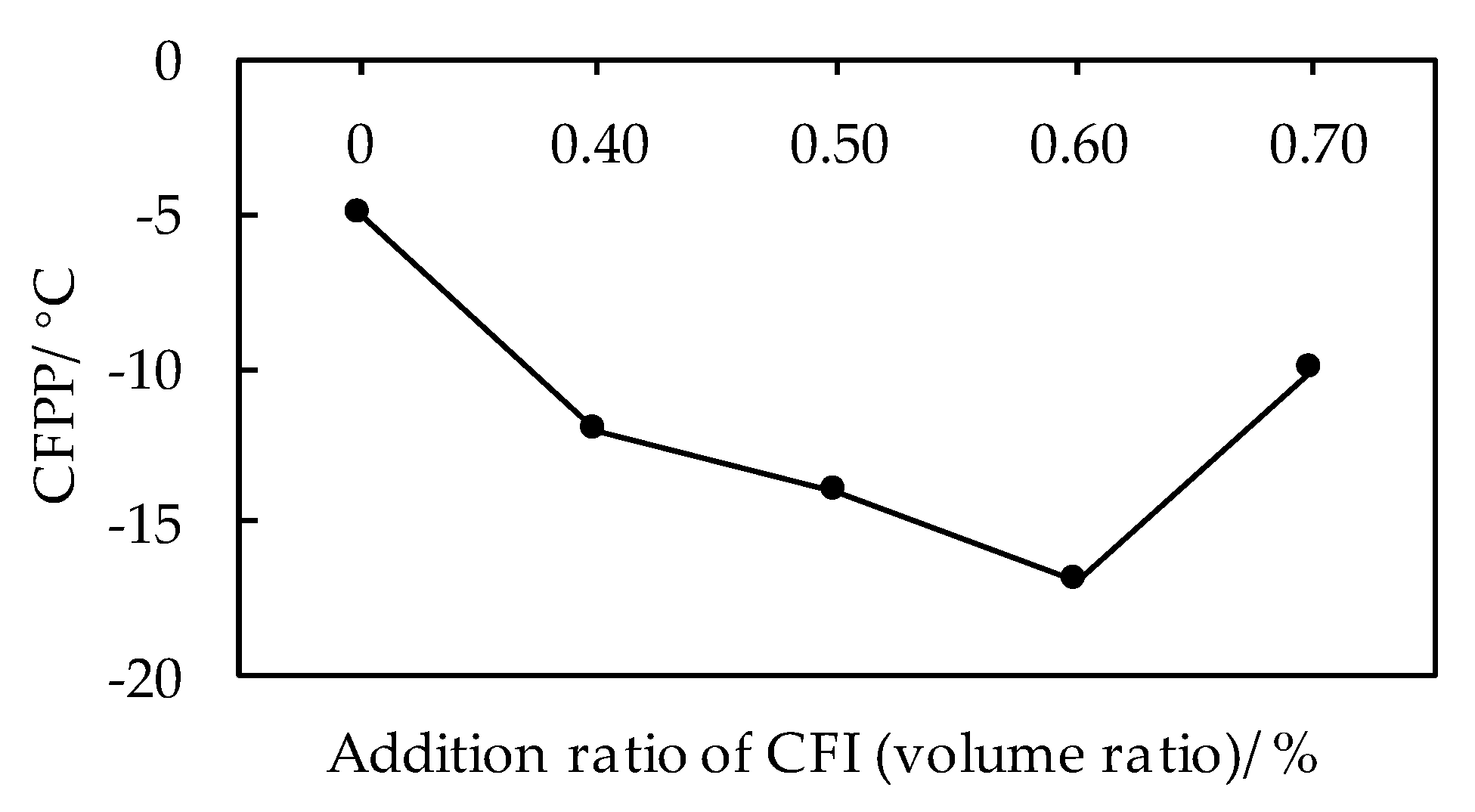
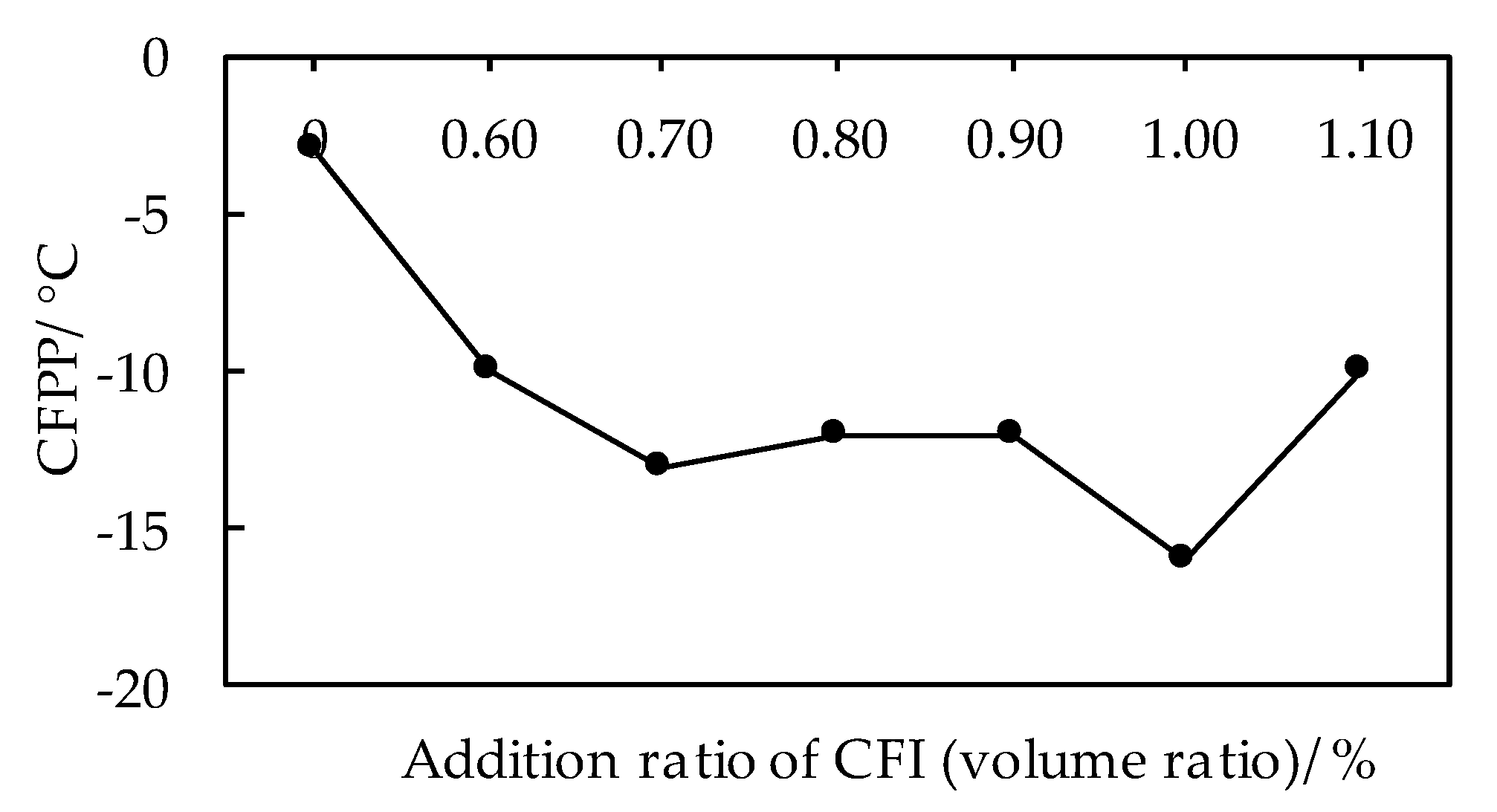
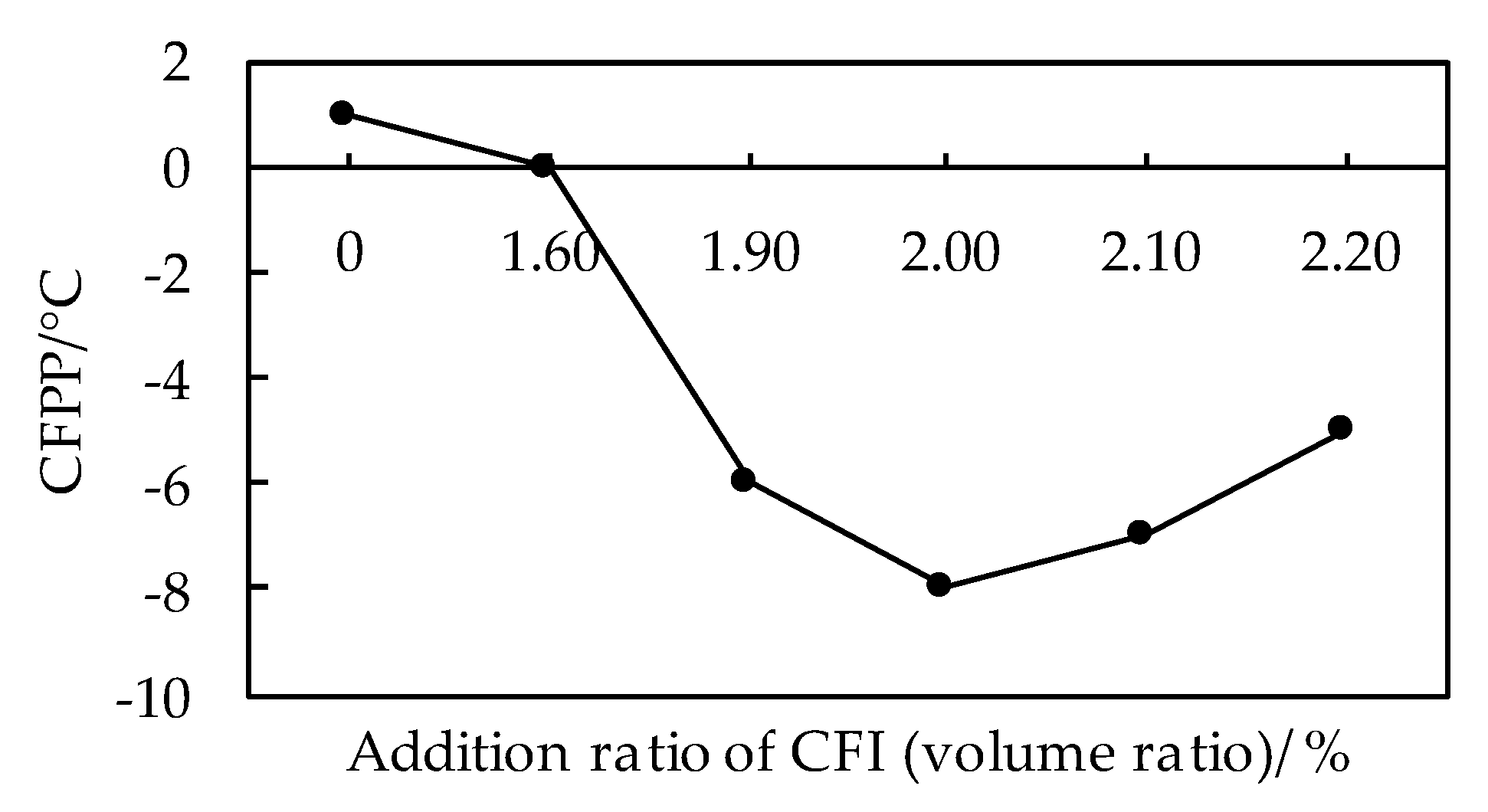
3.1.1. Effects of CFI on Low Temperature Fluidity of Biodiesel
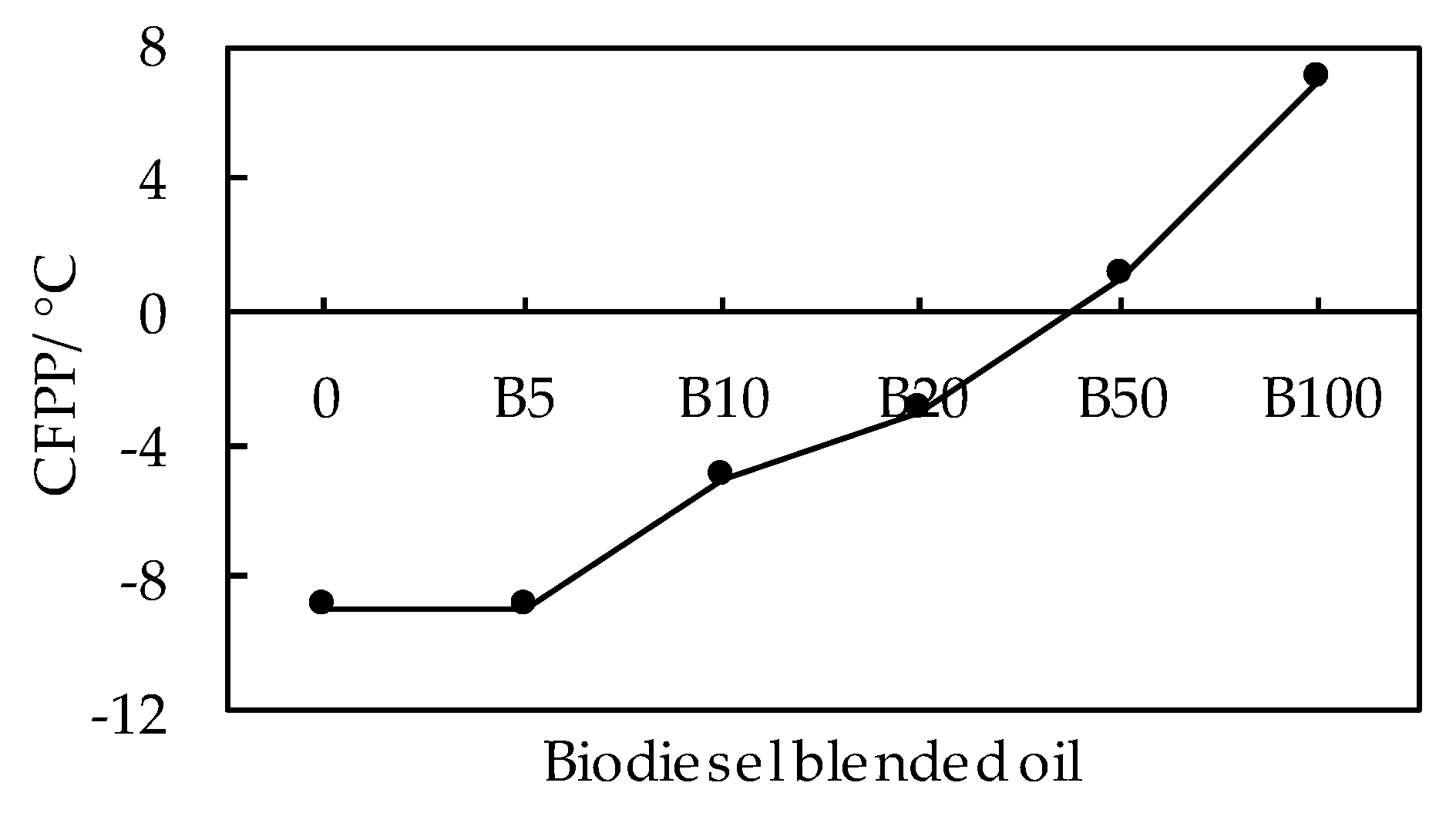
3.1.2. Effects of Blending Ratio on Low Temperature Fluidity of Biodiesel
3.2. Oxidation Stability Experiment Results
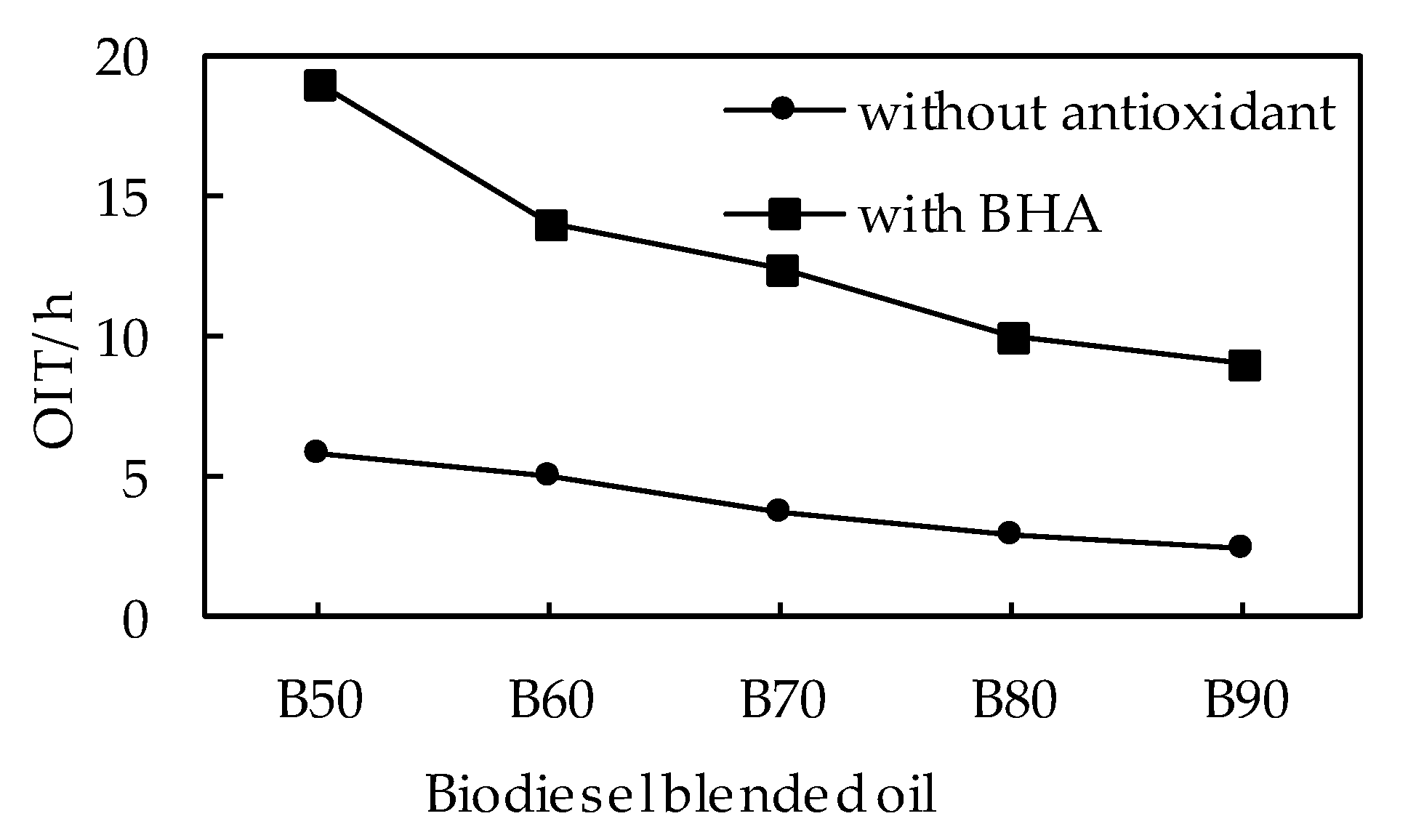
3.2.1. Effects of Antioxidants on OIT of Biodiesel
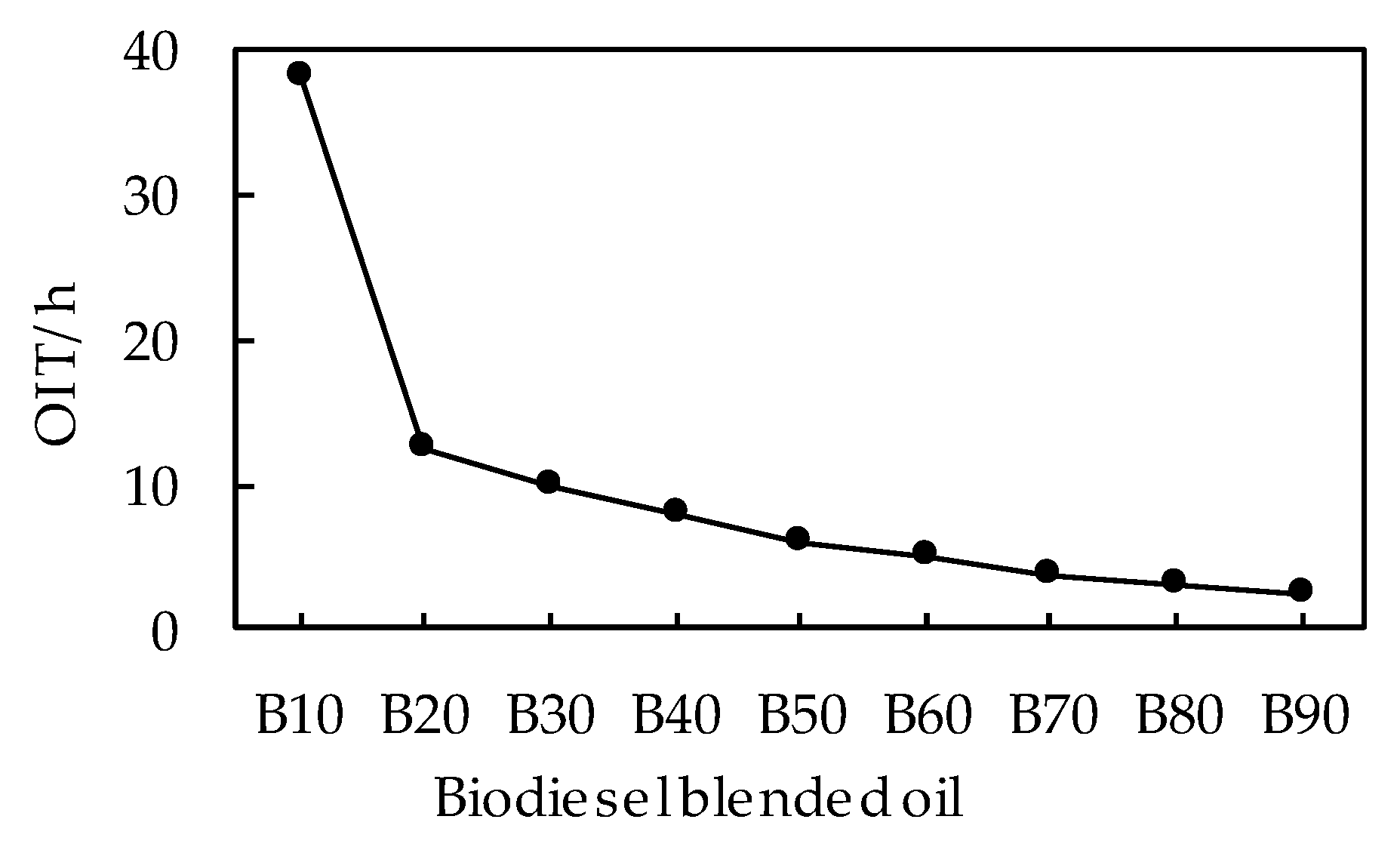
3.2.2. Effects of Blending Ratio on OIT of Biodiesel
3.3. Experiment Results of Coupling Relationship between Low Temperature Fluidity and Oxidation Stability
3.3.1. Coupling Relationship between Low Temperature Fluidity and Oxidation Stability of Biodiesel
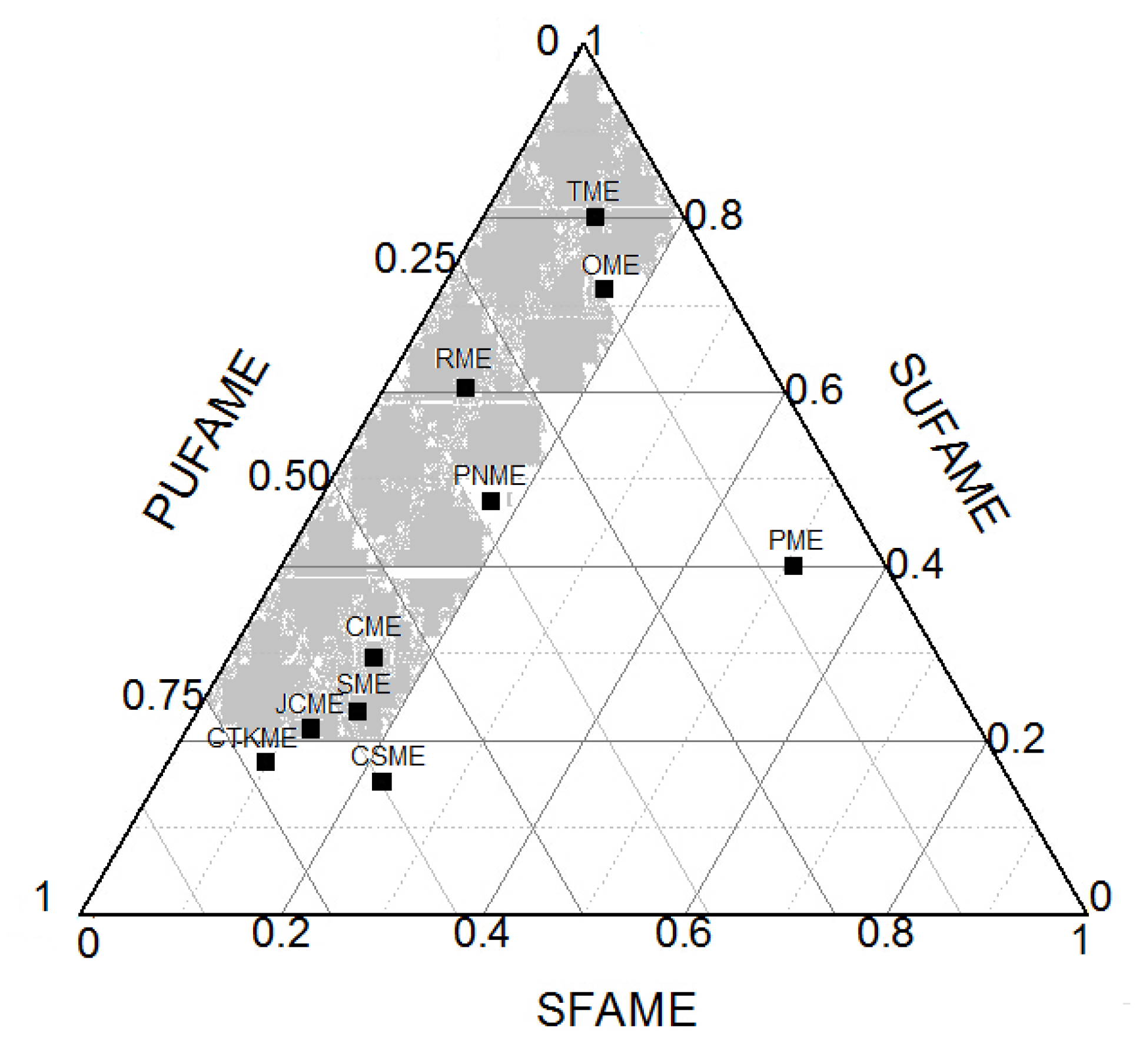
3.3.2. Biodiesel Fatty Acid Methyl Ester Data Acquisition
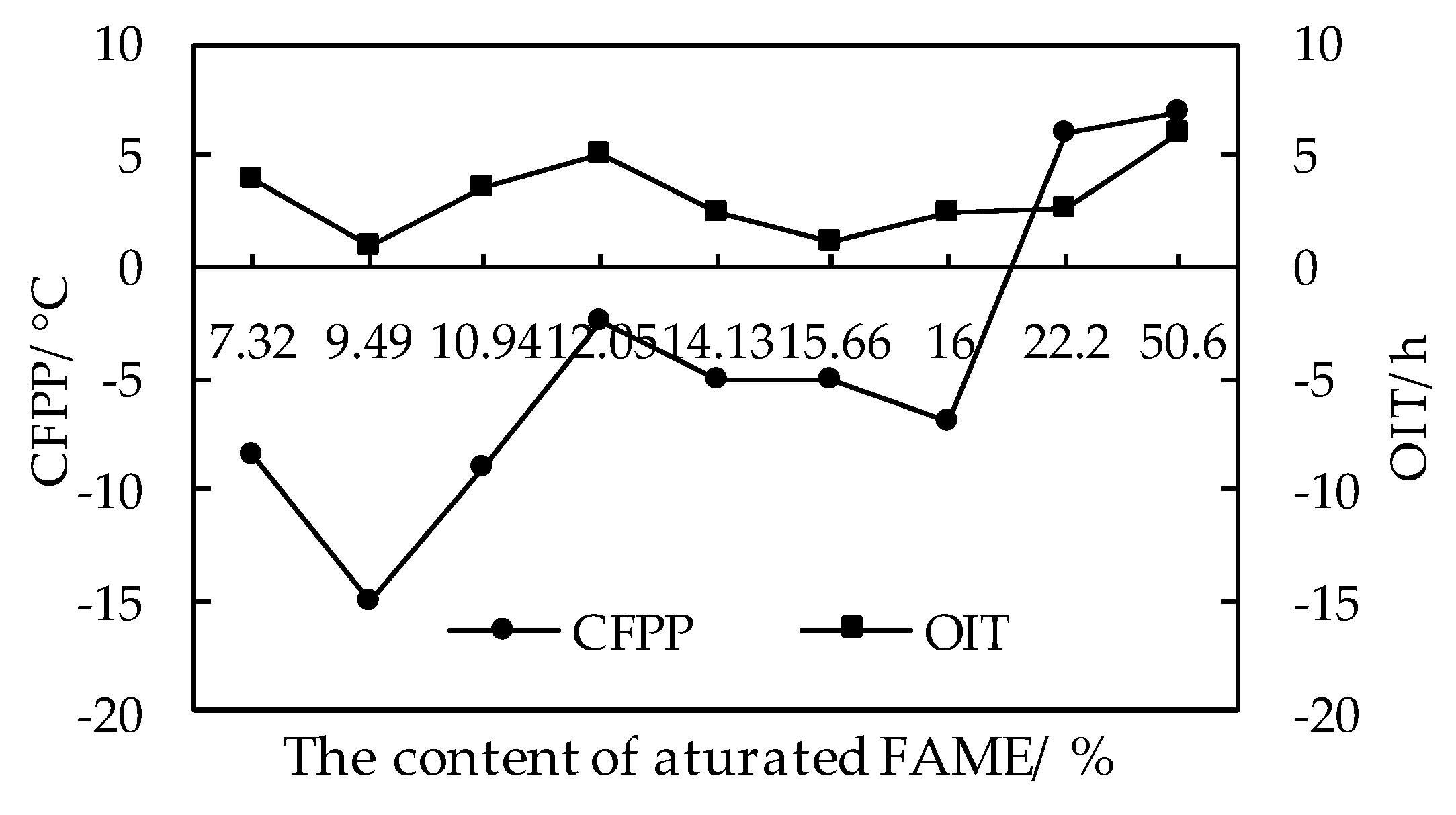
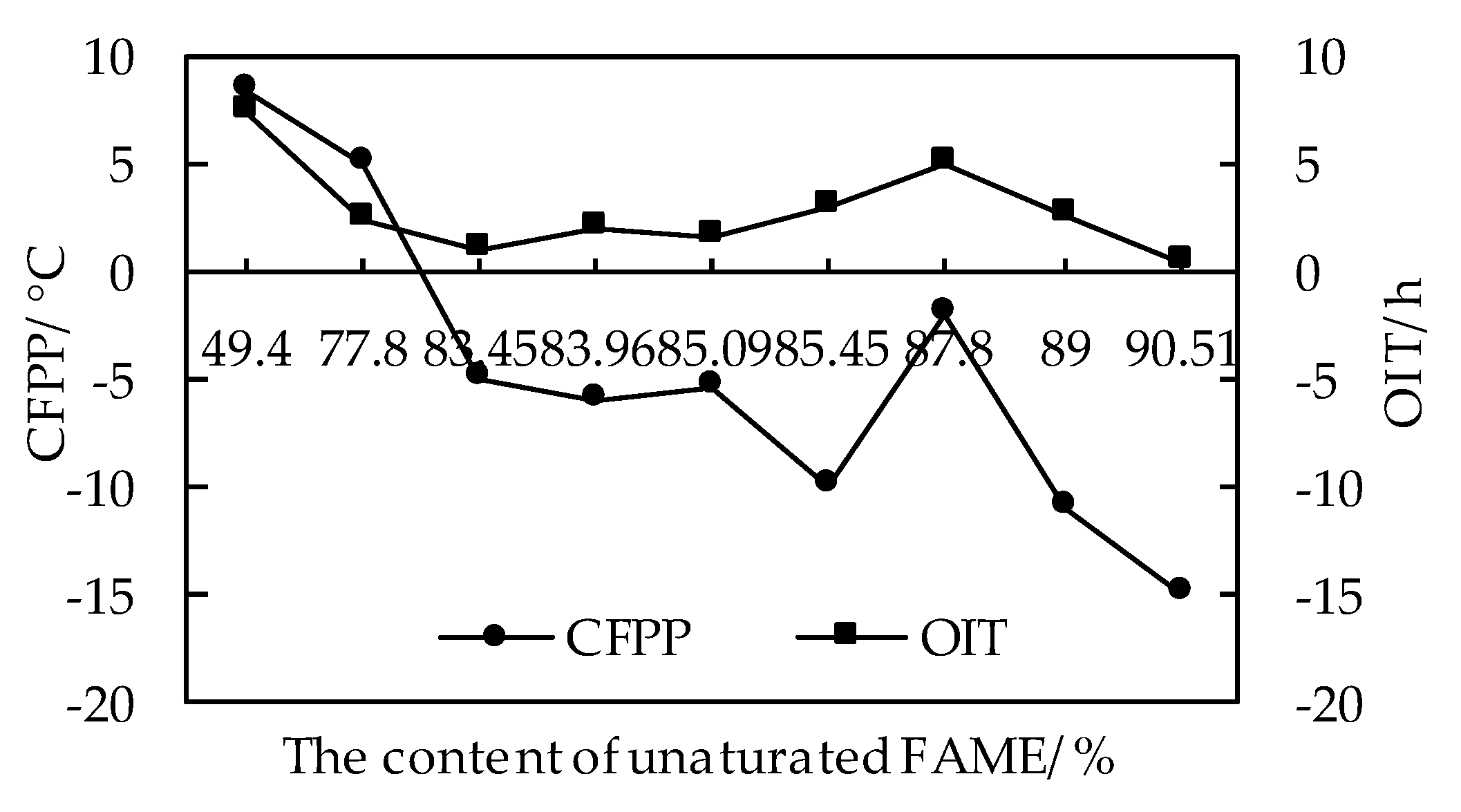
3.3.3. Effects of Fatty Acid Methyl Ester Composition on the Coupling Relationship between Low Temperature Fluidity and Oxidation Stability of Biodiesel
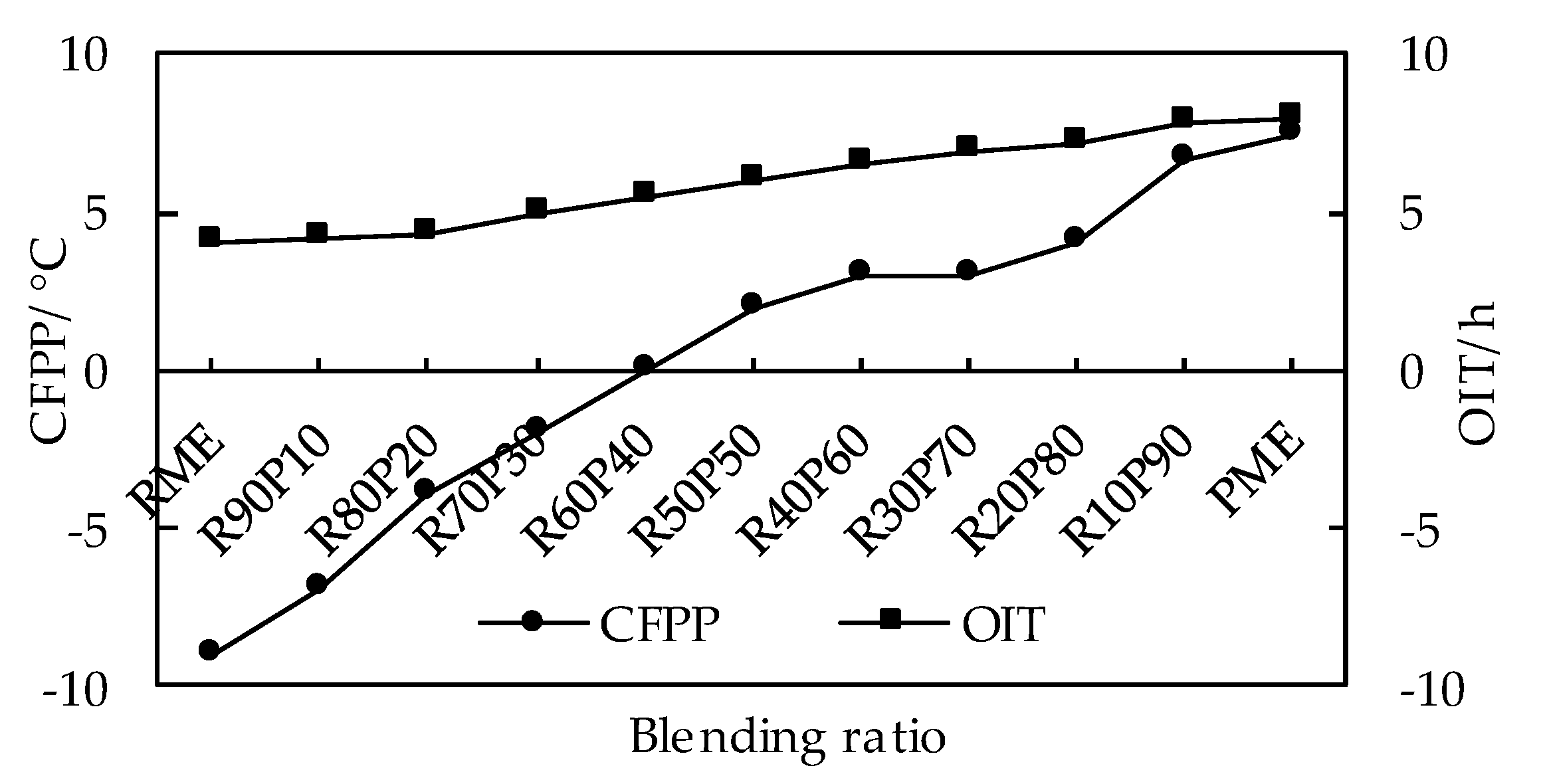
3.3.4. Effects of Blending Ratio on the Coupling Relationship between Low Temperature Fluidity and Oxidation Stability of Biodiesel
