Serial Disulfide Polymers as Cathode Materials in Lithium-Sulfur Battery: Materials Optimization and Electrochemical Characterization
Abstract
:1. Introduction
2. Experiment
2.1. Material Preparation
2.1.1. Synthesis of Poly (sulfur-diallyl-o-phthalate) (Poly(S-DAP)) Copolymer
2.1.2. Synthesis of Poly (sulfur-tung oil) (Poly(S-T)) Copolymer
2.1.3. Synthesis of Poly (sulfur-peanut oil) (Poly(S-P)) Copolymer
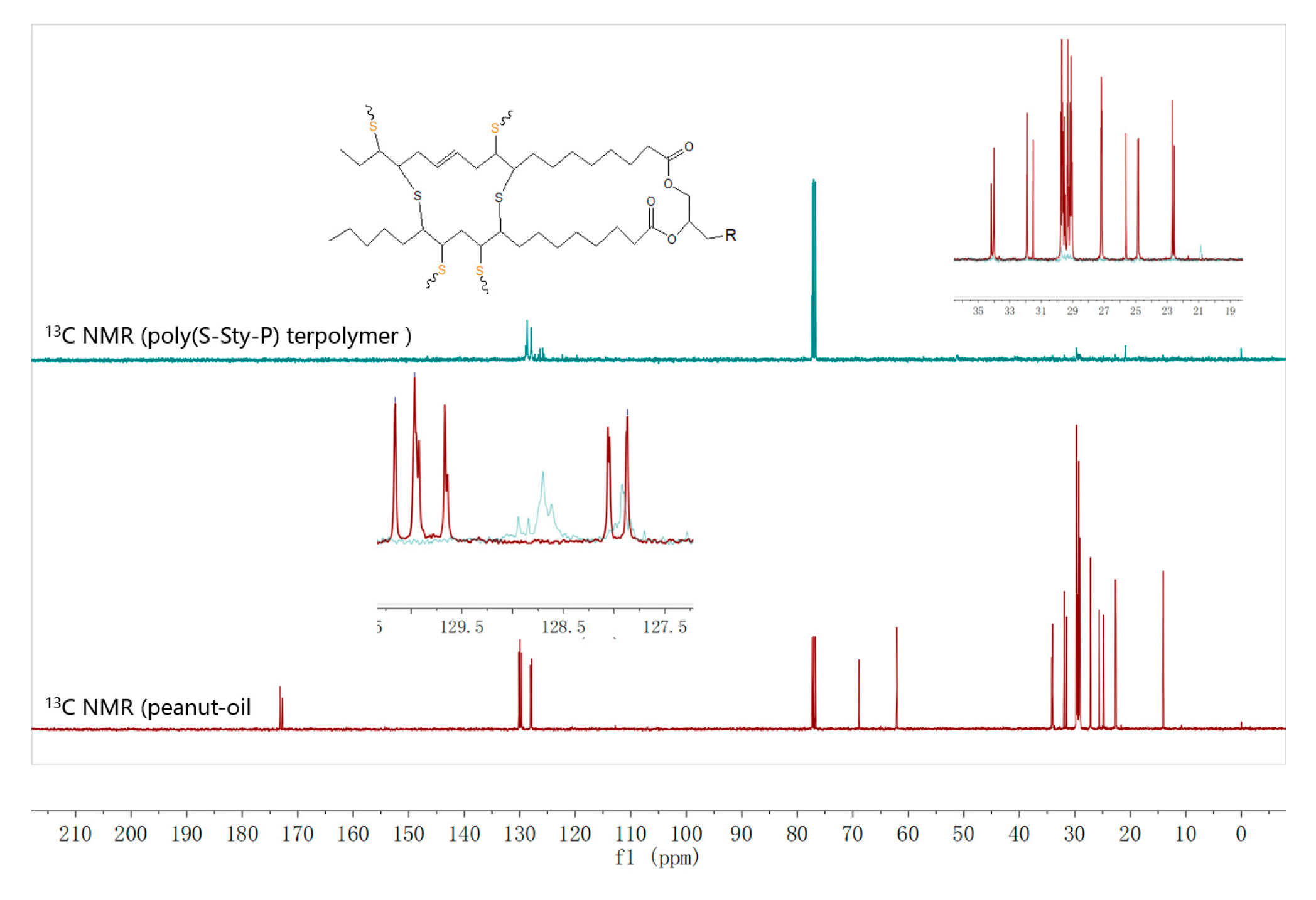
2.1.4. Synthesis of Poly (sulfur-styrene-peanut oil) (Poly(S-Sty-P)) Terpolymer
2.2. Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
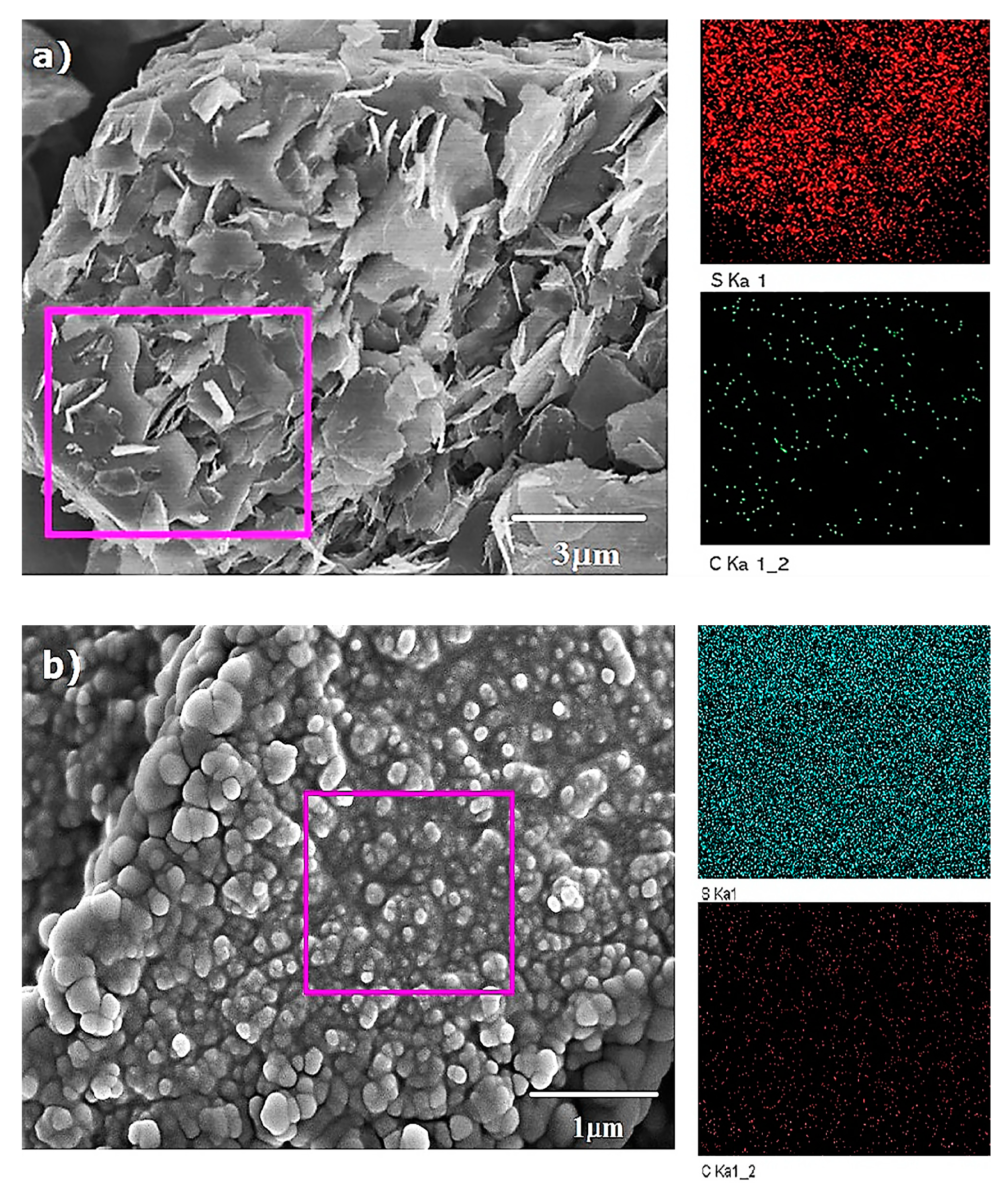
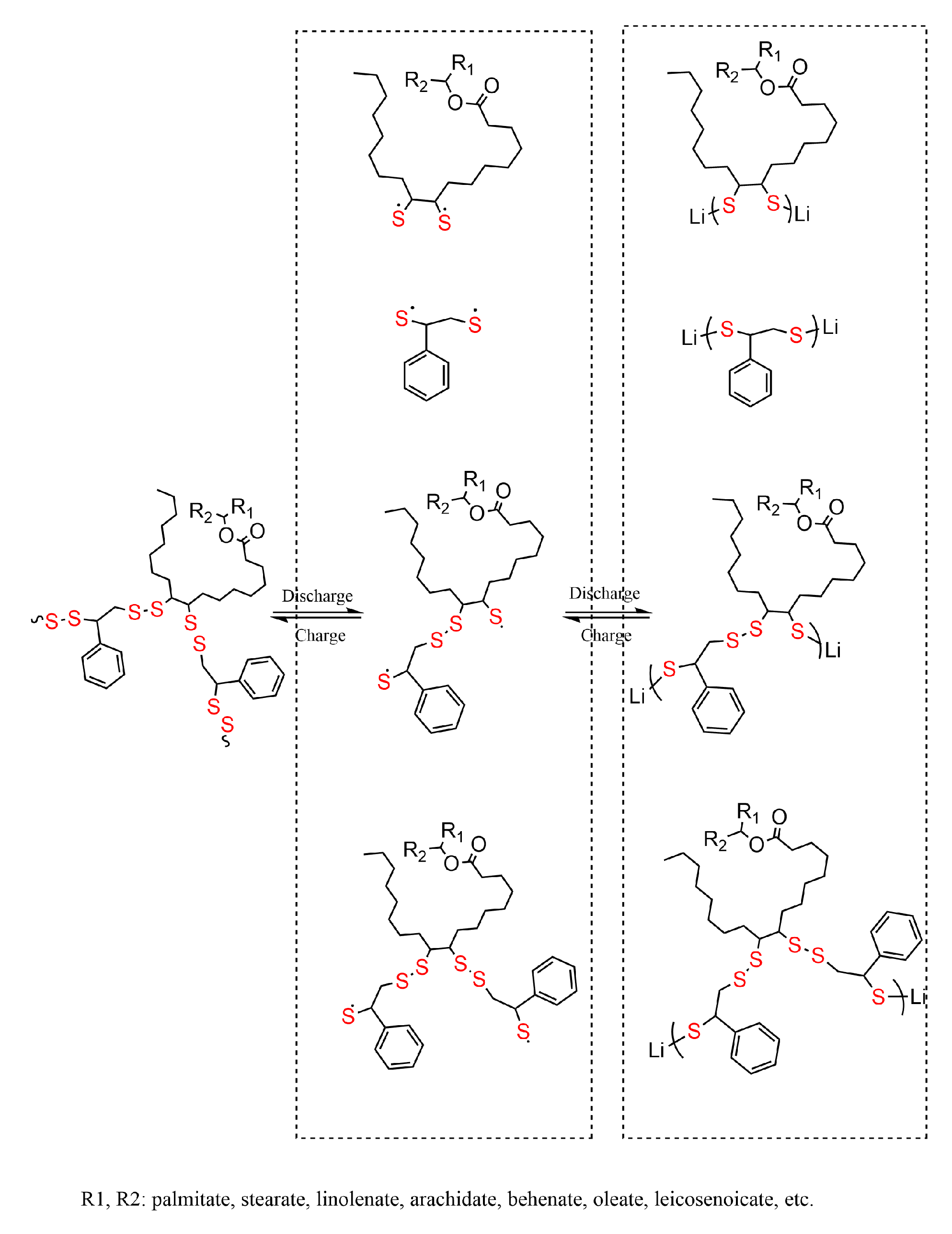
3.1. Characterization of Serial Disulfide Polymer
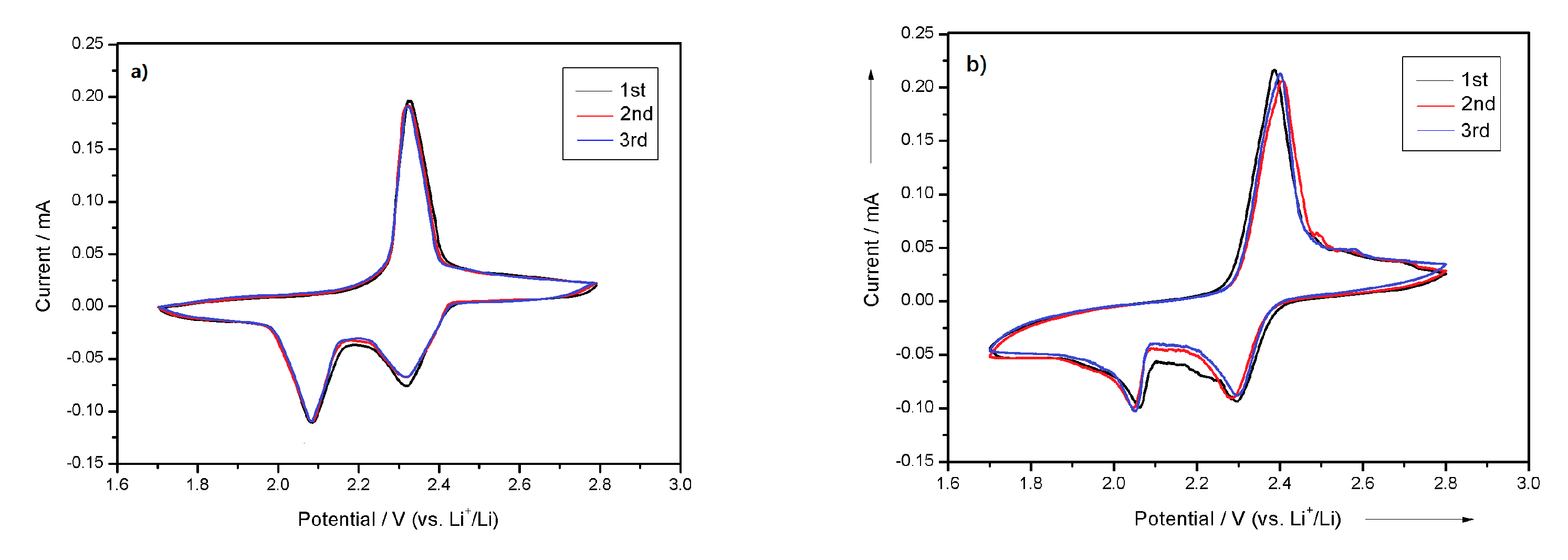
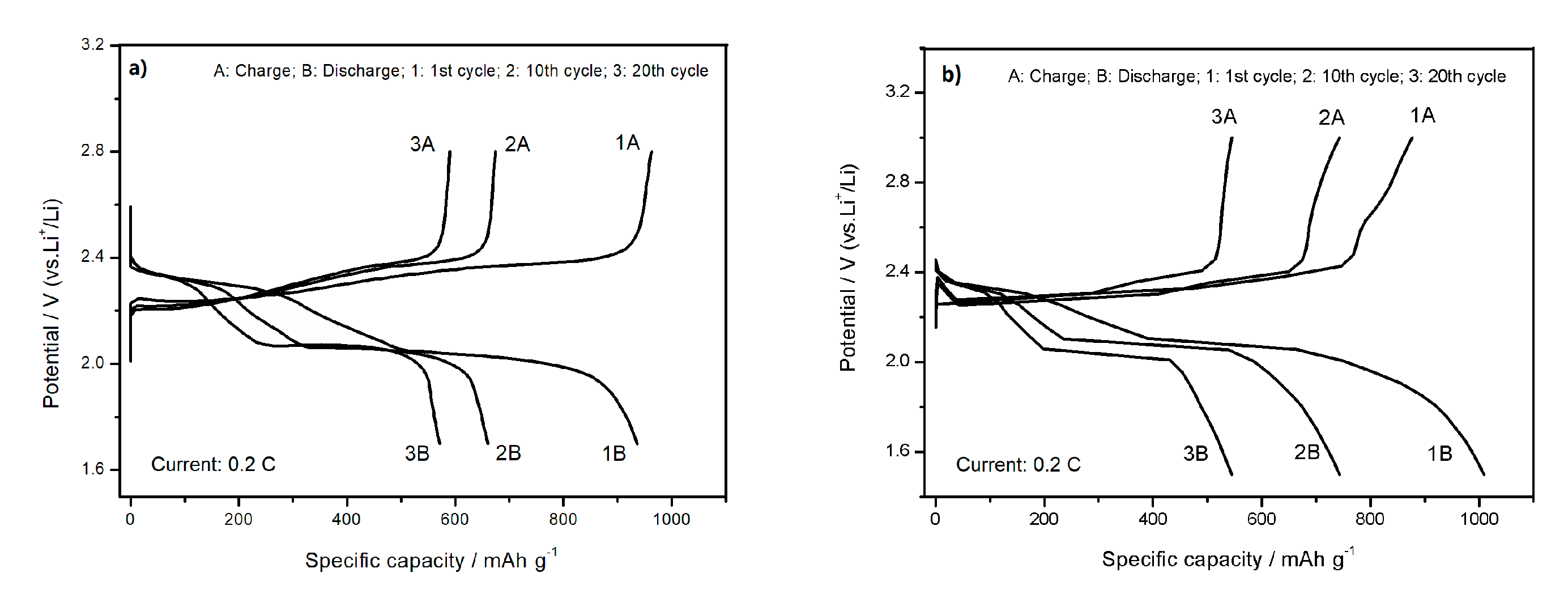
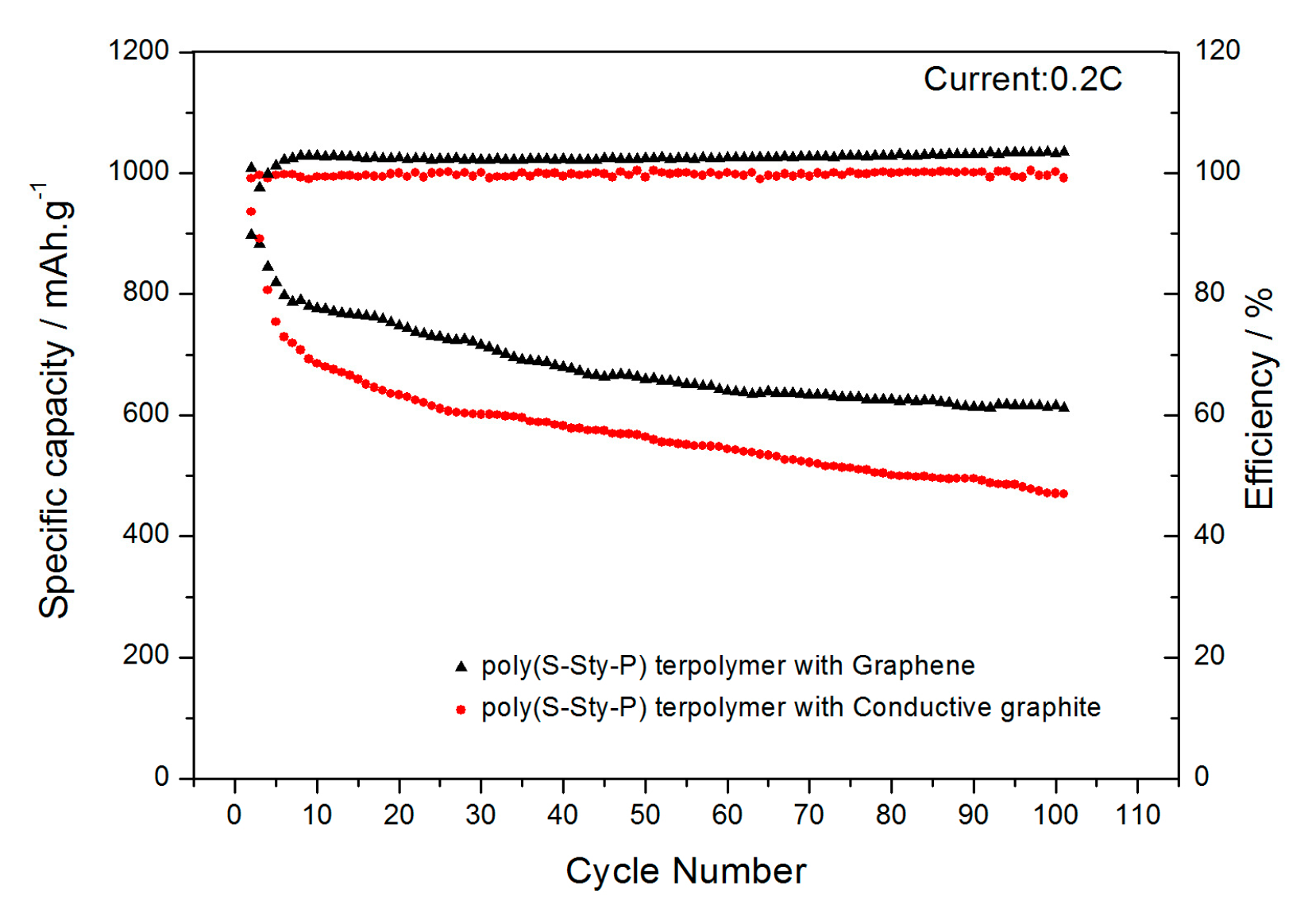
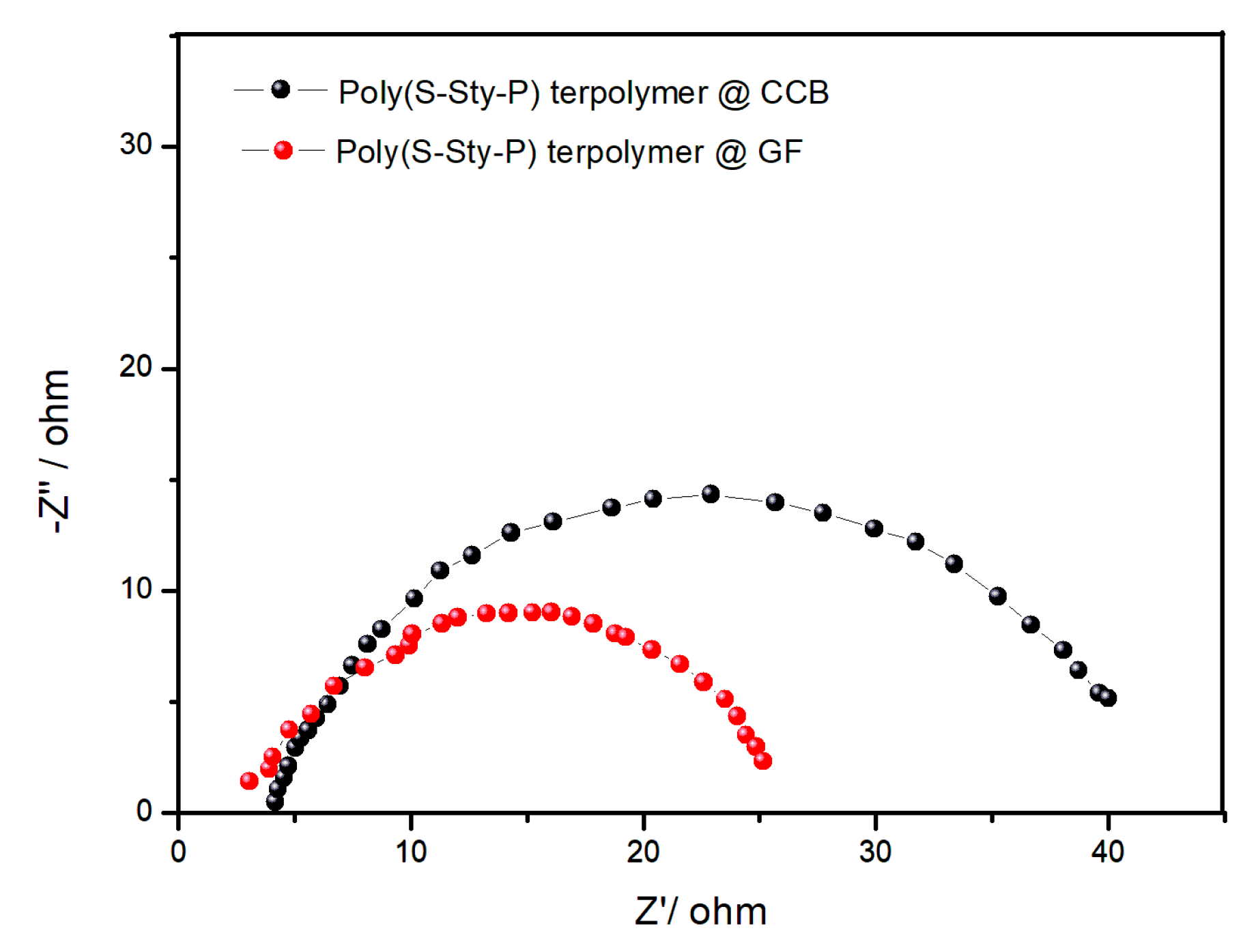
