Developments in Antibiotic-Eluting Scaffolds for the Treatment of Osteomyelitis
Abstract
:1. Osteomyelitis
2. Local Treatments
2.1. Polymethyl Methacrylate
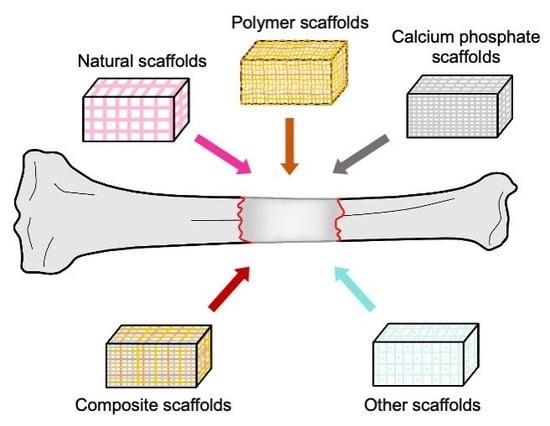
2.2. Scaffolds
3. Properties of an Antibiotic-Eluting Scaffold
3.1. Biocompatability
3.2. Biodegradability
3.3. Mechanical and Structural Properties
3.4. Bone Growth
3.5. Manufacturing Properties
4. The Choice of Antibiotic
5. Natural Scaffolds
5.1. Collagen
5.2. Chitosan
6. Synthetic Scaffolds
6.1. Polymers
6.2. Calcium Phosphates
7. Composite Scaffolds
8. Other Antimicrobial Materials
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lazzarini, L.; Mader, J.T.; Calhoun, J.H. Osteomyelitis in long bones. JBJS 2004, 86, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ma, Y.F.; Jiang, Y.; Zhao, X.Q.; Xie, G.P.; Hu, Y.J.; Qin, C.H.; Yu, B. Clinical characteristics and treatment of extremity chronic Osteomyelitis in Southern China. Medicine 2015, 94, e1874. [Google Scholar] [CrossRef] [PubMed]
- Leonidou, A.; Kiraly, Z.; Gality, H.; Apperley, S.; Vanstone, S.; Woods, D.A. The effect of the timing of antibiotics and surgical treatment on infection rates in open long-bone fractures: A 6-year prospective study after a change in policy. Strateg. Trauma Limb Reconstr. 2014, 9, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Springer, B.D.; Cahue, S.; Etkin, C.D.; Lewallen, D.G.; McGrory, B.J. Infection burden in total hip and knee arthroplasties: An international registry-based perspective. Arthroplast. Today 2017, 3, 137–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar] [PubMed]
- Panteli, M.; Giannoudis, P.V. Chronic osteomyelitis: What the surgeon needs to know. EFORT Open Rev. 2016, 1, 128–135. [Google Scholar] [CrossRef]
- Klenerman, L. A history of osteomyelitis from the Journal of Bone and Joint Surgery: 1948 to 2006. JBJS 2007, 89, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Beaman, F.D.; Von Herrmann, P.F.; Kransdorf, M.J.; Adler, R.S.; Amini, B.; Appel, M.; Arnold, E.; Bernard, S.A.; Greenspan, B.S.; Lee, K.S.; et al. Appropriateness Criteria ® Suspected Osteomyelitis, Septic Arthritis, or Soft Tissue Infection (Excluding Spine and Diabetic Foot) Expert Panel on Musculoskeletal Imaging. J. Am. Coll. Radiol. 2017, 14, 326–337. [Google Scholar] [CrossRef] [Green Version]
- NHS Osteomyelitis. Available online: https://www.nhs.uk/conditions/osteomyelitis/ (accessed on 25 February 2020).
