The Progress of Cobalt-Based Anode Materials for Lithium Ion Batteries and Sodium Ion Batteries
Abstract
1. Introduction
2. Li+/Na+ Storage Mechanism
2.1. Li+/Na+ Storage Mechanism in Co-Based Alloys
2.2. Li+/Na+ Storage Mechanism in CoxAy (A=O, S, P and Se)
3. Preparation Methods of Synthesizing Cobalt-based Active Materials
3.1. Hydrothermal/Solvothermal Methods
3.2. Galvanic Replacement
3.3. Heat Treatment
3.4. High-Energy Mechanical Milling
3.5. Electrodeposition
3.6. Other Methods
4. Application of Cobalt-Based Anode Materials in LIBs/SIBs
4.1. Cobalt-Based Alloys and Its Composites
4.2. Cobalt Oxides and Its Composites
4.3. Cobalt Sulfide and Its Composites
4.4. Cobalt Phosphide and Its Composites
4.5. Cobalt Selenide and Its Composites
4.6. Other Cobalt-Based Anode Materials
5. Conclusions and Perspectives
- (1)
- Developing a simple and feasible synthetic process for the preparation of cobalt-based anode materials with specific morphology and sizes, which can ensure satisfactory contact between the electrolyte and active material, and cycle performances of cobalt-based anode materials. In addition, the combination with the conductive material can improve the conductivity of the electrode material and improve the rate performances of the cobalt-based anode materials.
- (2)
- More advanced characterization and calculation methods should be used to further study the Li+/Na+ storage mechanisms of cobalt-based anode materials, which will be meaningful for designing a suitable morphology.
- (3)
- Considering commercial applications, the whole battery system, including cathode materials, binders, conductive agents, electrolytes and additives, should also be optimized. Appropriate matching materials can fully exploit the advantages of the high storage capacities of cobalt-based anode materials in LIBs/SIBs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xue, P.; Wang, N.; Wang, Y.; Zhang, Y.; Liu, Y.; Tang, B.; Bai, Z.; Dou, S. Nanoconfined SnS in 3D interconnected macroporous carbon as durable anodes for lithium/sodium ion batteries. Carbon 2018, 134, 222–231. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, N.; Zhang, Y.; Xue, P.; Guo, M.; Tang, B.; Bai, Z.; Dou, S. Pyrite FeS2@C nanorods as smart cathode for sodium ion battery with ultra-long lifespan and notable rate performance from tunable pseudocapacitance. Electrochim. Acta 2018, 260, 755–761. [Google Scholar] [CrossRef]
- Bai, Z.; Fan, N.; Ju, Z.; Guo, C.; Qian, Y.; Tang, B.; Xiong, S. Facile synthesis of mesoporous Mn3O4 nanotubes and their excellent performance for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 10985. [Google Scholar] [CrossRef]
- Bai, Z.; Ju, Z.; Guo, C.; Qian, Y.; Tang, B.; Xiong, S. Direct large-scale synthesis of 3D hierarchical mesoporous NiO microspheres as high-performance anode materials for lithium ion batteries. Nanoscale 2014, 6, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Liu, S.; Yang, J.; Qian, Y. Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: A high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale 2015, 7, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B. Hierarchical MoS2@Carbon Microspheres as Advanced Anodes for Li-Ion Batteries. Chem-Eur. J. 2015, 21, 18187–18191. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Sun, D. MOFs-derived porous Mn2O3 as high-performance anode material for Li-ion battery. J. Mater. Chem. A 2015, 3, 5266–5269. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Xu, X.; Liao, T.; Tang, B.; Bai, Z.; Dou, S. Synergistically Enhanced Interfacial Interaction to Polysulfide via N,O Dual-Doped Highly Porous Carbon Microrods for Advanced Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13573–13580. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Xu, X.; Liao, T.; Du, Y.; Bai, Z.; Dou, S. Defect Sites-Rich Porous Carbon with Pseudocapacitive Behaviors as an Ultrafast and Long-Term Cycling Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 9353–9361. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Wang, X.; Bai, Z. Porous ZnMn2O4 nanowires as an advanced anode material for lithium ion battery. Electrochim. Acta 2015, 182, 1140–1144. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Lu, B.-R.; Cao, F.-H.; Yu, Z.-L.; Cong, H.P.; Yu, S.H. Hierarchically structured Co3O4@carbon porous fibers derived from electrospun ZIF-67/PAN nanofibers as anodes for lithium ion batteries. J. Mater. Chem. A 2018, 6, 12962–12968. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Sun, C.; Lu, Z.; Xue, P.; Tang, B.; Bai, Z.; Dou, S. 3D spongy CoS2 nanoparticles/carbon composite as high-performance anode material for lithium/sodium ion batteries. Chem. Eng. J. 2018, 332, 370–376. [Google Scholar] [CrossRef]
