Evaluation of Tramadol Hydrochloride Toxicity to Juvenile Zebrafish—Morphological, Antioxidant and Histological Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Experiment
2.2. Histopathological Examination
2.3. Analysis of Selected Biomarkers in Whole-Body Homogenate
2.4. Analysis of Tramadol Hydrochloride in Water
2.5. Statistical Evaluation
3. Results and Discussion
3.1. Mortality Rate
3.2. Fish Growth
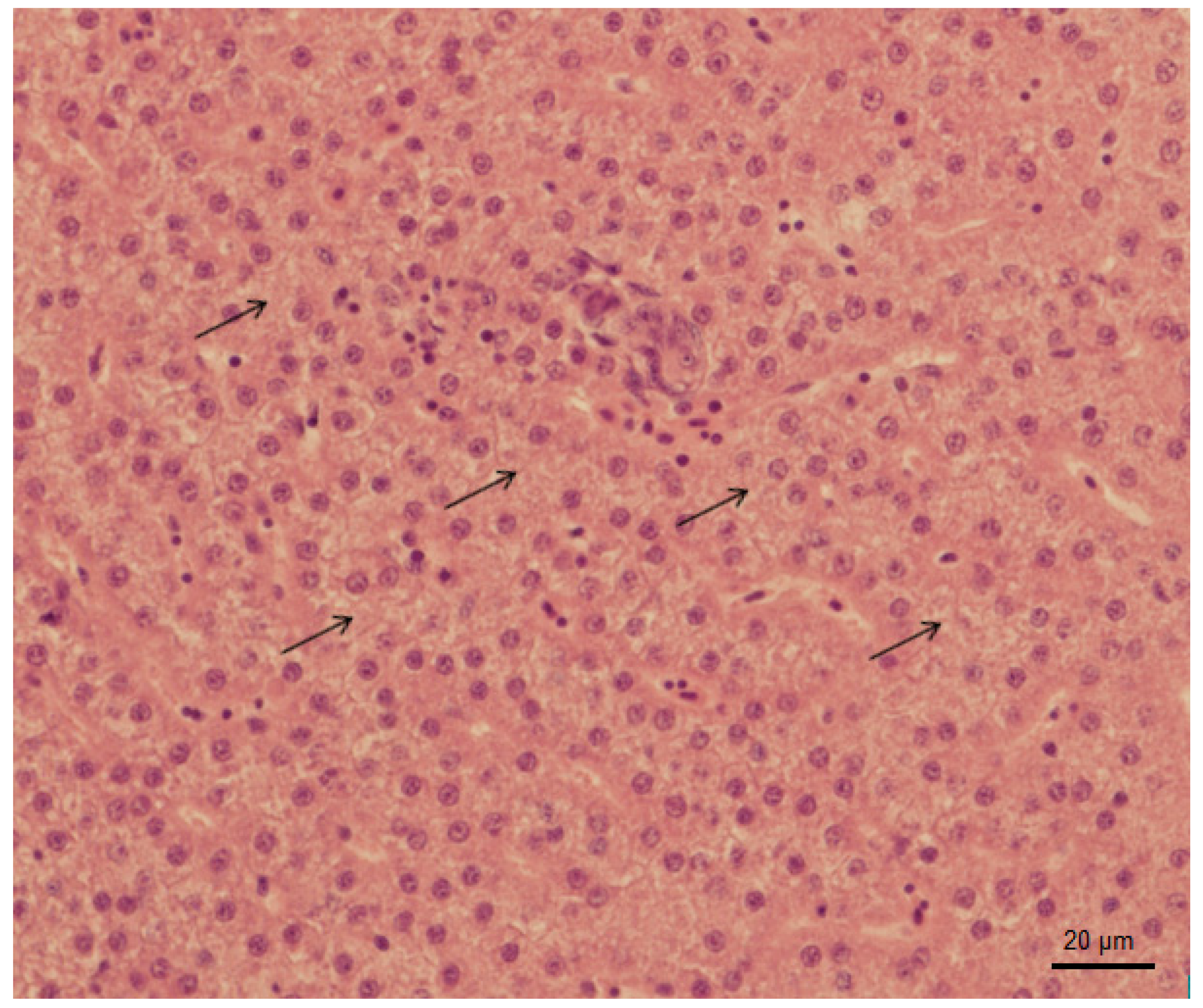
3.3. Histopathology
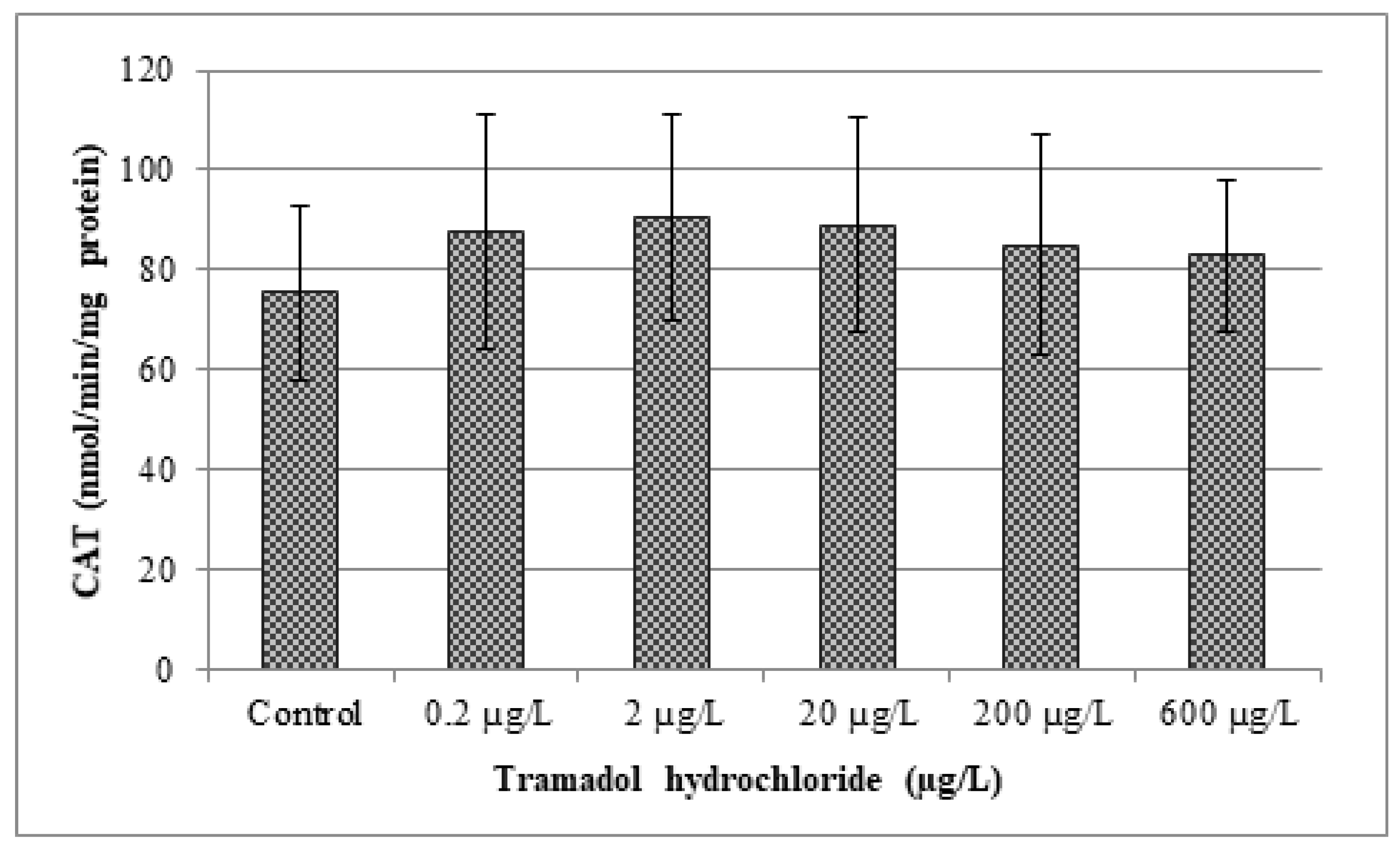
3.4. Oxidative Stress Indices
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Marsalek, P.; Prokes, M.; Tichy, F.; Skladana, M.; Fiorino, E.; Mikula, P.; et al. Effects of selected tricyclic antidepressants on early-life stages of common carp (Cyprinus carpio). Chemosphere 2017, 185, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Aliko, V.; Mehmeti, E.; Qirjo, M.; Faggio, C. Drink and sleep like a fish- goldfish as a behavior model to study pharmaceutical effects in freshwater ecosystem. J. Biol. Res. 2019, 92, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to Salicylic Acid. Aquat. Toxicol. 2019, in press. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Combined effects of salinity changes and salicylic acid exposure in Mytilus galloprovincialis. Sci. Total. Environ. 2020, in press. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Costa, S.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Toxic impacts induced by sodium lauryl sulfate in Mytilus galloprovincialis. Comp. Biochem. Phys. A 2020, in press. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugsduring wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Dai, G.; Huang, J.; Chen, W.; Wang, B.; Yu, G.; Deng, S. Major pharmaceuticals and personal care products (PPCPs) in wastewater treatment plant and receiving water in Beijing, China, and associated ecological risks. Bull. Environ. Contam. Toxicol. 2014, 92, 655–661. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrada, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Prokes, M.; Tichy, F.; Fiorino, E.; Faggio, C.; Svobodova, Z. Toxicity of naproxen sodium and its mixture with tramadol hydrochloride on fish early life stages. Chemosphere 2017, 188, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.J. Pollution from drug