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Nazir, M.S.; Mahdi, A.J.; Bilal, M.; Sohail, H.M.; Ali, N.; Iqbal, H.M. Environmental impact and pollution-related challenges of renewable wind energy paradigm—A review. Sci. Total Environ. 2019, 683, 436–444. [Google Scholar] [CrossRef]
- Fan, W.Y.; Hao, Y. An empirical research on the relationship amongst renewable energy consumption, economic growth and foreign direct investment in China. Renew. Energy 2020, 146, 598–609. [Google Scholar] [CrossRef]
- Anis, S.; Budiandono, G.N. Investigation of the effects of preheating temperature of biodiesel-diesel fuel blends on spray characteristics and injection pump performances. Renew. Energy 2019, 140, 274–280. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. An experimental assessment on semi-low temperature combustion using waste oil biodiesel/C3-C5 alcohol blends in a diesel engine. Fuel 2020, 260, 116357. [Google Scholar] [CrossRef]
- Kim, J.K.; Jeon, C.H.; Lee, H.W.; Park, Y.K.; Min, K.I.; Hwang, I.H.; Kim, Y.M. Effect of Accelerated High Temperature on Oxidation and Polymerization of Biodiesel from Vegetable Oils. Energies 2018, 11, 3514. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Wang, Y.; Liu, X.; Sun, L. Study on Low Temperature Oxidation Characteristics of Oil Shale Based on Temperature Programmed System. Energies 2018, 11, 2594. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Nie, X.A.; Chen, J.; Wang, Y.G. Preparation of Epoxy Dimeric Acid Methyl Ester Plasiticizer from Biodiesel and Its Application in PVC. Chem. Ind. Eng. Prog. 2016, 35, 2934–2940. (In Chinese) [Google Scholar]
- Tziourtzioumis, D.N.; Stamatelos, A.M. Experimental Investigation of the Effect of Biodiesel Blends on a DI Diesel Engine’s Injection and Combustion. Energies 2017, 10, 970. [Google Scholar] [CrossRef] [Green Version]
- Devi, A.; Das, V.K.; Deka, D. A green approach for enhancing oxidation stability including long storage periods of biodiesel via Thuja oreantalis L. as an antioxidant additive. Fuel 2019, 253, 1264–1273. [Google Scholar] [CrossRef]
- Sui, M.; Li, F. Effect of TEPA on oxidation stability and metal ion content of biodiesel. Renew. Energy 2019, 143, 352–358. [Google Scholar] [CrossRef]