- Zhang, K.; Park, M.; Zhang, J.; Lee, G.-H.; Shin, J.; Kang, Y.-M. Cobalt phosphide nanoparticles embedded in nitrogen-doped carbon nanosheets: Promising anode material with high rate capability and long cycle life for sodium-ion batteries. Nano Res. 2017, 10, 4337–4350. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Han, Y.; Du, H.; Peng, W.; Huan, Q.; Song, D.; Si, Y.; Wang, Y.; Yuan, H. CoS2 Hollow Spheres: Fabrication and Their Application in Lithium-Ion Batteries. J. Phy. Chem. C 2011, 115, 8300–8304. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Song, M.-S.; Kim, J.-H.; Kim, H.-S.; Park, M.-S.; Lee, J.-Y.; Liu, H.K.; Dou, S.X. A study on the charge–discharge mechanism of Co3O4 as an anode for the Li ion secondary battery. Electrochim. Acta 2005, 50, 3667–3673. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Tang, Y.; Lu, X.; Yang, C.; Qin, M.; Huang, F.; Li, X.; Zhang, X. Phase-controlled synthesis of cobalt sulfides for lithium ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4246–4250. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Y.; Wang, Y. Graphene-wrapped CoS nanoparticles for high-capacity lithium-ion storage. ACS Appl. Mater. Interfaces 2013, 5, 801–806. [Google Scholar] [CrossRef]
- DeMattei, R.C.; Watcharapasorn, A.; Feigelson, R.S. Conditions for the Electrochemical Synthesis of the CoPn (Pn=P, As, Sb) Skutterudites. J. Electrochem. Soc. 2001, 148, D109. [Google Scholar] [CrossRef]
- Ferguson, P.P.; Todd, A.D.W.; Dahn, J.R. Comparison of mechanically alloyed and sputtered tin–cobalt–carbon as an anode material for lithium-ion batteries. Electrochem. Commun. 2008, 10, 25–31. [Google Scholar] [CrossRef]
- Li, Z.; Xue, H.; Wang, J.; Tang, Y.; Lee, C.-S.; Yang, S. Reduced Graphene Oxide/Marcasite-Type Cobalt Selenide Nanocrystals as an Anode for Lithium-Ion Batteries with Excellent Cyclic Performance. ChemElectroChem 2015, 2, 1682–1686. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, C.; Su, L.; Zhang, X. Hydrothermal synthesis of Co3O4 microspheres as anode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 2507–2513. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, H.; Jia, X.; Li, X.; Qiu, W. A novel micro-spherical CoSn2/Sn alloy composite as high capacity anode materials for Li-ion rechargeable batteries. Electrochim. Acta 2007, 52, 4853–4857. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Xue, P.; Liu, Y.; Tang, B.; Bai, Z.; Dou, S. Co9S8 @carbon nanospheres as high-performance anodes for sodium ion battery. Chem. Eng. J. 2018, 343, 512–519. [Google Scholar] [CrossRef]
- Cui, C.; Wei, Z.; Zhou, G.; Wei, W.; Ma, J.; Chen, L.; Li, C. Quasi-reversible conversion reaction of CoSe2/nitrogen-doped carbon nanofibers towards long-lifetime anode materials for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 7088–7098. [Google Scholar] [CrossRef]
- Han, Z.; Wang, B.; Liu, X.; Wang, G.; Wang, H.; Bai, J. Peapod-like one-dimensional (1D) CoP hollow nanorods embedded into graphene networks as an anode material for lithium-ion batteries. J. Mater. Sci. 2018, 53, 8445–8459. [Google Scholar] [CrossRef]
- Qiu, W.; Jiao, J.; Xia, J.; Zhong, H.; Chen, L. A self-standing and flexible electrode of yolk-shell CoS2 spheres encapsulated with nitrogen-doped graphene for high-performance lithium-ion batteries. Chem-Eur. J. 2015, 21, 4359–4367. [Google Scholar] [CrossRef]
- Ge, X.; Li, Z.; Yin, L. Metal-organic frameworks derived porous core/shellCoP@C polyhedrons anchored on 3D reduced graphene oxide networks as anode for sodium-ion battery. Nano Energy 2017, 32, 117–124. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Ye, M.; Zhuo, K.; Fang, Z. In Situ Formation of Co9S8 /N-C Hollow Nanospheres by Pyrolysis and Sulfurization of ZIF-67 for High-Performance Lithium-Ion Batteries. Chem-Eur. J. 2017, 23, 9517–9524. [Google Scholar] [CrossRef]
- Dong, W.; Shen, D.; Yang, S.; Liang, B.; Wang, X.; Liu, Y.; Li, S. First-principles Study of Mechanical and Electronic Properties of Co-Sn Intermetallics for Lithium Ion Battery Anode. Chem. Res. Chin. Univ. 2018, 34, 235–240. [Google Scholar] [CrossRef]
- Hu, X.; Jia, J.; Wang, G.; Chen, J.; Zhan, H.; Wen, Z. Reliable and General Route to Inverse Opal Structured Nanohybrids of Carbon-Confined Transition Metal Sulfides Quantum Dots for High-Performance Sodium Storage. Adv. Energy Mater. 2018, 1801452. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.; Sun, B.; Chang, X.; Zheng, J.; Li, X. A Peapod-like CoP@C Nanostructure from Phosphorization in a Low-Temperature Molten Salt for High-Performance Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2018, 130, 10344–10348. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Li, S.; Huang, L.; Ji, X. Three-Dimensional Hierarchical Framework Assembled by Cobblestone-Like CoSe2@C Nanospheres for Ultrastable Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2018, 10, 14716–14726. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Bresser, D.; Ji, Y.; Geiger, D.; Kaiser, U.; Streb, C.; Varzi, A.; Passerini, S. Cobalt Disulfide Nanoparticles Embedded in Porous Carbonaceous Micro-Polyhedrons Interlinked by Carbon Nanotubes for Superior Lithium and Sodium Storage. ACS Nano 2018, 12, 7220–7231. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, J.; Mulas, G.; Panero, S.; Scrosati, B. Ternary Sn–Co–C Li-ion battery electrode material prepared by high energy ball milling. Electrochem. Commun. 2007, 9, 2075–2081. [Google Scholar] [CrossRef]
