Transport Properties of Film and Bulk Sr0.98Zr0.95Y0.05O3−δ Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bulk Samples of SZY
2.2. Preparation of SZY Films
2.3. Characterization
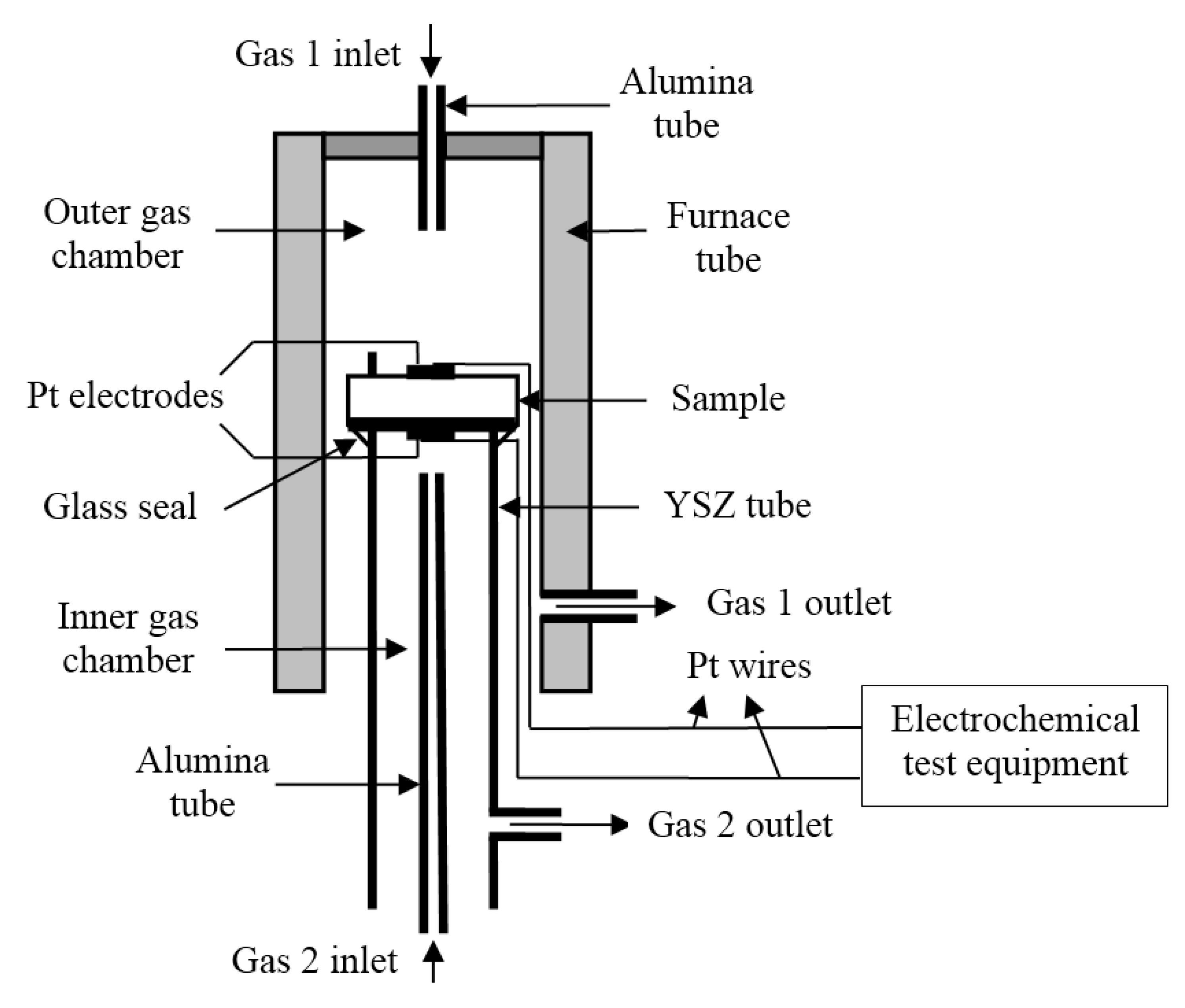
2.4. Measurement Description
3. Results and Discussion
3.1. Samples Characterization
3.2. Transport Numbers in SZY Membranes
3.2.1. Transport Numbers of Oxygen Ions in the Bulk and Film SZY Membranes
3.2.2. Conductivity and Transport Numbers of Oxygen Ions as Functions of the Oxygen Partial Pressure
3.2.3. Transport Numbers of Protons in the Bulk SZY Membrane in Oxidizing Atmospheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Ullah, M.K.; Usman, A. A brief description of high temperature solid oxide fuel cell’s operation, materials, design, fabrication, technologies and performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sust. Energ. Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Garbayo, I.; Baiutti, F.; Morata, A.; Tarancon, A. Engineering mass transport properties in oxide ionic and mixed ionic-electronic thin film ceramic conductors for energy applications. J. Eur. Ceram. Soc. 2019, 39, 101–114. [Google Scholar] [CrossRef]
- Tuller, H.L.; Litzelman, S.J.; Jung, W.; Saraf, L.; Shutthanandan, V.; McCready, D.E.; El-Azab, A.; Jaffe, J.E.; Engelhard, M.H.; Peden, C.H.F.; et al. Micro-ionics: Next generation power sources. Phys. Chem. Chem. Phys. 2009, 11, 3023–3034. [Google Scholar] [CrossRef]
- Kek, D.; Panjan, P.; Wanzenberg, E.; Jamnik, J. Electrical and microstructural investigations of cermet anode/YSZ thin film systems. J. Eur. Ceram. Soc. 2001, 21, 1861–1865. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Thevuthasan, S. State-of-the-art thin film electrolytes for solid oxide fuel cells. In Thin Film Structures in Energy Applications; Babu Krishna Moorthy, S., Ed.; Springer: Cham, Germany, 2015; pp. 167–214. [Google Scholar]
- Evans, A.; Bieberle-Hütter, A.; Rupp, J.L.M.; Gauckler, L.J. Review on microfabricated micro-solid oxide fuel cell membranes. J. Power Sources 2009, 194, 119–129. [Google Scholar] [CrossRef]
- Huang, H.; Nakamura, M.; Su, P.; Fasching, R.; Saito, Y.; Prinz, F.B. High-performance ultrathin solid oxide fuel cells for low-temperature operation. J. Electrochem. Soc. 2007, 154, B20–B24. [Google Scholar] [CrossRef]
- Shim, J.H.; Chao, C.-C.; Huang, H.; Prinz, F.B. Atomic layer deposition of yttria-stabilized zirconia for solid oxide fuel cells. Chem. Mater. 2007, 19, 3850–3854. [Google Scholar] [CrossRef]
- Su, P.C.; Chao, C.C.; Shim, J.H.; Fasching, R.; Prinz, F.B. Solid oxide fuel cell with corrugated thin film electrolyte. Nano Lett. 2008, 8, 2289–2292. [Google Scholar] [CrossRef]