- Kpan, J.D.A.; Krahl, J. The impact of adsorbents on the oxidative stability of biodiesel and its influence on the deterioration of engine oil. Fuel 2019, 256, 115984. [Google Scholar] [CrossRef]
- Liu, W.; Lu, G.H.; Yang, G.L.; Bi, Y.L. Improving oxidative stability of biodiesel by cis-trans isomerization of carbon-carbon double bonds in unsaturated fatty acid methyl esters. Fuel 2019, 242, 133–139. [Google Scholar] [CrossRef]
- Devi, A.; Das, V.K.; Deka, D. Evaluation of the effectiveness of potato peel extract as a natural antioxidant on biodiesel oxidation stability. Ind. Crop. Prod. 2018, 123, 454–460. [Google Scholar] [CrossRef]
- Bryan, R.M. Impact of fatty ester composition on low temperature properties of biodiesel–petroleum diesel blends. Fuel 2014, 115, 500–506. [Google Scholar]
- Wu, M.X. Research on Factors Affecting Low Temperature Flow Performance of Biodiesel. Ph.D. Thesis, Nanjing University of Science and Technology, Nanjing, China, 2008. (In Chinese). [Google Scholar]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar]
- Lv, Y.; Li, J.; Ouyang, F.S. Improvement of Low Temperature Flow Properties of Biodiesel Blending. J. Fuel Chem. Technol. 2011, 39, 189–193. [Google Scholar]