3.2. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manthiram, A.; Fu, Y.; Su Y, S. Challenges and prospects of lithium/sulfur batteries. Acc. Chem. Res. 2012, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Han, D.; Xiao, M. A Preparation Method of Sulfur Polymer as Cathode Material of Lithium Sulfur Battery. China Patent CN102664264A, 12 September 2012. [Google Scholar]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.; Sanders, J.K.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Jian, Z.; Li, H.; Cao, R.; Zhou, H.; Xu, H.; Zhao, G.; Xing, Y.; Zhang, S. Polydopamine-coated hierarchical tower-shaped carbon for high-performance lithium-sulfur batteries. Electrochim. Acta 2019, 319, 359–365. [Google Scholar] [CrossRef]
- Mustafa, A.; Baris, K.; Yusuf, Y. Combining Elemental Sulfur with Polybenzoxazines via Inverse Vulcanization. RSC Adv. 2019, 9, 31460–31465. [Google Scholar]
- Deng, Z.; Hoefling, A.; Théato, P.; Lienkamp, K. Surface properties and antimicrobial activity of poly(sulfur-co-1,3-diisopropenylbenzene) copolymers. Macromol. Chem. Phys. 2018, 219, 1700497. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Shahrukh, Z.; Khawaja, S.; Vijay, K.; Kishore, K.; Jena, S.M. Alhassan. Flexible sulfur film from inverse vulcanization technique. Mater. Lett. 2017, 203, 58–61. [Google Scholar]
- Dirlam, P.T.; Glass, R.S.; Char, K.; Pyun, J. The use of polymers in Li-S batteries: A review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1635–1668. [Google Scholar] [CrossRef] [Green Version]
- Philip, T.D.; Adam, G.S.; Shallcross, R.C.; Kyle, J.A. Improving the Charge Conductance of Elemental Sulfur via Tandem Inverse Vulcanization and Electropolymerization. ACS Macro Lett. 2015, 4, 111–114. [Google Scholar]
- Zhenjie, S.; Min, X.; Shuanjin, W.; Dongmei, H. Sulfur-rich polymeric materials with semi- interpenetrating network structure as a novel lithium-sulfur cathode. J. Mater. Chem. A 2014, 2, 9280–9286. [Google Scholar]