- Ferguson, J.; Wong, T.H.N.; Atkins, L.B.; McNally, M. Osteomyelitis. BMJ Best Pract. 2018, 1–44. [Google Scholar]
- Cierny, G.; Mader, J.T.; Penninck, J.J. A clinical staging system for adult osteomyelitis. Clin. Orthop. Relat. Res. 2003, 414, 7–24. [Google Scholar] [CrossRef] [Green Version]
- McNally, M.; Nagarajah, K. Osteomyelitis. Orthop. Trauma 2010, 24, 416–429. [Google Scholar] [CrossRef]
- Fritz, J.M.; McDonald, J.R. Osteomyelitis: Approach to diagnosis and treatment. Phys. Sportsmed. 2008, 36, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.L. The Current Status of Material Used for Depot Delivery of Drugs. Clin. Orthop. Relat. Res. 2004, 427, 72–78. [Google Scholar] [CrossRef]
- Gogia, J.; Meehan, J.; Di Cesare, P.; Jamali, A. Local Antibiotic Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-based Therapies for Fractures and Bone defects. Front. Bioeng. Biotechnol. 2018, 31, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Wahlig, H.; Dingeldein, E.; Bergmann, R.; Reuss, K. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. JBJS 1978, 60B, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.P.; Nelson, C.L. Gentamicin-impregnated polymethylmethacrylate beads compared with systemic antibiotic therapy in the treatment of chronic osteomyelitis. Clin. Orthop. Relat. Res. 1993, 295, 37–42. [Google Scholar] [CrossRef]
- Barth, R.E.; Vogely, H.C.; Hoepelman, A.I.M.; Peters, E.J.G. “To bead or not to bead?” Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains. Int. J. Antimicrob. Agents 2011, 38, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.L.; Evans, R.P.; Blaha, J.D.; Calhoun, J.; Henry, S.L.; Patzakis, M.J. A comparison of gentamicin-impregnated polymethylmethacrylate bead implantation to conventional parenteral antibiotic therapy in infected total hip and knee arthroplasty. Clin. Orthop. Relat. Res. 1993, 295, 96–101. [Google Scholar] [CrossRef]
- Shih, H.-N.; Shih, L.-Y.; Wong, Y.-C. Diagnosis and Treatment of Subacute Osteomyelitis. J. Trauma Infect. Crit. Care 2005, 58, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.J.; Thomson, H.E.; Harper, N.J.; Kenny, N.W. Bone cement implantation syndrome. Br. J. Anaesth. 2009, 102, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, N.; Rezzadeh, K.S.; Lee, J.C. Biomimetic Scaffolds for Osteogenesis. Recept. Clin. Investig. 2015, 2, 898. [Google Scholar]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Ward, W.G.; Gautreaux, M.D.; Lippert, D.C.; Boles, C. HLA sensitization and allograft bone graft incorporation. Clin. Orthop. Relat. Res. 2008, 466, 1837–1848. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, E.D.; Battle, R.K.; Zahra, S.; Keating, J.F.; Marson, L.P.; Turner, D.M. Allosensitization Following Bone Graft. Am. J. Transplant. 2017, 17, 2207–2211. [Google Scholar] [CrossRef] [Green Version]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res. 2018, 67, 335–348. [Google Scholar] [CrossRef]
- Neut, D.; van de Belt, H.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials 2003, 24, 1829–1831. [Google Scholar] [CrossRef]
- Hope, P.G.; Kristinsson, K.G.; Norman, P.; Elson, R.A. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. JBJS 1989, 71, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Stravinskas, M.; Horstmann, P.; Ferguson, J.; Hettwer, W.; Nilsson, M.; Tarasevicius, S.; Petersen, M.M.; McNally, M.A.; Lidgren, L. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute in vitro and clinical release studies. JBJS 2016, 5, 427–435. [Google Scholar]