- Li, H.-H.; Li, Z.-Y.; Wu, X.L.; Zhang, L.-L.; Fan, C.-Y.; Wang, H.-F.; Li., X.Y.; Wang, K.; Sun, H.-Z.; Zhang, J.-P. Shale-like Co3O4 for high performance lithium/sodium ion batteries. J. Mater. Chem. 2013, 4, 8242–8248. [Google Scholar]
- Juan Xiang, T.S. One-Pot Synthesis of Multicomponent (Mo, Co) Metal Sulfide/Carbon Nanoboxes as anode materials for Improving Na-ion Strorage. Chem. Commun. 2013, 53, 10820–10823. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Guan, J.; Li, S.; Lan, Z.; Chen, C.; Shang, C.; Zhang, L.; Wang, X.; Gu, L.; et al. Insight into Enhanced Cycling Performance of Li-O2 Batteries Based on Binary CoSe2/CoO Nanocomposite Electrodes. J. Phys. Chem. Lett. 2014, 5, 615–621. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, S. Enhanced Electrochemical Performance of Sn–Co Nanoarchitectured Electrode for Lithium Ion Batteries. J. Phys. Chem. C 2011, 115, 23603–23609. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Zhu, Y.; Wei, D.; Fan, L.; Qian, Y. Synthesis of Co2SnO4 hollow cubes encapsulated in graphene as high capacity anode materials for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 2728. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, A.; Yang, H.; Meng, H.; Dou, P.; Ma, D.; Xu, X. Facile synthesis of hollow Sn–Co@PMMA nanospheres as high performance anodes for lithium-ion batteries via galvanic replacement reaction and in situ polymerization. Appl. Surf. Sci. 2015, 347, 624–631. [Google Scholar] [CrossRef]
- Huang, B.; Yang, J.; Li, Y.; Xiao, S.; Chen, Q. Carbon encapsulated Sn-Co alloy: A stabilized tin-based material for sodium storage. Mater. Lett. 2018, 210, 321–324. [Google Scholar] [CrossRef]
- Lee, S.-I.; Yoon, S.; Park, C.-M.; Lee, J.-M.; Kim, H.; Im, D.; Doo, S.-G.; Sohn, H.-J. Reaction mechanism and electrochemical characterization of a Sn–Co–C composite anode for Li-ion batteries. Electrochim. Acta 2008, 54, 364–369. [Google Scholar] [CrossRef]
- Fan, X.-Y.; Ke, F.-S.; Wei, G.-Z.; Huang, L.; Sun, S.-G. Sn–Co alloy anode using porous Cu as current collector for lithium ion battery. J. Alloy. Compd. 2009, 476, 70–73. [Google Scholar] [CrossRef]
- Qin, J.; Liu, D.; Zhang, X.; Zhao, N.; Shi, C.; Liu, E.Z.; He, F.; Ma, L.; Li, Q.; Li, J.; et al. One-step synthesis of SnCo nanoconfined in hierarchical carbon nanostructures for lithium ion battery anode. Nanoscale 2017, 9, 15856–15864. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, J.; Ochal, P.; Panero, S.; Mulas, G.; Bonatto Minella, C.; Scrosati, B. The effect of CoSn/CoSn2 phase ratio on the electrochemical behaviour of Sn40Co40C20 ternary alloy electrodes in lithium cells. J. Power Sources 2008, 180, 568–575. [Google Scholar] [CrossRef]
- López, M.C.; Ortiz, G.F.; Tirado, J.L. A Functionalized Co2P Negative Electrode for Batteries Demanding High Li-Potential Reaction. J. Electrochem. Soc. 2012, 159, A1253–A1261. [Google Scholar] [CrossRef]
- Shadike, Z.; Cao, M.H.; Ding, F.; Sang, L.; Fu, Z.W. Improved electrochemical performance of CoS2-MWCNT nanocomposites for sodium-ion batteries. Chem. Commun. 2015, 51, 10486–10489. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.; Hao, X.; Wang, Y.; Liu, Y.; Hou, Y.; Yang, Q.; Wang, X.; Qiu, J. Engineering hollow polyhedrons structured from carbon-coated CoSe2 nanospheres bridged by CNTs with boosted sodium storage performance. J. Mater. Chem. A 2017, 5, 13591–13600. [Google Scholar] [CrossRef]
- Li, Z.; Feng, W.; Lin, Y.; Liu, X.; Fei, H. Flaky CoS2 and graphene nanocomposite anode materials for sodium-ion batteries with improved performance. RSC Adv. 2016, 6, 70632–70637. [Google Scholar] [CrossRef]
- Zheng, X.-M.; Xiao, Y.; Huang, L.; Ke, F.-S.; He, Y.; Li, J.-T.; Wei, G.-Z.; Sun, S.-G. Fabrication and electrochemical properties of novel ternary Sb–Co–P alloy electrodes for lithium-ion batteries. Electrochem. Commun. 2009, 11, 1803–1806. [Google Scholar] [CrossRef]
- Jiu, H.; Ren, N.; Jiang, L.; Zhang, Q.; Gao, Y.; Meng, Y.; Zhang, L. Hierarchical porous CoMn2O4 microspheres with sub-nanoparticles as advanced anode for high-performance lithium-ion batteries. J. Solid State Electr. 2018, 22, 2747–2755. [Google Scholar] [CrossRef]
- Zou, G.; Hou, H.; Zhao, G.; Ge, P.; Yin, D.; Ji, X. N-rich carbon coated CoSnO3 derived from in situ construction of a Co–MOF with enhanced sodium storage performance. J. Mater. Chem. A 2018, 6, 4839–4847. [Google Scholar] [CrossRef]