- Arslan, M.; Kiskan, B.; Cengiz, E.C.; Demir-cakan, R.; Yagci, Y. Inverse vulcanization of bismaleimide and divinylbenzene by elemental sulfur for lithium sulfur batteries. Eur. Polym. J. 2016, 80, 70–77. [Google Scholar] [CrossRef]
- Inaki, G.; David, M.; Alberto, B.J.; Olatz, L.; Hicham, B.Y.; Chunmei, L.; Juan, L.G.; Oleksandr, B.; Lide, R.M. Inverse vulcanization of sulfur with divinylbenzene: Stable and easy processable cathode material for lithium-sulfur batteries. J. Power Sources 2016, 329, 72–78. [Google Scholar]
- Swapnil, S.; Arnab, G.; Prasun, K.R.; Sagar, M.; Bimlesh, L. Cardanol benzoxazines e A sustainable linker for elemental sulphur based copolymers via inverse vulcanization. Polymer 2016, 99, 349–357. [Google Scholar]
- Zhang, Y.Y.; Konopka, K.M.; Glass, R.S.; Char, K.; Pyun, J. Chalcogenide hybrid inorganic/organic polymers (CHIPs) via inverse vulcanization and dynamic covalent polymerizations. Polym. Chem. 2017, 8, 5167–5173. [Google Scholar] [CrossRef]
- Liu, J.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Calatldo, F. A study on the structure and properties of polymeric sulfur. Angew. Makromol. Chem. 1997, 249, 137–149. [Google Scholar] [CrossRef]
- Adam, G.S.; Jared, J.G.; Jungjin, P.; Kwi, R.K. Inverse vulcanization of element sulfur to prepare polymer electrode materials for Li-S batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar]
- Zeng, S.; Li, L.; Zhao, D.; Liu, J. Polymer-Capped Sulfur Copolymers as Lithium-Sulfur Battery Cathode: Enhanced Performance by Combined Contributions of Physical and Chemical Confinements. J. Phys. Chem. C 2017, 12, 2495–2503. [Google Scholar] [CrossRef]