- Kendall, R.W.; Duncan, C.P.; Smith, J.A.; Ngui-Yen, J.H. Persistence of bacteria on antibiotic loaded acrylic depots: A reason for caution. Clin. Orthop. Relat. Res. 1996, 329, 273–280. [Google Scholar] [CrossRef]
- Wheelton, A.; Mace, J.; Khan, W.S.; Anand, S. Biomaterials and Fabrication to Optimise Scaffold Properties for Musculoskeletal Tissue Engineering. Curr. Stem Cell Res. Ther. 2016, 11, 578–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdizadeh, H.; Sumo, S.; Bayrak, E.S.; Brey, E.M.; Cinar, A. Three-dimensional modeling of angiogenesis in porous biomaterial scaffolds. Biomaterials 2013, 34, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Mestres, G.; Fernandez-Yague, M.A.; Pastorino, D.; Montufar, E.B.; Canal, C.; Manzanares-Céspedes, M.C.; Ginebra, M.P. In vivo efficiency of antimicrobial inorganic bone grafts in osteomyelitis treatments. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 84–95. [Google Scholar] [CrossRef]
- Rasyid, H.N.; Soegijoko, S. Influence of soluble fillers in improving porosity of handmade antibiotic-impregnated polymethyl methacrylate (PMMA) beads: An in-vitro study. Malays. Orthop. J. 2016, 10, 6–10. [Google Scholar]
- Schlickewei, C.W.; Yarar, S.; Rueger, J.M. Eluting antibiotic bone graft substitutes for the treatment of osteomyelitis in long bones. A review: Evidence for their use? Orthop. Res. Rev. 2014, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Van Der Stok, J.; Van Lieshout, E.M.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the Netherlands—A systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Hosseini, F.S.; Soleimanifar, F.; Ardeshirylajimi, A.; Vakilian, S.; Mossahebi-Mohammadi, M.; Enderami, S.E.; Khojasteh, A.; Zare Karizi, S. In vitro osteogenic differentiation of stem cells with different sources on composite scaffold containing natural bioceramic and polycaprolactone. Artif. Cells Nanomed. Biotechnol. 2019, 47, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.H.; Kim, M.J.; Park, J.Y.; Pati, R.G.; Yun, Y.P.; Kim, S.E.; Song, H.R.; Cho, D.W. Three-dimensional printing of antibiotics-loaded poly-ε-caprolactone/poly(lactic-co-glycolic acid) scaffolds for treatment of chronic osteomyelitis. Tissue Eng. Regen. Med. 2015, 12, 283–293. [Google Scholar] [CrossRef]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; Bentley, K.L.M.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Calcium phosphate spacers for the local delivery of sitafloxacin and rifampin to treat orthopedic infections: Efficacy and proof of concept in a mouse model of single-stage revision of device-associated osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaren, J.S.; White, L.J.; Cox, H.C.; Ashraf, W.; Rahman, C.V.; Blunn, G.W.; Goodship, A.E.; Quirk, R.A.; Shakesheff, K.M.; Bayston, R.; et al. A biodegradable antibiotic-impregnated scaffold to prevent osteomyelitis in a contaminated in vivo bone defect model. Eur. Cell. Mater. 2014, 27, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.A.; Cassat, J.E. Advances in the local and targeted delivery of anti-infective agents for management of osteomyelitis. Expert Rev. Anti Infect. Ther. 2017, 15, 851–860. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold. Int. J. Nanomed. 2017, 12, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.P.; Higham, P.A.; Dumbleton, J.H. Effectiveness of bone cement containing tobramycin. An in vitro susceptibility study of 99 organisms found in infected joint arthroplasty. J. Bone Jt. Surg. Br. 1999, 81, 440–443. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Mrestani, Y.; Neubert, R.H.H.; Bernstein, A.; Mayr, H.O. Release Kinetics and Antibacterial Efficacy of Microporous β-TCP Coatings. J. Nanomater. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Restricting the Selection of Antibiotic-Resistant Mutants: A General Strategy Derived from Fluoroquinolone Studies. Clin. Infect. Dis. 2001, 33, S147–S156. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Van Vugt, T.A.G.; Walraven, J.M.B.; Geurts, J.A.P.; Arts, J.J.C. Antibiotic-Loaded Collagen Sponges in Clinical Treatment of Chronic Osteomyelitis: A Systematic Review. J. Bone Jt. Surg. Am. 2018, 100, 2153–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, T.S.; Sørensen, L.I.; Merser, S. Rapid release of gentamicin from collagen sponge: In vitro comparison with plastic beads. Acta Orthop. 1990, 61, 353–356. [Google Scholar] [CrossRef]
- Hapach, L.A.; VanderBurgh, J.A.; Miller, J.P.; Reinhart-King, C.A. Manipulation of in vitro collagen matrix architecture for scaffolds of improved physiological relevance. Phys. Biol. 2015, 12, 061002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Hafeman, A.E.; Zienkiewicz, K.J.; Carney, E.; Litzner, B.; Stratton, C.; Wenke, J.C.; Guelcher, S.A. Local delivery of tobramycin from injectable biodegradable polyurethane scaffolds. J. Biomater. Sci. Polym. Ed. 2010, 21, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Brown, K.V.; Wenke, J.C.; Guelcher, S.A. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J. Control. Release 2010, 145, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.D.; Kuthe, A.M.; Quazi, S.; Gupta, V.; Jaiswal, S.; Jyothilal, S.; Lande, N.; Nagdeve, S. State of the Art Technology for Bone Tissue Engineering and Drug Delivery. IRBM 2019, 40, 133–144. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.L.; Chen, C.K.; Lee, J.W.; Lee, Y.L.; Ju, C.P.; Lin, J.H.C. Structure, properties and animal study of a calcium phosphate/calcium sulfate composite cement. Mater. Sci. Eng. C 2014, 37, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.Y.; Dudareva, M.; Riley, N.D.; Stubbs, D.; Atkins, B.L.; McNally, M.A. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: A series of 195 cases. Bone Jt. J. 2014, 96B, 829–836. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Wild, L.M.; Schemitsch, E.H.; Waddell, J.P. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: Early results of a prospective trial. J. Orthop. Trauma 2002, 16, 622–627. [Google Scholar] [CrossRef]
- Vugt, T.A.G.; Geurts, J.; Arts, J.J. Clinical Application of Antimicrobial Bone Graft Substitute in Osteomyelitis Treatment: A Systematic Review of Different Bone Graft Substitutes Available in Clinical Treatment of Osteomyelitis. BioMed Res. Int. 2016, 2016, 6984656. [Google Scholar] [CrossRef] [Green Version]
- McKee, M.D.; Li-Bland, E.A.; Wild, L.M.; Schemitsch, E.H. A Prospective, Randomized Clinical Trial Comparing an Antibiotic-Impregnated Bioabsorbable Bone Substitute With Standard Antibiotic-Impregnated Cement Beads in the Treatment of Chronic Osteomyelitis and Infected Nonunion. J. Orthop. Trauma 2010, 24, 483–490. [Google Scholar] [CrossRef]
- Flierl, M.A.; Culp, B.M.; Okroj, K.T.; Springer, B.D.; Levine, B.R.; Della Valle, C.J. Poor Outcomes of Irrigation and Debridement in Acute Periprosthetic Joint Infection With Antibiotic-Impregnated Calcium Sulfate Beads. J. Arthroplast. 2017, 32, 2505–2507. [Google Scholar] [CrossRef]