- Nasir Mahmood, C.Z.; Fei, L.; Jinghan, Z.; Yanglong, H. Hybrid of Co3Sn2@Co Nanoparticles and Nitrogen-Doped Graphene as a Lithium Ion Battery Anode. ACS Nano 2013, 7, 10307–10318. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Geng, B.; Guo, Y. Porous Co3O4 nanosheets with extraordinarily high discharge capacity for lithium batteries. Chem-Eur. J. 2009, 15, 6169–6174. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, H.; Wang, J.; Wang, J.; Chen, J. Hydrothermal synthesis and electrochemical properties of nano-sized Co–Sn alloy anodes for lithium ion batteries. J. Alloy. Compd. 2010, 508, 629–635. [Google Scholar] [CrossRef]
- Wang, X.L.; Han, W.Q.; Chen, J.; Graetz, J. Single-crystal intermetallic M-Sn (M = Fe, Cu, Co, Ni) nanospheres as negative electrodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2010, 2, 1548–1551. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, W.; Tao, X.; Xia, Y.; Yu, Z.; Fang, J.; Gan, Y.; Zhang, W. Nanocrystal-constructed mesoporous single-crystalline Co3O4 nanobelts with superior rate capability for advanced lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 5974–5980. [Google Scholar] [CrossRef]
- Xia, H.; Li, K.; Guo, Y.; Guo, J.; Xu, Q.; Zhang, J. CoS2 nanodots trapped within graphitic structured N-doped carbon spheres with efficient performances for lithium storage. J. Mater. Chem. A 2018, 6, 7148–7154. [Google Scholar] [CrossRef]
- Jin, R.; Cui, Y.; Gao, S.; Zhang, S.; Yang, L.; Li, G. CNTs@NC@CuCo2S4 nanocomposites: An advanced electrode for high performance lithium-ion batteries and supercapacitors. Electrochim. Acta 2018, 273, 43–52. [Google Scholar] [CrossRef]
- Ali, Z.; Tang, T.; Huang, X.; Wang, Y.; Asif, M.; Hou, Y. Cobalt selenide decorated carbon spheres for excellent cycling performance of sodium ion batteries. Energy Storage Mater. 2018, 13, 19–28. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Hu, R.; Liu, J.; Ouyang, L.; Zhu, M. Self-Supported CoP Nanorod Arrays Grafted on Stainless Steel as an Advanced Integrated Anode for Stable and Long-Life Lithium-Ion Batteries. Chem-Eur. J. 2017, 23, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Xu, J.; Dravid, V.P. Synergistic sodiation of cobalt oxide nanoparticles and conductive carbon nanotubes (CNTs) for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 8669–8675. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, P.; Li, F.; Chen, M.; Zhou, H. Monodispersed hierarchical Co3O4spheres intertwined with carbon nanotubes for use as anode materials in sodium-ion batteries. J. Mater. Chem. A 2014, 2, 13805. [Google Scholar] [CrossRef]
- Xiao, S.; Li, A.; Chen, X.H.; Zhou, J.S.; Ma, Z.K. Sn-Co nano alloy embedded in porous N-doped carbon microboxes as a stable anode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 5873–5879. [Google Scholar]
- Lou, X.W.; Deng, D.; Lee, J.Y.; Feng, J.; Archer, L.A. Self-Supported Formation of Needlelike Co3O4 Nanotubes and Their Application as Lithium-Ion Battery Electrodes. Adv. Mater. 2008, 20, 258–262. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, R.; Xia, W.; Ma, J.; Qiu, B.; Mahmood, A.; Zhao, R.; Yang, Y.; Xia, D.; Xu, Q. Facile Synthesis of Ultrasmall CoS2 Nanoparticles within Thin N-Doped Porous Carbon Shell for High Performance Lithium-Ion Batteries. Small 2015, 11, 2511–2517. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, F.; Wang, Y. Confined Volume Change in Sn-Co-C Ternary Tube-in-Tube Composites for High-Capacity and Long-Life Lithium Storage. Adv. Funct. Mater. 2013, 23, 893–899. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Lu, T.; Yao, Y.; Pan, L. An advanced CoSe embedded within porous carbon polyhedra hybrid for high performance lithium-ion and sodium-ion batteries. Chem. Eng. J. 2017, 325, 14–24. [Google Scholar] [CrossRef]
- Wang, J.; Yang, N.; Tang, H.; Dong, Z.; Jin, Q.; Yang, M.; Kisailus, D.; Zhao, H.; Tang, Z.; Wang, D. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 2013, 52, 6417–6420. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Ge, X.; Li, C.; Dong, S.; Wang, C.; Yin, L. Core-shell structured CoP/FeP porous microcubes interconnected by reduced graphene oxide as high performance anodes for sodium ion batteries. Nano Energy 2017, 32, 494–502. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.K.; Chan Kang, Y. Metal–organic framework-derived CoSe2/(NiCo)Se2 box-in-box hollow nanocubes with enhanced electrochemical properties for sodium-ion storage and hydrogen evolution. J. Mater. Chem. A 2017, 5, 18823–18830. [Google Scholar] [CrossRef]