- Elam, J.W.; Dasgupta, N.P.; Prinz, F.B. ALD for clean energy conversion, utilization, and storage. MRS Bull. 2011, 36, 899–906. [Google Scholar] [CrossRef]
- Solov’ev, A.A.; Sochugov, N.S.; Shipilova, A.V.; Efimova, K.B.; Tumashevskay, A.E. Mid-Temperature Solid Oxide Fuel Cells with Thin Film ZrO2:Y2O3 Electrolyte. Russ. J. Electrochem. 2011, 47, 494–502. [Google Scholar] [CrossRef]
- Noh, H.-S.; Yoon, K.J.; Kim, B.-K.; Je, H.-J.; Lee, H.-W.; Lee, J.-H.; Son, J.-W. The potential and challenges of thin-film electrolyte and nanostructured electrode for yttria-stabilized zirconia-base anode-supported solid oxide fuel cells. J Power Sources 2014, 247, 105–111. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Pankratov, A.A.; Gorelov, V.P.; Brouzgou, A.; Tsiakaras, P. Deposition and Characterization of Y-doped CaZrO3 Electrolyte Film on a Porous SrTi0.8Fe0.2O3−δ Substrate. Electrochim. Acta 2016, 202, 39–46. [Google Scholar] [CrossRef]
- Litzelman, S.J.; De Souza, R.A.; Butz, B.; Tuller, H.L.; Martin, M.; Gerthsen, D. Heterogeneously doped nanocrystalline ceria films by grain boundary diffusion: Impact on transport properties. J. Electroceram. 2009, 22, 405–415. [Google Scholar] [CrossRef]
- Agrirusis, C.; Kilo, M.; Muller, A.C.; Weber, A.; Borchardt, G.; Ivers-Tiffee, E. Interdiffusion between electrode and electrolyte materials for cathode supported SOFC’s with thin film electrolytes. In Proceedings of the XIII International Conference on High Temperature Materials Chemistry, Julich, Germany, 10–14 April 2000; Volume 4, pp. 1–5. [Google Scholar]
- Yamaji, K.; Xiong, Y.; Kishimoto, H.; Horita, T.; Brito, M.E.; Yokokawa, H. Electronic Conductivity and Efficiency of SOFC Electrolytes. ECS Trans. 2008, 12, 317–322. [Google Scholar]
- Li, Z.-P.; Mori, T.; Aucheterlonie, G.J.; Zou, J.; Drennan, J.; Miyayama, M. Diffusion and segregation along grain boundary at the electrolyte–anode interface in IT-SOFC. Solid State Ion. 2011, 191, 55–60. [Google Scholar] [CrossRef]
- Mills, E.M.; Kleine-Boymann, M.; Janek, J.; Yang, H.; Browning, N.D.; Takamura, Y.; Kim, S. YSZ thin films with minimized grain boundary resistivity. Phys. Chem. Chem. Phys. 2016, 18, 10486–10491. [Google Scholar] [CrossRef] [Green Version]
- Reisert, M.; Aphale, A.; Singh, P. Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches. Materials 2018, 11, 2169. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Suzuki, H.; Yogo, T.; Iwahara, H. Protonic conduction in SrZrO3-based oxides. Solid State Ion. 1992, 51, 101–107. [Google Scholar] [CrossRef]