- Lv, S.; Yang, T.; She, D.; Liu, Y.; Li, Y.; Wang, X.; Li, S.; Ni, H. Research on Improvement of Low Temperature Fluidity of Biodiesel. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Beijing, China, 19–21 June 2019; Volume 371. [Google Scholar]
- Freitas, O.N.; Rial, R.C.; Cavalheiro, L.F.; Barbosa, J.M.S.; Nazário, C.E.D.; Vianaa, L.H. Evaluation of the oxidative stability and cold filter plugging point of soybean methyl biodiesel/bovine tallow methyl biodiesel blends. Ind. Crop. Prod. 2019, 140, 111667. [Google Scholar] [CrossRef]
- Lin, C.Y.; Cheng, H.H. Application of mesoporous catalysts over palm-oil biodiesel for adjusting fuel properties. Energy Convers. Manag. 2012, 53, 128–134. [Google Scholar] [CrossRef]
- Roosta, A. New group interaction parameters of the UNIFAC model for the solubility of water in fatty acid methyl esters and biodiesel. Fuel 2018, 220, 339–344. [Google Scholar] [CrossRef]
- Fan, J.; Zhua, Z.X.; Wang, X.P.; Song, F.H.; Zhang, L.H. Experimental studies on the liquid thermal conductivity of three saturated fatty acid methyl esters components of biodiesel. J. Chem. Thermodyn. 2018, 125, 200–204. [Google Scholar] [CrossRef]