- Alcantara, R.; Jaraba, M.; Lavela, P.; Tirado, J.L. NiCo2O4 Spinel: First Report on a Transition Metal Oxide for the Negative Electrode of Sodium-Ion Batteries. Chem. Mater. 2002, 14, 7. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Chen, B.D.; Wu, J.B.; Ma, X.Y.; Liu, Z.H.; Zhang, Y.Q.; Yang, D.R.; Huang, X.H.; Tu, J.P. Porous Co3O4 Nanotubes Derived From Co4(CO)12 Clusters on Carbon Nanotube Templates: A Highly Efficient Material For Li-Battery Applications. Adv. Mater. 2007, 19, 4505–4509. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Shioyama, H.; Mukai, T.; Sakai, T.; Xu, Q. Converting cobalt oxide subunits in cobalt metal-organic framework into agglomerated Co3O4 nanoparticles as an electrode material for lithium ion battery. J. Power Sources 2010, 195, 857–861. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zou, Y.; Jiao, Z.; Zhao, B.; He, Y.; Wu, M. Macroporous Co3O4 platelets with excellent rate capability as anodes for lithium ion batteries. Electrochem. Commun. 2010, 12, 101–105. [Google Scholar] [CrossRef]
- Hu, L.; Yan, N.; Chen, Q.; Zhang, P.; Zhong, H.; Zheng, X.; Li, Y.; Hu, X. Fabrication based on the Kirkendall effect of Co3O4 porous nanocages with extraordinarily high capacity for lithium storage. Chem-Eur. J. 2012, 18, 8971–8977. [Google Scholar] [CrossRef]
- Yan, N.; Hu, L.; Li, Y.; Wang, Y.; Zhong, H.; Hu, X.; Kong, X.; Chen, Q. Co3O4 Nanocages for High-Performance Anode Material in Lithium-Ion Batteries. J. Phy. Chem. C 2012, 116, 7227–7235. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, R.Z.; Xu, D.; Wang, Z.L.; Wang, H.G.; Xu, J.J.; Wu, Z.; Liu, Q.C.; Zhang, Y.; Zhang, X.B. Homogeneous CoO on Graphene for Binder-Free and Ultralong-Life Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 4345–4353. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Rui, X.; Tan, H.; Cai, R.; Hoster, H.E.; Yu, D.Y.; Hng, H.H.; Yan, Q. Synthesis of cobalt phosphides and their application as anodes for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 1093–1099. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X.; Wang, G.; Long, X.; Li, Y.; Zhang, W.; Zhang, P. Carbon-coated CoP3 nanocomposites as anode materials for high-performance sodium-ion batteries. Appl. Surf. Sci. 2018, 445, 167–174. [Google Scholar] [CrossRef]
- Li, J.; Le, D.B.; Ferguson, P.P.; Dahn, J.R. Lithium polyacrylate as a binder for tin–cobalt–carbon negative electrodes in lithium-ion batteries. Electrochim. Acta 2010, 55, 2991–2995. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Nuli, Y.; Wang, B.; Xu, J. CoPx synthesis and lithiation by ball-milling for anode materials of lithium ion cells. Solid State Ionics 2005, 176, 693–697. [Google Scholar] [CrossRef]
- Lavela, P.; Nacimiento, F.; Ortiz, G.F.; Tirado, J.L. Sn–Co–C composites obtained from resorcinol-formaldehyde gel as anodes in lithium-ion batteries. J. Solid State Electr. 2009, 14, 139–148. [Google Scholar] [CrossRef]
- González, J.R.; Nacimiento, F.; Alcántara, R.; Ortiz, G.F.; Tirado, J.L. Electrodeposited CoSn2 on nickel open-cell foam: Advancing towards high power lithium ion and sodium ion batteries. CrystEngComm 2013, 15, 9196. [Google Scholar] [CrossRef]
- Ke, F.S.; Huang, L.; Solomon, B.C.; Wei, G.Z.; Xue, L.-J.; Zhang, B.; Li, J.T.; Zhou, X.D.; Sun, S.G. Three-dimensional nanoarchitecture of Sn–Sb–Co alloy as an anode of lithium-ion batteries with excellent lithium storage performance. J. Mater. Chem. 2012, 22, 17511. [Google Scholar] [CrossRef]
- Xue, L.J.; Xu, Y.F.; Huang, L.; Ke, F.S.; He, Y.; Wang, Y.X.; Wei, G.Z.; Li, J.T.; Sun, S.G. Lithium storage performance and interfacial processes of three dimensional porous Sn–Co alloy electrodes for lithium-ion batteries. Electrochim. Acta 2011, 56, 5979–5987. [Google Scholar] [CrossRef]
- Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Electrochemical deposition of porous Co3O4 nanostructured thin film for lithium-ion battery. J. Power Sources 2008, 182, 359–364. [Google Scholar] [CrossRef]
- Liu, H.C.; Yen, S.K. Characterization of electrolytic Co3O4 thin films as anodes for lithium-ion batteries. J. Power Sources 2007, 166, 478–484. [Google Scholar] [CrossRef]
- Groult, H.; El Ghallali, H.; Barhoun, A.; Briot, E.; Julien, C.M.; Lantelme, F.; Borensztjan, S. Study of Co–Sn and Ni–Sn alloys prepared in molten chlorides and used as negative electrode in rechargeable lithium battery. Electrochim. Acta 2011, 56, 2656–2664. [Google Scholar] [CrossRef]
- Jang, B.O.; Park, S.H.; Lee, W.J. Electrospun Co–Sn alloy/carbon nanofibers composite anode for lithium ion batteries. J. Alloy. Compd. 2013, 574, 325–330. [Google Scholar] [CrossRef]
- Zhang, W.; Yue, Z.; Miao, W.; Liu, S.; Fu, C.; Li, L.; Zhang, Z.; Wang, H. Carbon-Encapsulated Tube-Wire Co3O4/MnO2 Heterostructure Nanofibers as Anode Material for Sodium-Ion Batteries. Part. Part. Syst. Char. 2018, 35, 1800138. [Google Scholar] [CrossRef]