manufacturing: review and perspectives. Philos. Trans. R. Soc. B 2014, 369, 20130571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buřič, M.; Grabicová, K.; Kubec, J.; Kouba, A.; Kuklina, I.; Kozák, P.; Grabic, R.; Randák, T. Environmentally relevant concentrations of tramadol and citalopram alter behaviour of an aquatic invertebrate. Aquat. Toxicol. 2018, 200, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ložek, F.; Kuklina, I.; Grabicová, K.; Kubec, J.; Buřič, M.; Grabic, R.; Randák, T.; Císař, P.; Kozák, P. Behaviour and cardiac response to stress in signal crayfish exposed to environmental concentrations of tramadol. Aquat. Toxicol. 2019, 213, 105217. [Google Scholar] [CrossRef]
- Vazzana, M.; Andreani, T.; Fangueiro, J.; Faggio, C.; Silva, C.; Santini, A.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 2015, 70, 234–238. [Google Scholar] [CrossRef]
- Zhuo, H.Q.; Huang, L.; Huang, H.Q.; Cai, Z. Effects of chronic tramadol exposure on the zebrafish brain: a proteomic study. J. Proteom. 2012, 75, 3351–3364. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Pypendop, B.H.; Ilkiw, J.E. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J. Vet. Pharmacol. Ther. 2008, 31, 52–59. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Occurrence and removal of lidocaine, tramadol, venlafaxine, and their metabolites in German wastewater treatment plants. Environ. Sci. Pollut. Res. Int. 2012, 19, 689–699. [Google Scholar]
- Rúa-Gómez, P.C.; Püttmann, W. Impact of wastewater treatment plant discharge of lidocaine, tramadol, venlafaxine and their metabolites on the quality of surface waters and groundwater. J. Environ. Monit. 2012, 14, 1391–1399. [Google Scholar]
- Fedorova, G.; Randak, T.; Golovko, O.; Kodes, V.; Grabicova, K.; Grabic, R. A passive sampling method for detecting analgesics, psycholeptics, antidepressants and illicit drugs in aquatic environments in the Czech Republic. Sci. Total. Environ. 2014, 487, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, T.A. Fate of beta blockers andpsycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, R.; Margiotta-Casaluci, L.; Huerta, B.; Runnalls, T.J.; Eguchi, A.; Nomiyama, K.; Kunisue, T.; Tanabe, S.; Sumpter, J.P. Protecting the environment from psychoactive drugs: Problems for regulators illustrated by the possible effects of tramadol on fish behaviour. Sci. Total. Environ. 2019, 664, 915–926. [Google Scholar] [CrossRef]