- Razavi, R.; Bemani, A.; Baghban, A.; Mohammadi, A.H.; Habibzadeh, S. An insight into the estimation of fatty acid methyl ester based biodiesel properties using a LSSVM model. Fuel 2019, 243, 133–141. [Google Scholar] [CrossRef]

| Reagent Name | Manufacturer |
|---|---|
| BHT (2,6-di-tert-butyl-4-methylphenol) | Shanghai Pengxiang Chemical Co., Ltd. (Shanghai, China) |
| BHA (2-tert-butyl-4-methoxyphenol) | Shanghai Pengxiang Chemical Co., Ltd. (Shanghai, China) |
| TBHQ (Tert-butyl hydroquinone) | Zhongshan Jiahui Food Additive Co., Ltd. (Zhongshan, China) |
| PG (3,4,5-trihydroxybenzoic acid) | Shanghai Yanxintang Biological Technology Co., Ltd. (Shanghai, China) |
| FAME | RME | TME | SME | OME | PNME | JCME | CSME | CTKME | CME | PME |
|---|---|---|---|---|---|---|---|---|---|---|
| Methyl laurate (C 12:0) | 0.26 | |||||||||
| Methyl myristate (C 14:0) | 0.40 | 1.09 | ||||||||
| Methyl palmitate (C 16:0) | 5.54 | 8.54 | 11.35 | 11.20 | 10.90 | 5.77 | 20.40 | 7.21 | 12.14 | 44.81 |
| Methyl stearate (C 18:0) | 1.78 | 1.78 | 4.31 | 1.96 | 2.70 | 5.73 | 1.40 | 2.28 | 1.99 | 4.09 |
| Methyl aramate (C 20:0) | 0.62 | 2.45 | 1.10 | 0.55 | 0.35 | |||||
| Methyl behenate (C 22:0) | 0.39 | 1.70 | ||||||||
| Methyl oleate (C 24:0) | 0.60 | |||||||||