- Shin, J.; Ryu, W.-H.; Park, K.-S.; Kim, I.-D. Morphological Evolution of Carbon Nanofibers Encapsulating SnCo Alloys and Its Effect on Growth of the Solid Electrolyte Interphase Layer. ACS Nano 2013, 7, 7330–7341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Cao, D.; Lu, X.; Han, X.; Niu, C. Epitaxial Growth of Urchin-Like CoSe2 Nanorods from Electrospun Co-Embedded Porous Carbon Nanofibers and Their Superior Lithium Storage Properties. Part. Part. Syst. Char. 2017, 34, 1700185. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, Y.; Liu, Y.; Li, D.; Zhu, X.; Ji, Q.; Quan, F.; Xia, Y. Synthesis of Co/Co3O4 nanoparticles embedded in porous carbon nanofibers for high performance lithium-ion battery anodes. J. Porous. Mat. 2016, 24, 551–557. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.H.; Tu, J.P.; Xiong, Q.Q.; Yu, Y.X.; Wang, X.L.; Gu, C.D. Co3O4–C core–shell nanowire array as an advanced anode material for lithium ion batteries. J. Mater. Chem. 2012, 22, 15056. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, D.H.; Cha, S.M.; Sun, Y.K.; Yoon, C.S. Electrochemical behaviour of Heusler alloy Co2MnSi for secondary lithium batteries. J. Power Sources 2009, 188, 281–285. [Google Scholar] [CrossRef]
- Wang, X.; Kong, D.; Huang, Z.X.; Wang, Y.; Yang, H.Y. Nontopotactic Reaction in Highly Reversible Sodium Storage of Ultrathin Co9Se8/rGO Hybrid Nanosheets. Small 2017, 13. [Google Scholar] [CrossRef]
- Barreca, D.; Cruz-Yusta, M.; Gasparotto, A.; Maccato, C.; Morales, J.; Pozza, A.; Sada, C.; Sa’nchez, L.; Tondello, E. Cobalt Oxide Nanomaterials by Vapor-Phase Synthesis for Fast and Reversible Lithium Storage. J. Phys. Chem. C 2010, 114, 10054–10060. [Google Scholar] [CrossRef]
- Jena, A.; Munichandraiah, N.; Shivashankar, S.A. Metal-organic chemical vapor-deposited cobalt oxide films as negative electrodes for thin film Li-ion battery. J. Power Sources 2015, 277, 198–204. [Google Scholar] [CrossRef]
- Liu, H.J.; Bo, S.H.; Cui, W.J.; Li, F.; Wang, C.X.; Xia, Y.Y. Nano-sized cobalt oxide/mesoporous carbon sphere composites as negative electrode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 6497–6503. [Google Scholar] [CrossRef]
- Liao, C.L.; Lee, Y.H.; Chang, S.T.; Fung, K.Z. Structural characterization and electrochemical properties of RF-sputtered nanocrystalline Co3O4 thin-film anode. J. Power Sources 2006, 158, 1379–1385. [Google Scholar] [CrossRef]
- Huang, L.; Cai, J.S.; He, Y.; Ke, F.S.; Sun, S.G. Structure and electrochemical performance of nanostructured Sn–Co alloy/carbon nanotube composites as anodes for lithium ion batteries. Electrochem. Commun. 2009, 11, 950–953. [Google Scholar] [CrossRef]
- Li, M.Y.; Liu, C.L.; Shi, M.R.; Dong, W.S. Nanostructure Sn–Co–C composite lithium ion battery electrode with unique stability and high electrochemical performance. Electrochim. Acta 2011, 56, 3023–3028. [Google Scholar] [CrossRef]
- Zhai, C.; Du, N.; Zhang, H.; Yu, J.; Wu, P.; Xiao, C.; Yang, D. Assembling CoSn3 nanoparticles on multiwalled carbon nanotubes with enhanced lithium storage properties. Nanoscale 2011, 3, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Chen, H.; Bai, J.; Han, W.Q. CoSn5 Phase: Crystal Structure Resolving and Stable High Capacity as Anodes for Li Ion Batteries. J. Phys. Chem. Lett. 2012, 3, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, L.; Wang, Y. Graphene wrapped SnCo nanoparticles for high-capacity lithium ion storage. J. Power Sources 2013, 222, 526–532. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, D. Amorphous Bimetallic Co3Sn2 Nanoalloys Are Better Than Crystalline Counterparts for Sodium Storage. J. Phys. Chem. C 2015, 119, 21323–21328. [Google Scholar] [CrossRef]
- Cui, W.; Wang, F.; Wang, J.; Wang, C.; Xia, Y. Nanostructural CoSnC anode prepared by CoSnO3 with improved cyclability for high-performance Li-ion batteries. Electrochim. Acta 2011, 56, 4812–4818. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, G.; Zhou, Y.; Wang, H. Flexible free-standing ternary CoSnO3 /graphene/carbon nanotubes composite papers as anodes for enhanced performance of lithium-ion batteries. Energy 2017, 118, 172–180. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.-H.; Kim, S.-W.; Kim, J.; Kang, K. Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Shao, J.; Li, G.; Qu, M.; Yin, G. Co3O4@graphene composites as anode materials for high-performance lithium ion batteries. Inorg. Chem. 2011, 50, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Jones, W.D.; Moganty, S.S.; Archer, L.A. Composite lithium battery anodes based on carbon@Co3O4 nanostructures: Synthesis and characterization. J. Power Sources 2012, 200, 53–58. [Google Scholar] [CrossRef]
- Tao, L.; Zai, J.; Wang, K.; Zhang, H.; Xu, M.; Shen, J.; Su, Y.; Qian, X. Co3O4 nanorods/graphene nanosheets nanocomposites for lithium ion batteries with improved reversible capacity and cycle stability. J. Power Sources 2012, 202, 230–235. [Google Scholar] [CrossRef]
- Wang, R.T.; Kong, L.B.; Lang, J.W.; Wang, X.W.; Fan, S.Q.; Luo, Y.C.; Kang, L. Mesoporous Co3O4 materials obtained from cobalt–citrate complex and their high capacitance behavior. J. Power Sources 2012, 217, 358–363. [Google Scholar] [CrossRef]
- Huang, S.; Jin, Y.; Jia, M. Preparation of graphene/Co3O4 composites by hydrothermal method and their electrochemical properties. Electrochim. Acta 2013, 95, 139–145. [Google Scholar] [CrossRef]