- Richmond, E.K.; Rosi, E.J.; Walters, D.M.; Fick, J.; Hamilton, S.K.; Brodin, T.; Sundelin, A.; Grace, M.R. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat. Commun. 2018, 9, 4491. [Google Scholar] [CrossRef] [Green Version]
- Bachour, R.L.; Golovko, O.; Kellner, M.; Pohl, J. Behavioral effects of citalopram, tramadol, and binary mixture in zebrafish (Danio rerio) larvae. Chemosphere 2020, 238, 124587. [Google Scholar] [CrossRef]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Berankova, P.; Doubkova, V.; Prokes, M.; Tichy, F.; Vecerek, V.; Svobodova, Z. The effect of tramadol hydrochloride on early life stages of fish. Environ. Toxicol. Phar. 2016, 44, 151–157. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals: Fish, Juvenile Growth Test; OECD 215: Paris, France, 2000; 16p. [Google Scholar]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Segner, H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2009, 149, 187–195. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [Green Version]
- Hoo, J.Y.; Kumari, Y.; Shaikh, M.F.; Hue, S.M.; Goh, B.H. Zebrafish: A Versatile Animal Model for Fertility Research. Biomed. Res. Int. 2016, 2016, 9732780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bambino, K.; Chu, J. Zebrafish in Toxicology and Environmental Health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar] [PubMed] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Emryonic Development of the Zebrafish. Develop. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jacoby, W.B. Glutathione S-transferases. First enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Lushchak, O.V.; Storey, J.M.; Storey, K.B. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int. J. Biochem. Cell. Biol. 2005, 37, 1319–1330. [Google Scholar] [CrossRef]
- Lushchak, V.; Semchyshyn, H.M. Oxidative Stress—Molecular Mechanisms and Biological Effects; IntechOpen: Rijeka, Croatia, 2012; 362p. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria. Med. J. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Dong, X.; Xie, H.; Wang, J.; Su, J.; Yu, C. DNA damage and effects on glutathione-S-transferase activity induced by atrazine exposure in zebrafish (Danio rerio). Environ. Toxicol. 