- Gharbage, B.; Marques, F.M.B.; Frade, J.R. Protonic conduction in Sr1−y(Zr1−xDyx)O3−δ. Ceramics. J. Eur. Ceram. Soc. 1996, 16, 1149–1156. [Google Scholar] [CrossRef]
- Huang, P.; Petric, A. Electrical conduction of Y-doped strontium zirconate. J. Mater. Chem. 1995, 5, 53–56. [Google Scholar] [CrossRef]
- Huang, H.H.; Ishigame, M.; Shin, S. Protonic conduction in the single-crystals of Y-doped SrZrO3. Solid State Ion. 1991, 47, 251–255. [Google Scholar] [CrossRef]
- Muller, J.; Kreuer, K.D.; Maier, J.; Matsuo, S.; Ishigame, M. A conductivity and thermal gravimetric analysis of a Y-doped SrZrO3 single crystal. Solid State Ion. 1997, 97, 421–427. [Google Scholar] [CrossRef]
- Zajac, W.; Rusinek, D.; Zheng, K.; Molenda, J. Applicability of Gd-doped BaZrO3, SrZrO3, BaCeO3 and SrCeO3 proton conducting perovskites as electrolytes for solid oxide fuel cells. Cent. Eur. J. Chem. 2013, 11, 471–484. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Khaliullina, A.S.; Meshcherskikh, A.N.; Pankratov, A.A.; Osinkin, D.A. Effect of A-site nonstoichiometry on defect chemistry and electrical conductivity of undoped and Y-doped SrZrO3. Materials 2019, 12, 1258. [Google Scholar] [CrossRef] [Green Version]
- Dunyushkina, L.; Smirnov, S.; Kuimov, V.; Gorelov, V. Electrical conductivity of CaZr0.9Y0.1O3−δ films deposited from liquid solutions. Int. J. Hydrog. Energ. 2014, 39, 18385–18391. [Google Scholar] [CrossRef]
- Courtin, E.; Boy, P.; Piquero, T.; Vulliet, J.; Poirot, N.; Laberty-Robert, C. A composite sol–gel process to prepare a YSZ electrolyte for solid oxide fuel cells. J. Power Sources 2012, 206, 77–83. [Google Scholar] [CrossRef]
- Boukamp, B. A package for impedance/admittance data analysis. Solid State Ion. 1986, 18–19, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Boukamp, B. A Nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liao, H.L.; Li, W.Y.; Zhang, G.; Coddet, C.; Li, C.J.; Li, C.X.; Ning, X.J. Characterization of YSZ solid oxide fuel cells electrolyte deposited by atmospheric plasma spraying and low pressure plasma spraying. J. Therm. Spray Technol. 2006, 15, 598–603. [Google Scholar] [CrossRef]
- Liu, M.; Hu, H. Effect of interfacial resistance on determination of transport properties of mixed-conducting electrolytes. J. Electrochem. Soc. 1996, 143, L109–L112. [Google Scholar] [CrossRef]
- Agarwal, V.; Liu, M. Electrochemical properties of BaCe0.8Gd0.2O3 electrolyte films deposited on Ni-BaCe0.8Gd0.2O3 substrates. J. Electrochem. Soc. 1997, 144, 1035–1040. [Google Scholar] [CrossRef]
- Kim, S.; Maier, J. On the conductivity mechanism of nanocrystalline ceria. J. Electrochem. Soc. 2002, 149, J73–J83. [Google Scholar] [CrossRef]