- Rai, A.K.; Gim, J.; Anh, L.T.; Kim, J. Partially reduced Co3O4/graphene nanocomposite as an anode material for secondary lithium ion battery. Electrochim. Acta 2013, 100, 63–71. [Google Scholar] [CrossRef]
- Wen, J.W.; Zhang, D.W.; Zang, Y.; Sun, X.; Cheng, B.; Ding, C.X.; Yu, Y.; Chen, C.H. Li and Na storage behavior of bowl-like hollow Co3O4 microspheres as an anode material for lithium-ion and sodium-ion batteries. Electrochim. Acta 2014, 132, 193–199. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Sun, B.; Ao, Z.; Xie, X.; Wei, Y.; Wang, G. Microwave-assisted synthesis of mesoporous Co3O4 nanoflakes for applications in lithium ion batteries and oxygen evolution reactions. ACS Appl. Mater. Interfaces 2015, 7, 3306–3313. [Google Scholar] [CrossRef]
- DeliWang, Y.Y.; Huan, H.; Jie, W.; WeiDong, Z.; Hector, D. Abruna Template-Free Synthesis of Hollow-Structured Co3O4 Nanoparticles as High-Performance Anodes for Lithium-Ion Batteries. ACS Nano 2015, 9, 1775–1781. [Google Scholar]
- Deng, Q.; Wang, L.; Li, J. Electrochemical characterization of Co3O4/MCNTs composite anode materials for sodium-ion batteries. J. Mater. Sci. 2015, 50, 4142–4148. [Google Scholar] [CrossRef]
- Gu, D.; Li, W.; Wang, F.; Bongard, H.; Spliethoff, B.; Schmidt, W.; Weidenthaler, C.; Xia, Y.; Zhao, D.; Schuth, F. Controllable Synthesis of Mesoporous Peapod-like Co3O4@Carbon Nanotube Arrays for High-Performance Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 7060–7064. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Sultana, I.; Chen, Z.; Srikanth, M.; Li, L.H.; Dai, X.J.; Chen, Y. Ex situ electrochemical sodiation/desodiation observation of Co3O4 anchored carbon nanotubes: A high performance sodium-ion battery anode produced by pulsed plasma in a liquid. Nanoscale 2015, 7, 13088–13095. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Xu, J.; Ruan, B.; Liu, Q.; Pan, Y.; Sun, Z.; Dou, S.X. Atomic Layer-by-Layer Co3O4/Graphene Composite for High Performance Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501835. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, Y.; Liu, H.; Huang, Z. Superior sodium-ion storage performance of Co3O4@nitrogen-doped carbon: Derived from a metal–organic framework. J. Mater. Chem. A 2016, 4, 5428–5435. [Google Scholar] [CrossRef]
- Yan, C.; Chen, G.; Zhou, X.; Sun, J.; Lv, C. Template-Based Engineering of Carbon-Doped Co3O4 Hollow Nanofibers as Anode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 1428–1436. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, J.; He, Y.-B.; Wang, Z.; Tanaka, S.; Hossain, M.S.A.; Pan, Z.Z.; Xiang, B.; Yang, Q.-H.; Yamauchi, Y. Fabrication of an MOF-derived heteroatom-doped Co/CoO/carbon hybrid with superior sodium storage performance for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 15356–15366. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, J.K.; Kim, J.H.; Kang, Y.C. Metal–organic framework-templated hollow Co3O4 nanosphere aggregate/N-doped graphitic carbon composite powders showing excellent lithium-ion storage performances. Mater. Charact. 2017, 132, 320–329. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, F.; Li, S.; Shen, Y.; Xie, A. Novel porous starfish-like Co3O4@nitrogen-doped carbon as an advanced anode for lithium-ion batteries. Nano Res. 2017, 10, 3457–3467. [Google Scholar] [CrossRef]
- Wu, Y.; Meng, J.; Li, Q.; Niu, C.; Wang, X.; Yang, W.; Li, W.; Mai, L. Interface-modulated fabrication of hierarchical yolk–shell Co3O4/C dodecahedrons as stable anodes for lithium and sodium storage. Nano Res. 2017, 10, 2364–2376. [Google Scholar] [CrossRef]
- Xin, D.; Dai, J.; Liu, J.; Wang, Q.; Li, W. Mesocrystal hexagonal Co3O4 nanosheets for high performance lithium and sodium-ion batteries. Mater. Lett. 2017, 209, 388–391. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Ning, F.; Zhou, L.; Zhang, R.; Shao, M.; Wei, M. Ultrathin Mesoporous Co3O4 Nanosheet Arrays for High-Performance Lithium-Ion Batteries. ACS Omega. 2018, 3, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Pan, L. Metal-organic frameworks converted flower-like hybrid with Co3O4 nanoparticles decorated on nitrogen-doped carbon sheets for boosted lithium storage performance. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, H.; Zhu, M.; Feng, J.; Yuan, A. Facile synthesis of metal-organic framework-derived Co3O4 with different morphologies coated graphene foam as integrated anodes for lithium-ion batteries. J. Alloy. Compd. 2018, 768, 1049–1057. [Google Scholar] [CrossRef]
- Kwon, H.T.; Kim, J.H.; Jeon, K.J.; Park, C.M. CoxP compounds: Electrochemical conversion/partial recombination reaction and partially disproportionated nanocomposite for Li-ion battery anodes. RSC Adv. 2014, 4, 43227–43234. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Sun, C.; Liu, H.; Li, L.; Si, W.; Huang, W.; Yan, Q.; Dong, X. Graphene and cobalt phosphide nanowire composite as an anode material for high performance lithium-ion batteries. Nano Res. 2016, 9, 612–621. [Google Scholar] [CrossRef]
