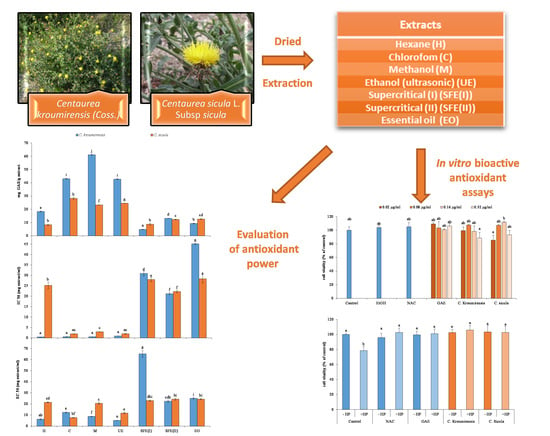
Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plants Material
2.3. Preparation of the Extracts
2.3.1. Hydrodistillation Procedure (HD)
2.3.2. Conventional Extraction with Soxhlet Apparatus (SE)
2.3.3. Ultrasound-Assisted Extraction (UAE)
2.3.4. Supercritical Fluid Extraction (SFE-CO2)
2.4. Total Phenolic Content
2.5. Antioxidant Properties
2.5.1. DPPH Scavenging Activity
2.5.2. Reducing Power Activity (RP)
2.6. Cell Culture
2.7. Protective Effect, against Induced Oxidative Stress, in Human Skin Fibroblast (HS-68) Cells
2.8. Statistical Analysis
3. Results and Discussions
3.1. Total Phenolic Content (TPC)
3.2. Antioxidant Properties
3.2.1. DPPH Scavenging Activity
3.2.2. Reducing Power Activity
3.3. Correlation between Total Phenolic Content and Antioxidant Properties
3.4. In Vitro Bioactive Antioxidant Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albayrak, S.; Atasagun, B.; Aksoy, A. Comparison of phenolic components and biological activities of two Centaurea sp. obtained by three extraction techniques. Asian Pac. J. Trop. Med. 2017, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.; Niokhor, P.; Stevanovic, T. Polyphenol contents and radical scavenging capacities of red maple (Acer rubrum L.) extracts. Food Chem. Toxicol. 2011, 49, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Soni, B.; Dalwadi, N. Free radical scavenging and antiatherogenic activities of Sesamum indicum seed extracts in chemical and biological model systems. Food Chem. Toxicol. 2009, 47, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aktumsek, A.; Guler, G.O.; Cakmak, S.; Yildiztugay, E. Antioxidant Properties of Methanolic Extract and Fatty Acid Composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec. Nat. Prod. 2011, 2, 123–132. [Google Scholar]
- Asirvatham, R.; Christina, A.J.M.; Murali, A. In vitro antioxidant and anticancer activity studies on Drosera indica L. (droseraceae). Adv. Pharm. Bull. 2013, 3, 115–120. [Google Scholar]
- Auten, R.L.; Davis, J.M. The Role of Oxygen in Health and Disease-A Series of Reviews Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Karawita, R.; Siriwardhana, N.; Lee, K.W.; Heo, M.S.; Yeo, I.K.; Lee, Y.D.; Jeon, Y.J. Reactive oxygen species scavenging, metal chelation, reducing power and lipid peroxidation inhibition properties of different solvent fractions from Hizikia fusiformis. Eur. Food Res. Technol. 2005, 220, 363–371. [Google Scholar] [CrossRef]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Zengin, G.; Selim, Y.; Ozmen, G.; Aktumsek, A. In vitro antioxidant capacities and fatty acid compositions of three Centaurea species collected from Central Anatolia region of Turkey. Food Chem. Toxicol. 2010, 48, 2638–2641. [Google Scholar] [CrossRef] [PubMed]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, I.; Yesilada, E. Evaluation of the anti-ulcerogenic effect of sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis by using various in vivo and biochemical techniques. J. Ethnopharmacol. 2007, 112, 284–291. [Google Scholar]
- Deng, C.; Li, S.; Feng, C.; Hong, Y.; Huang, H.; Wang, J.; Wang, L.; Dai, S. Plant Physiology and Biochemistry Metabolite and gene expression analysis reveal the molecular mechanism for petal colour variation in six Centaurea cyanus cultivars. Plant Physiol. Biochem. 2019, 142, 22–33. [Google Scholar] [CrossRef]
- Dalar, A.; Uzun, Y.; Mukemre, M.; Turker, M.; Konczak, I. Centaurea karduchorum Boiss. from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. J. Herb. Med. 2015, 5, 211–216. [Google Scholar] [CrossRef]
- Ifantis, T.M.; Solujić, S.; Pavlović-Muratspahić, D.; Skaltsa, H. Secondary metabolites from the aerial parts of Centaurea pannonica (Heuff.) Simonk. from Serbia and their chemotaxonomic importance. Phytochemistry 2013, 94, 159–170. [Google Scholar] [CrossRef]
- Shoeb, M.; Celik, S.; Jaspars, M.; Kumarasamy, Y.; Macmanus, S.M.; Nahar, L.; Thoo-lin, P.K.; Sarker, S.D. Isolation, structure elucidation and bioactivity of schischkiniin, a unique indole alkaloid from the seeds of Centaurea schischkinii. Tetrahedron 2005, 61, 9001–9006. [Google Scholar] [CrossRef]