- Fleiter, N.; Walter, G.; Bösebeck, H.; Vogt, S.; Büchner, H.; Hirschberger, W.; Hoffmann, R. Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis. Bone Jt. Res. 2014, 3, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Humm, G.; Noor, S.; Bridgeman, P.; David, M.; Bose, D. Adjuvant treatment of chronic osteomyelitis of the tibia following exogenous trauma using OSTEOSET®-T: A review of 21 patients in a regional trauma centre. Strateg. Trauma Limb Reconstr. 2014, 9, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, M.A.; Ferguson, J.Y.; Lau, A.C.K.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: A prospective series of 100 cases. Bone Jt. J. 2016, 98B, 1289–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, K.E.; Maier, G.S.; Schmidtmann, I.; Eigner, U.; Hübner, W.D.; Peters, F.; Drees, P.; Maus, U. Release of Antibiotics Out of a Moldable Collagen-β-Tricalciumphosphate-Composite Compared to Two Calcium Phosphate Granules. Materials 2019, 12, 4056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.; Qu, H.; Zhang, G.; Zhang, X. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Qin, J.; Zhang, B.; Zheng, Y.; Yang, L.; Shen, Y.; Zuo, B.; Zhang, F. Gentamicin-loaded silk/ nanosilver composite scaffolds for MRSA-induced chronic osteomyelitis. R. Soc. Open Sci. 2019, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhou, X.G.; Wang, J.W.; Zhou, H.; Dong, J. Treatment of osteomyelitis defects by a vancomycin-loaded gelatin/β-tricalcium phosphate composite scaffold. Bone Jt. Res. 2018, 7, 46–57. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Liu, W.; He, X.; Zhou, N.; Zhang, D.; Gu, H.; Li, J.; Jiang, J.; Huang, W. Levofloxacin loaded mesoporous silica microspheres/nanohydroxyapatite/ polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Kamboj, N.; Rodríguez, M.A.; Rahmani, R.; Gokuldoss Prashanth, K.; Hussainova, I. Bioceramic scaffolds by additive manufacturing for controlled delivery of the antibiotic vancomycin. Proc. Est. Acad. Sci. 2019, 68, 185–190. [Google Scholar] [CrossRef]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2019. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Fille, M.; Hausdorfer, J.; Putzer, D.; Nogler, M. Efficacy of antibacterial bioactive glass S53P4 against S. aureus biofilms grown on titanium discs in vitro. J. Orthop. Res. 2014, 32, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Toward Strong and Tough Glass and Ceramic Scaffolds for Bone Repair. Adv. Funct. Mater. 2013, 23, 5461–5476. [Google Scholar] [CrossRef]
- Lindfors, N.; Geurts, J.; Drago, L.; Arts, J.J.; Juutilainen, V.; Hyvönen, P.; Suda, A.J.; Domenico, A.; Artiaco, S.; Alizadeh, C.; et al. Antibacterial bioactive glass S53P4 for chronic bone infections—A multinational study. Adv. Exp. Med. Biol. 2017, 971, 81–92. [Google Scholar]
- Nandi, S.K.; Mahato, A.; Kundu, B.; Mukherjee, P. Doped Bioactive Glass Materials in Bone Regeneration. Adv. Tech. Bone Regen. 2016, 13, 276–327. [Google Scholar]
- Mueller, B.; Treccani, L.; Rezwan, K. Antibacterial active open-porous hydroxyapatite/lysozyme scaffolds suitable as bone graft and depot for localized drug delivery. J. Biomat. Appl. 2017, 31, 1123–1134. [Google Scholar] [CrossRef]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered Polymer Nanoparticles with Unprecedented Antimicrobial Efficacy and Therapeutic Indices against Multidrug-Resistant Bacteria and Biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for bone tissue engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef] [Green Version]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Yao, Q.; Li, L.; Zhang, X.; Wei, B.; Yuan, L.; Wang, L. Antimicrobial Activity of 3D-Printed Poly(ε-Caprolactone) (PCL) Composite Scaffolds Presenting Vancomycin-Loaded Polylactic Acid-Glycolic Acid (PLGA) Microspheres. Med. Sci. Monit. 2018, 24, 6934–6945. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 2. [Google Scholar] [CrossRef] [Green Version]