- Guo, X.; Ding, Y. Grain boundary space charge effect in zirconia. Experimental Evidence. J. Electrochem. Soc. 2004, 151, J1–J7. [Google Scholar] [CrossRef] [Green Version]
- Iguchi, F.; Sata, N.; Yugami, H. Proton transport properties at the grain boundary of barium zirconate based proton conductors for intermediate temperature operating SOFC. J. Mater. Chem. 2010, 20, 6265–6270. [Google Scholar] [CrossRef]
- Shirpour, M.; Merkle, R.; Maier, J. Space charge depletion in grain boundaries of BaZrO3 proton conductors. Solid State Ion. 2012, 225, 304–307. [Google Scholar] [CrossRef]
- Gerstl, M.; Navickas, E.; Friedbacher, G.; Kubel, F.; Ahrens, M.; Fleig, J. The separation of grain and grain boundary impedance in thin yttria stabilized zirconia (YSZ) layers. Solid State Ion. 2011, 185, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Heras-Juaristi, G.; Pérez-Coll, D.; Mather, G.C. Effect of sintering conditions on the electrical-transport properties of the SrZrO3-based protonic ceramic electrolyser membrane. J. Power Sources 2016, 331, 435–444. [Google Scholar] [CrossRef]
- Labrincha, J.A.; Marques, F.M.B.; Frade, J.R. Protonic and oxygen-ion conduction in SrZrO3-based materials. J. Mater. Sci. 1995, 30, 2785–2792. [Google Scholar] [CrossRef]
- Kreuer, K.D. Strategies in the development of proton conducting oxides for fuel cell applications in ionic and mixed conducting ceramics. J. Electrochem. Soc. 1998, 97, 17–27. [Google Scholar]
- Pérez-Coll, D.; Heras-Juaristi, G.; Fagg, D.P.; Mather, G.C. Transport-number determination of a protonic ceramic electrolyte membrane via electrode-polarisation correction with the Gorelov method. J. Power Sources 2014, 245, 445–455. [Google Scholar] [CrossRef] [Green Version]











| σ, S cm−1 | S cm−1 | σh, S cm−1 | σexp, S cm−1 | Ea(σ), eV | Ea(σh), eV | Ea(σ exp), eV | ||
|---|---|---|---|---|---|---|---|---|
| Our data | 9.1 × 10−5 | 7.6 × 10−5 | 5.4 × 10−6 | 1.2 × 10−4 | 1.03 | 0.94 | 1.58 | 0.92 |
| Ref. [42]1 | 7.7 × 10−5 | 1.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliullina, A.; Dunyushkina, L.; Pankratov, A. Transport Properties of Film and Bulk Sr0.98Zr0.95Y0.05O3−δ Membranes. Appl. Sci. 2020, 10, 2229. https://doi.org/10.3390/app10072229
Khaliullina A, Dunyushkina L, Pankratov A. Transport Properties of Film and Bulk Sr0.98Zr0.95Y0.05O3−δ Membranes. Applied Sciences. 2020; 10(7):2229. https://doi.org/10.3390/app10072229
Chicago/Turabian StyleKhaliullina, Adelya, Liliya Dunyushkina, and Alexander Pankratov. 2020. "Transport Properties of Film and Bulk Sr0.98Zr0.95Y0.05O3−δ Membranes" Applied Sciences 10, no. 7: 2229. https://doi.org/10.3390/app10072229