| Methyl myrcene (C 12:1) | 3.16 | |||||||||
| Methyl oil palmitate (C 16:1) | 0.30 | 0.20 | ||||||||
| Methyl oleate (C 18:1) | 39.96 | 80.10 | 23.25 | 71.44 | 46.50 | 20.49 | 15.10 | 14.61 | 29.49 | 39.99 |
| Methyl eicosenoate (C 20:1) | 0.54 | 0.70 | 0.98 | |||||||
| Methyl erucate (C 22:1) | 16.18 | 0.30 | ||||||||
| Methyl linoleate (C 18:2) | 22.35 | 8.50 | 53.58 | 10.60 | 35.40 | 66.11 | 62.40 | 31.72 | 55.03 | 8.94 |
| Methyl linolenate (C 18:3) | 6.96 | 0.40 | 6.62 | 1.38 | 0.10 | 0.22 | 41.02 | 0.57 | 0.27 | |
| Total | 92.77 | 99.94 | 99.11 | 99.96 | 100.00 | 99.85 | 100.00 | 100.00 | 99.92 | 100.00 |
| SFAME | 7.32 | 10.94 | 15.66 | 16.00 | 17.00 | 12.05 | 22.20 | 9.49 | 14.13 | 50.60 |
| SU FAME | 56.14 | 80.10 | 23.25 | 71.98 | 47.50 | 21.47 | 15.40 | 17.77 | 29.49 | 40.19 |
| PU FAME | 29.31 | 8.90 | 60.20 | 11.98 | 35.50 | 66.33 | 62.40 | 72.74 | 55.60 | 9.21 |
| RME | TME | SME | OME | PNME | JCME | CSME | CTKME | CME | PME | |
|---|---|---|---|---|---|---|---|---|---|---|
| CFPP/°C | −9 | −9 | −5 | 8 | 12 | −3 | 6 | −14 | −5 | 8 |
| OIT/h | 3.72 | 3.57 | 2.46 | 3.68 | 2.79 | 5.07 | 3.22 | 1.47 | 2.48 | 7.38 |
| Ratio | Categories | ||
|---|---|---|---|