| Authors | Number of Patients | Material | Systemic Antibiotics Used | Mean Follow-up (yrs) | Eradication Rate | Other Outcomes |
|---|---|---|---|---|---|---|
| McKee et al., 2002 [68] | 25 | OSTEOSET®-T (calcium sulfate + tobramycin) | Yes | 2.3 | 92% | 12% fracture, 32% wound leak, 36% had autologous bone grafting. |
| McKee et al., 2010 [70] | 15 | OSTEOSET®-T (calcium sulfate + tobramycin) | Yes | 3.2 | 86% (same result as PMMA) | 14% fracture, 21% wound leak, 33% underwent further surgical procedures. |
| Fleiter et al., 2014 [72] | 20 | HERAFILL® G (calcium sulfate + calcium carbonate + gentamicin) | No | 0.5 | 80% | No adverse outcomes reported, sufficient gentamicin elution rates measured. |
| Humm et al., 2014 [73] | 21 | OSTEOSET®-T (calcium sulfate + tobramycin) | Yes | 1.3 | 95% | 33.3% wound discharge, 100% union rate, 24% delayed wound-healing or pin-site infections. |
| Ferguson et al., 2014 [67] | 195 | OSTEOSET ®-T (calcium sulfate + tobramycin) | Yes | 3.7 | 91% | 4.7% fracture (at a mean of 1.9 years), 15.4% wound leak, radiographic bone filling absent in 36.6%, partial in 59% and complete in 8%. |
| McNally et al., 2016 [74] | 100 | CERAMENT® G (calcium sulfate + hydroxyapatite + gentamicin) | Yes | 1.6 | 96% | 3% fracture, 6% wound leak. |
| Authors | Study Type | Materials | Main Finding [s] |
|---|---|---|---|
| Cheng et al., 2017 [76] | In vitro | Bioglass + PLGA + vancomycin | Supported the fewest viable bacteria compared to controls after 24 h of S. aureus culture. Effect was maintained even after 6 cycles of exposure. |
| Wang et al., 2017 [79] | In vivo (rabbits) | Silica microspheres + nano-HA + polyurethane + levofloxacin (lev) | Increased bone formation compared to controls and lev-PMMA at 6- and 12-weeks. After this time, the scaffold began to degrade. |
| Zhou et al., 2018 [78] | In vivo (rabbits) | Gelatin + β-TCP + vancomycin | At 8 weeks, the radiological and histopathological severities were significantly better than controls (7.3× and 3.66× respectively). |
| Kamboj et al., 2019 [80] | In vitro | Silicon–calcium silicate + polycaprolactone + vancomycin (3D-printed) | Observed a two-step, controlled antibiotic release profile: ~50% during the first 40 h, then sustained release of 20% over the next 6 days. |
| Kuang et al., 2019 [81] | In vitro | Silica microspheres + nano-HA + polyurethane + levofloxacin | Observed increased osteogenic differentiation of bone marrow stem cells at 14 days, a lower number of bacterial colony units at 12 days, decreased apoptosis of osteoblast precursors and decreased microbial adhesion compared to controls. |
| Zhang et al., 2019 [77] | In vivo (rats) | Silk + nanosilver + gentamicin | Lower colony count at 3 weeks compared to controls. Four of the six cases in this group inhibited bacterial growth completely. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriacou, H.; Kamaraj, A.; Khan, W.S. Developments in Antibiotic-Eluting Scaffolds for the Treatment of Osteomyelitis. Appl. Sci. 2020, 10, 2244. https://doi.org/10.3390/app10072244
Kyriacou H, Kamaraj A, Khan WS. Developments in Antibiotic-Eluting Scaffolds for the Treatment of Osteomyelitis. Applied Sciences. 2020; 10(7):2244. https://doi.org/10.3390/app10072244
Chicago/Turabian StyleKyriacou, Harry, Achi Kamaraj, and Wasim S. Khan. 2020. "Developments in Antibiotic-Eluting Scaffolds for the Treatment of Osteomyelitis" Applied Sciences 10, no. 7: 2244. https://doi.org/10.3390/app10072244
APA StyleKyriacou, H., Kamaraj, A., & Khan, W. S. (2020). Developments in Antibiotic-Eluting Scaffolds for the Treatment of Osteomyelitis. Applied Sciences, 10(7), 2244. https://doi.org/10.3390/app10072244