- Deng, S.R.; Kong, L.B.; Hu, G.Q.; Wu, T.; Li, D.; Zhou, Y.H.; Li, Z.Y. Benzene-based polyorganodisulfide cathode materials for secondary lithium batteries. Electrochim. Acta 2006, 13, 2589–2593. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, C.; Wu, J. Research status quo of sulfur electrode for lithium/ sulfur battery. Battery Bimon. 2010, 40, 232–235. [Google Scholar]
- Zeng, S.; Li, L.; Xie, L.; Zhao, D.; Zhou, N.; Wang, N.; Chen, S.W. Graphene-supported highly crosslinked organosulfur nanoparticles as cathode materials for high-rate, long-life lithium-sulfur battery. Carbon 2017, 122, 106–113. [Google Scholar] [CrossRef]
- Robert, D.; Miran, G.; Jernej, D.; Marjan, B.; Stane, P.; Janko, J. The role of carbon black distribution in cathodes for Li ion batteries. J. Power Sources 2003, 119, 770–773. [Google Scholar]
- Wojtecki, R.; Meador, M.; Rowan, S. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater 2011, 10, 14–27. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Aruna, V. Hydrazine-reduction of graphite-and grapheneoxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Hou, T.Z.; Xu, W.T.; Chen, X.; Peng, H.J.; Huang, J.Q.; Zhang, Q. Lithium Bond Chemistry in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2017, 56, 8178–8182. [Google Scholar] [CrossRef]
- Xavier, B. Electrically Conductive Grades of Carbon Black: Structure and Properties. Carbon 1993, 31, 287–302. [Google Scholar]
- Dan, L.; Marc, B.M.; Scott, G.; Richard, K. Processable aqueous dispersion of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar]
- Arnab, G.; Swapnil, S.; Gaganpreet, S.K.; Bimlesh, L. Sustainable Sulfur-rich Copolymer/Graphene Composite as Lithium-Sulfur Battery Cathode with Excellent Electrochemical Performance. Sci. Rep. 2016, 6, 25207. [Google Scholar]
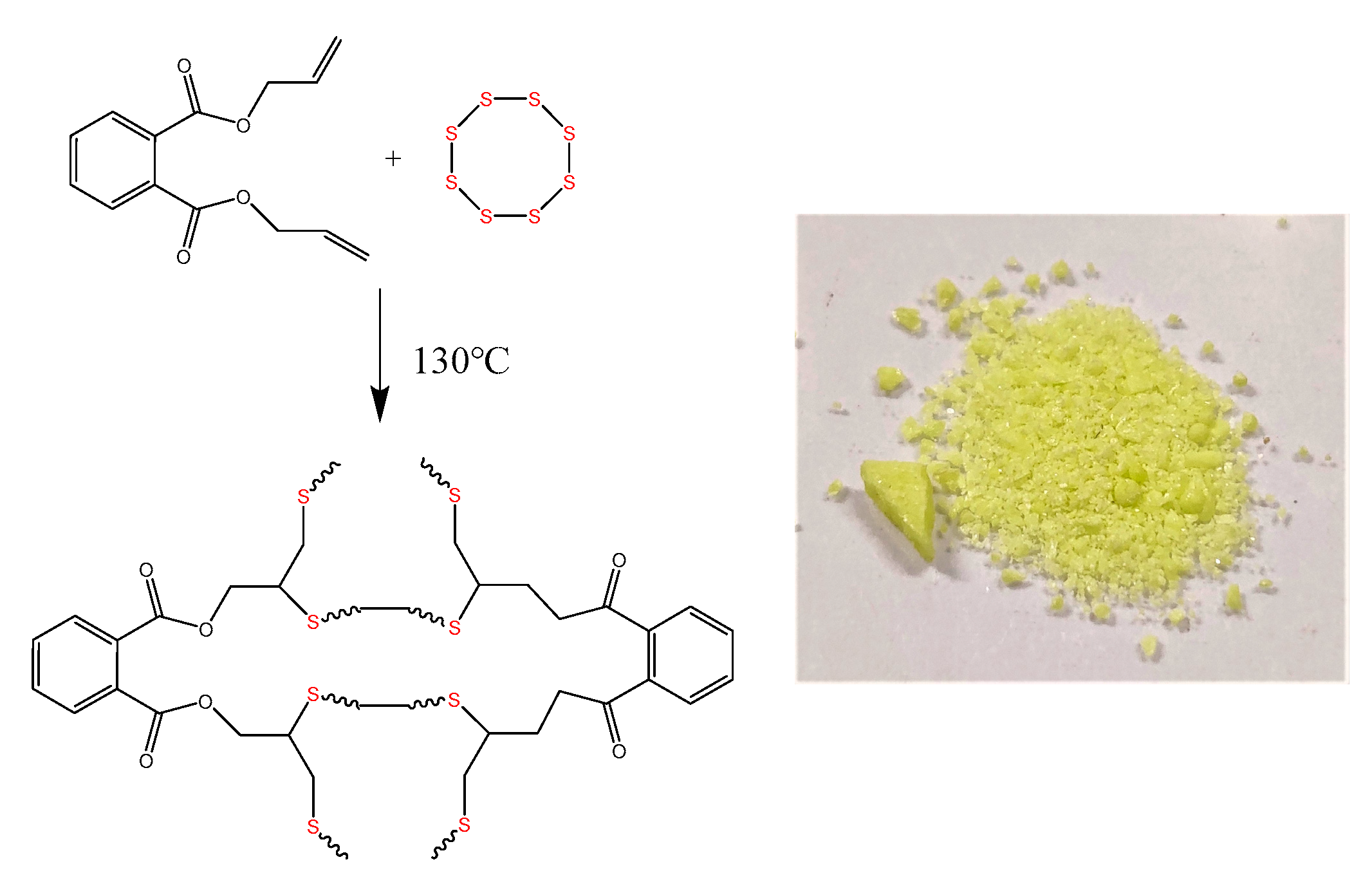





| Acetone | Dichloromethane | Trichloromethane | Cyclohexane | Toluene | Tetrahydrofuran | |
|---|---|---|---|---|---|---|
| S-DAP | × | × | × | × | × | × |
| S-T | × | × | × | × | × | ○ |
| S-P | × | ○ | ○ | ○ | ○ | ○ |
| S-Sty-P | × | ○ | ○ | ○ | ○ | √ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, S. Serial Disulfide Polymers as Cathode Materials in Lithium-Sulfur Battery: Materials Optimization and Electrochemical Characterization. Appl. Sci. 2020, 10, 2538. https://doi.org/10.3390/app10072538
Wang J, Zhang S. Serial Disulfide Polymers as Cathode Materials in Lithium-Sulfur Battery: Materials Optimization and Electrochemical Characterization. Applied Sciences. 2020; 10(7):2538. https://doi.org/10.3390/app10072538
Chicago/Turabian StyleWang, Jing, and Shichao Zhang. 2020. "Serial Disulfide Polymers as Cathode Materials in Lithium-Sulfur Battery: Materials Optimization and Electrochemical Characterization" Applied Sciences 10, no. 7: 2538. https://doi.org/10.3390/app10072538