2011, 26, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Zivna, D.; Blahova, J.; Siroka, Z.; Plhalova, L.; Marsalek, P.; Doubkova, V.; Zelinska, G.; Vecerek, V.; Tichy, F.; Sehonova, P.; et al. The effects of salicylic acid on juvenile zebrafish Danio rerio under flow-through conditions. Bull. Environ. Contam. Toxicol. 2016, 97, 323–330. [Google Scholar] [CrossRef] [PubMed]




| Parent Ion (m/z) | Product Ion (m/z) | Collision Energy (V) | Fragmentation Values (V) |
|---|---|---|---|
| 264.5 | 58.1 | 36 | 87 |
| Tramadol Hydrochloride Test Group (µg/L) | ||||||
|---|---|---|---|---|---|---|
| Parameters | Control | 0.2 | 2 | 20 | 200 | 600 |
| Total length (mm) | 19.95 ± 2.83 | 19.89 ± 2.58 | 19.52 ± 2.99 | 19.68 ± 2.73 | 19.57 ± 2.94 | 19.42 ± 2.78 |
| Body weight (mg) | 63.80 ± 9.37 | 65.17 ± 6.21 | 61.58 ± 8.21 | 69.58 ± 9.35 | 63.90 ± 6.19 | 63.57 ± 6.58 |
| Specific growth rate | 2.50 ± 0.17 | 2.53 ± 0.18 | 2.39 ± 0.16 | 2.50 ± 0.17 | 2.49 ± 0.19 | 2.47 ± 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plhalova, L.; Sehonova, P.; Blahova, J.; Doubkova, V.; Tichy, F.; Faggio, C.; Berankova, P.; Svobodova, Z. Evaluation of Tramadol Hydrochloride Toxicity to Juvenile Zebrafish—Morphological, Antioxidant and Histological Responses. Appl. Sci. 2020, 10, 2349. https://doi.org/10.3390/app10072349
Plhalova L, Sehonova P, Blahova J, Doubkova V, Tichy F, Faggio C, Berankova P, Svobodova Z. Evaluation of Tramadol Hydrochloride Toxicity to Juvenile Zebrafish—Morphological, Antioxidant and Histological Responses. Applied Sciences. 2020; 10(7):2349. https://doi.org/10.3390/app10072349
Chicago/Turabian StylePlhalova, Lucie, Pavla Sehonova, Jana Blahova, Veronika Doubkova, Frantisek Tichy, Caterina Faggio, Petra Berankova, and Zdenka Svobodova. 2020. "Evaluation of Tramadol Hydrochloride Toxicity to Juvenile Zebrafish—Morphological, Antioxidant and Histological Responses" Applied Sciences 10, no. 7: 2349. https://doi.org/10.3390/app10072349
APA StylePlhalova, L., Sehonova, P., Blahova, J., Doubkova, V., Tichy, F., Faggio, C., Berankova, P., & Svobodova, Z. (2020). Evaluation of Tramadol Hydrochloride Toxicity to Juvenile Zebrafish—Morphological, Antioxidant and Histological Responses. Applied Sciences, 10(7), 2349. https://doi.org/10.3390/app10072349