| SFAME | SUFAME | PUFAME | |
| RME | 7.32 | 56.14 | 29.13 |
| R90P10 | 11.648 | 54.545 | 27.138 |
| R80P20 | 15.976 | 52.95 | 25.146 |
| R70P30 | 20.304 | 51.355 | 23.154 |
| R60P40 | 24.632 | 49.76 | 21.162 |
| R50P50 | 28.96 | 48.165 | 19.17 |
| R40P60 | 33.288 | 46.57 | 17.178 |
| R30P70 | 37.616 | 44.975 | 15.186 |
| R20P80 | 41.944 | 43.38 | 13.194 |
| R10P90 | 46.272 | 41.785 | 11.202 |
| PME | 50.6 | 40.19 | 9.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Zhang, J.; Ni, H.; Wang, X.; Zhu, Y.; Chen, L. Study on the Coupling Relationship of Low Temperature Fluidity and Oxidation Stability of Biodiesel. Appl. Sci. 2020, 10, 1757. https://doi.org/10.3390/app10051757
Lv S, Zhang J, Ni H, Wang X, Zhu Y, Chen L. Study on the Coupling Relationship of Low Temperature Fluidity and Oxidation Stability of Biodiesel. Applied Sciences. 2020; 10(5):1757. https://doi.org/10.3390/app10051757
Chicago/Turabian StyleLv, Shuaishuai, Jiaqiao Zhang, Hongjun Ni, Xingxing Wang, Yu Zhu, and Lei Chen. 2020. "Study on the Coupling Relationship of Low Temperature Fluidity and Oxidation Stability of Biodiesel" Applied Sciences 10, no. 5: 1757. https://doi.org/10.3390/app10051757