- Demir, S.; Karaalp, C.; Bedir, E. Phytochemistry Specialized metabolites from the aerial parts of Centaurea polyclada DC. Phytochemistry 2017, 143, 12–18. [Google Scholar] [CrossRef]
- Escher, G.B.; Santos, J.S.; Rosso, N.D.; Marques, M.B.; Azevedo, L.; do Carmo, M.A.V.; Daguer, H.; Molognoni, L.; do Prado-Silva, L.; Sant’Ana, A.S.; et al. Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol. 2018, 118, 439–453. [Google Scholar] [CrossRef]
- Zengin, G.; Llorent-martínez, E.J.; Ibrahime, K.; Yıldıztugay, E.; Picot-allain, C.; Mahomoodally, M.F. Chemical profiling of Centaurea bornmuelleri Hausskn. aerial parts by HPLC-MS/MS and their pharmaceutical effects: From nature to novel perspectives. J. Pharm. Biomed. Anal. 2019, 174, 406–413. [Google Scholar] [CrossRef]
- Zengin, G.; Zheleva-dimitrova, D.; Gevrenova, R.; Glamoˇ, J.; Nedialkov, P.; Mocan, A.; Ciri, A.; Glamočlija, J.; Sokovi, M.; Aktumsek, A.; et al. Identification of phenolic components via LC-MS analysis and biological activities of two Centaurea species: C. drabifolia subsp. drabifolia and C. lycopifolia. J. Pharm. Biomed. Anal. 2018, 149, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X1987417. [Google Scholar] [CrossRef] [Green Version]
- Griveau, J.F.; Dumont, E.; Renard, P.; Callegari, J.P.; Le Lannou, D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J. Reprod. Fertil. 1995, 103, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folin, O.; Ciocalteu, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar]
- Messina, C.M.; Troia, A.; Arena, R.; Manuguerra, S.; Ioannou, T.; Curcuraci, E.; Renda, G.; Hellio, C.; Santulli, A. Species-Specific Antioxidant Power and Bioactive Properties of the Extracts Obtained from Wild Mediterranean Calendula Spp. (Asteraceae). Appl. Sci. 2019, 9, 4627. [Google Scholar] [CrossRef] [Green Version]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The antioxidant power of horseradish, Armoracia rusticana, underlies antimicrobial and antiradical effects, exerted in vitro. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In vitro antioxidant activity and in vivo topical efficacy of lipid nanoparticles co-loadingm idebenone and tocopheryl acetate. Appl. Sci. 2019, 9, 845. [Google Scholar] [CrossRef] [Green Version]
- Erol-Dayi, Ö.; Pekmez, M.; Bona, M.; Aras-Perk, A.; Arda, N. Total Phenolic Contents, Antioxidant Activities Cytotoxicity of Three Centaurea Species: C. calcitrapa subsp. calcitrapa, C. ptosimopappa C. spicata. Free Radic. Antioxid. 2011, 1, 31–36. [Google Scholar] [CrossRef]
- Messina, C.M.; Pizzo, F.; Santulli, A.; Bušelić, I.; Boban, M.; Orhanović, S.; Mladineo, I. Anisakis pegreffii (Nematoda: Anisakidae) products modulate oxidative stress and apoptosis-related biomarkers in human cell lines. Parasite Vector 2016, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, F.A.; Ozcan, M.; Kurt, A.; Karayigit, B.; Ozogul, Y.; Glew, R.; Ozogul, F. Fatty acid composition and antioxidant capacity of cypselas in Centaurea s. l. taxa (Asteraceae, Cardueae) from NE Anatolia. South Afr. J. Bot. 2017, 112, 474–482. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Ozmen, G.; Cakmak, Y.S.; Duran, A. Screening for in vitro antioxidant properties and fatty acid profiles of five Centaurea L. species from Turkey flora. Food Chem. Toxicol. 2011, 49, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aktumsek, A.; Guler, G.O.; Kan, Y. Composition of essential oil and antioxidant capacity of Centaurea drabifolia Sm. subsp. detonsa (Bornm) Wagenitz, endemic to Turkey. Nat. Prod. Res. 2012, 26, 1–10. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Severino, J.F.; Stich, K.; Soja, G. Ozone stress and antioxidant substances in Trifolium repens and Centaurea jacea leaves. Environ. Pollut. 2007, 146, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lockowandt, L.; Pinela, J.; Lobo, C.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; José, M.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crop. Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Ugur, A.; Duru, M.E.; Ceylan, O.; Sarac, N.; Varol, O.; Kivrak, I. Chemical composition, antimicrobial and antioxidant activities of Centaurea ensiformis Hub. -Mor. (Asteraceae), a species endemic to Mugla (Turkey). Nat. Prod. Res. 2009, 23, 149–167. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Senkardes, I.; Sadeer, N.B.; Fawzi, M.; Lucini, L. Metabolomics-based pro fi ling with chemometric approach to delineate the bio-pharmaceutical properties of fruit extracts from Ligustrum vulgare L. Ind. Crop. Prod. 2019, 140, 111635. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Guler, G.O.; Mahomoodally, M.F.; Glamočlija, J.; Ćirić, A.; Soković, M. Shedding light on the biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). Food Funct. 2017, 8, 1152–1165. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, F.; Esmaeili, S.; Bashash, D.; Dehghan-nayeri, N.; Mashati, P.; Gharehbaghian, A. Centaurea albonitens extract enhances the therapeutic effects of Vincristine in leukemic cells by inducing apoptosis. Biomed. Pharmacother. 2018, 99, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2017, 105, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostad, S.N.; Rajabi, A.; Khademi, R.; Farjadmand, F.; Eftekhari, M.; Hadjiakhoondi, A.; Khanavi, M. Cytotoxic Potential of Centaurea bruguierana ssp. Belangerana: The MTT Assay. Acta Med Iran. 2016, 54, 583–589. [Google Scholar]
- De Bona, K.S.; Bonfanti, G.; Bitencourt, P.E.R.; Da Silva, T.P.; Borges, R.M.; Boligon, A.; Pigatto, A.; Athayde, M.L.; Moretto, M.B. Protective effect of gallic acid and Syzygium cumini extract against oxidative stress-induced cellular injury in human lymphocytes. Drug Chem. Toxicol. 2016, 39, 256–263. [Google Scholar] [CrossRef]





| Plants | Extracts | TPC (mg GAE/g) | DPPH IC50 (mg/mL) | RP EC50 (mg/mL) |
|---|---|---|---|---|
| Centaurea kroumirensis (Coss.) | Hexane (H) | 18.34a ± 0.31 | 0.49a ± 0.01 | 6.28a ± 0.77 |
| Chlorofom (C) | 43.06b ± 0.16 | 0.58a ± 0.02 | 12.36b ± 0.41 | |
| Methanol (M) | 61.12c ± 0.60 | 0.59a ± 0.01 | 8.77c ± 0.16 | |
| Ethanol (ultrasonic) (UE) | 42.73b ± 0.36 | 0.94a ± 0.01 | 5.15a ± 0.09 | |
| supercritical (I) (SFE(I)) | 4.79d ± 0.15 | 30.93b ± 1.24 | 65.17d ± 2.93 | |
| supercritical (II) (SFE(II)) | 13.17e ± 0.05 | 21.22c ± 0.50 | 22.44e ± 1.12 | |
| essential oil (EO) | 12.64e ± 0.20 | 45.10c ± 0.27 | 24.32e ± 0.46 | |
| Centaurea sicula L. subsp sicula | Hexane (H) | 8.32a ± 0.50 | 25.20a ± 1.55 | 21.50a ± 0.33 |
| Chlorofom (C) | 28.25b ± 0.72 | 1.96b ± 0.05 | 7.62b ± 0.15 | |
| Methanol (M) | 23.27c ± 0.077 | 2.94b ± 0.02 | 20.55a ± 0.76 | |
| Ethanol (ultrasonic) (UE) | 24.56d ± 0.05 | 2.00b ± 0.10 | 11.75c ± 0.58 | |
| supercritical (I) (SFE(I)) | 8.75a ± 0.87 | 28.01c ± 1.08 | 22.98d ± 0.59 | |
| supercritical (II) (SFE(II)) | 12.29e ± 0.21 | 22.25d ± 0.62 | 24.30e ± 0.90 | |
| essential oil (EO) | 9.28f ± 0.27 | 28.36d ± 2.01 | 25.05f ± 0.68 |
| Centaurea Specie | Variables | TPC | DPPH (IC50) | RP (EC50) |
|---|---|---|---|---|
| C. kroumirensis (Coss.) | TPC | _ | −0.758 * | −0.646 * |
| DPPH (IC50) | _ | _ | 0.676 * | |
| C. sicula L. subsp sicula | TPC | _ | −0.959 * | −0.846 * |
| DPPH (IC50) | _ | _ | 0.802 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhouibi, N.; Manuguerra, S.; Arena, R.; Mahdhi, A.; Messina, C.M.; Santulli, A.; Dhaouadi, H. Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula). Appl. Sci. 2020, 10, 2267. https://doi.org/10.3390/app10072267
Dhouibi N, Manuguerra S, Arena R, Mahdhi A, Messina CM, Santulli A, Dhaouadi H. Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula). Applied Sciences. 2020; 10(7):2267. https://doi.org/10.3390/app10072267
Chicago/Turabian StyleDhouibi, Nedra, Simona Manuguerra, Rosaria Arena, Abdelkarim Mahdhi, Concetta Maria Messina, Andrea Santulli, and Hatem Dhaouadi. 2020. "Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula)" Applied Sciences 10, no. 7: 2267. https://doi.org/10.3390/app10072267
APA StyleDhouibi, N., Manuguerra, S., Arena, R., Mahdhi, A., Messina, C. M., Santulli, A., & Dhaouadi, H. (2020). Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula). Applied Sciences, 10(7), 2267. https://doi.org/10.3390/app10072267