- Guo, Q.; Ru, Q.; Wang, B.; Mo, Y.; Wang, Z.; Zhang, P.; Hou, X.; Hu, S. The electrochemical confrontation between CoP microflake and Co3O4 microsphere via a similar synthesis process as anodes for lithium ion batteries. J. Alloy. Compd. 2017, 728, 910–916. [Google Scholar] [CrossRef]
- Guo, Q.; Ru, Q.; Wang, B.; Zhang, P.; Hou, X.; Ling, F.C.-C. Design and Synthesis of Mesoporous Honeycomb-Like CoP/Co2P Hybrids as Anode with a High Cyclic Stability in Lithium-Ion Batteries. Energy Technol. 2017, 5, 2294–2299. [Google Scholar] [CrossRef]
- Wang, B.; Ru, Q.; Guo, Q.; Chen, X.; Wang, Z.; Hou, X.; Hu, S. Fabrication of One-Dimensional Mesoporous CoP Nanorods as Anode Materials for Lithium-Ion Batteries. Eur. J. Inorg. Chem. 2017, 2017, 3729–3735. [Google Scholar] [CrossRef]
- Xie, Q.; Zeng, D.; Gong, P.; Huang, J.; Ma, Y.; Wang, L.; Peng, D.-L. One-pot fabrication of graphene sheets decorated Co2P-Co hollow nanospheres for advanced lithium ion battery anodes. Electrochim. Acta 2017, 232, 465–473. [Google Scholar] [CrossRef]
- Xing, Y.M.; Zhang, X.H.; Liu, D.H.; Li, W.H.; Sun, L.N.; Geng, H.B.; Zhang, J.P.; Guan, H.Y.; Wu, X.L. Porous Amorphous Co2P/N,B-Co-doped Carbon Composite as an Improved Anode Material for Sodium-Ion Batteries. ChemElectroChem 2017, 4, 1395–1401. [Google Scholar] [CrossRef]
- Bai, J.; Xi, B.; Mao, H.; Lin, Y.; Ma, X.; Feng, J.; Xiong, S. One-Step Construction of N,P-Codoped Porous Carbon Sheets/CoP Hybrids with Enhanced Lithium and Potassium Storage. Adv. Mater. 2018, e1802310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, K.; Fang, Y.; Wang, H.; Ye, K.; Yan, J.; Wang, G.; Cheng, K.; Zhou, L.; Cao, D. Coralloidal carbon-encapsulated CoP nanoparticles generated on biomass carbon as a high-rate and stable electrode material for lithium-ion batteries. J. Colloid Interface Sci. 2018, 530, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Li, X.; Sun, Y.; Shan, H.; Fan, L.; Li, D.; Sun, X. Metal-Organic Frameworks-Derived Co2P@N-C@rGO with Dual Protection Layers for Improved Sodium Storage. ACS Appl. Mater. Interfaces 2018, 10, 14641–14648. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, Y.; Chen, W.; Yan, Y.; Xue, L.; Zhang, W. CoP3@PPy microcubes as anode for lithium-ion batteries with improved cycling and rate performance. Chem. Eng. J. 2018, 347, 455–461. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Xie, H.; Chen, T.; Yang, C.; Wang, J. MOF-driven ultra-small hollow Co9S8 nanoparticles embedded in porous carbon for lithium-ion batteries. J. Mater. Res. 2018, 33, 1496–1505. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Sun, Y.; Liu, X. CoS@sulfur doped onion-like carbon nanocapsules with excellent cycling stability and rate capability for sodium-ion batteries. Ceram. Int. 2018, 44, 17113–17117. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, Q.; Li, H.; Xu, J.; Wang, H.; Zhao, G.; Lu, L.; Lin, X.; Li, H.; et al. Improved Electrochemical Performance Based on Nanostructured SnS2@CoS2–rGO Composite Anode for Sodium-Ion Batteries. Nano-Micro Lett. 2018, 10. [Google Scholar] [CrossRef]
- Wang, H.C.; Cui, Z.; Fan, C.Y.; Liu, S.Y.; Shi, Y.H.; Wu, X.L.; Zhang, J.P. 3 D Porous CoS2 Hexadecahedron Derived from MOC toward Ultrafast and Long-Lifespan Lithium Storage. Chem-Eur. J. 2018, 24, 6798–6803. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Liu, Q.; Wang, Y.; Lu, Z.; Li, M.; Wang, J.; Wang, D. Ethylenediamine-assisted synthesis of microsized cobalt sulfide as advanced anode materials for sodium ion batteries. J. Alloy. Compd. 2018, 735, 765–772. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Q.; Tian, J.; Jiang, F. TiO2 Nanobelt@Co9S8 Composites as Promising Anode Materials for Lithium and Sodium Ion Batteries. Nanomaterials 2017, 7, 252. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Sheng, J.; An, Q.; Tao, Z.; Kang, Y.M.; Chen, J.; Mai, L. Structural and chemical synergistic effect of CoS nanoparticles and porous carbon nanorods for high-performance sodium storage. Nano Energy 2017, 35, 281–289. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, J. Facile synthesis of multi-walled carbon nanotubes/Co9S8 composites with enhanced performances for sodium-ion battery. Mater. Lett. 2017, 195, 26–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Pang, Q.; Wei, Y.; Wei, L.; Ju, Y.; Zou, B.; Gao, Y.; Chen, G. Co9S8/Co as a High-Performance Anode for Sodium-Ion Batteries with an Ether-Based Electrolyte. ChemSusChem 2017, 10, 4778–4785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Wang, G. Cobalt sulfide nanoparticles anchored in three-dimensional carbon nanosheet networks for lithium and sodium ion batteries with enhanced electrochemical performance. J. Colloid Interface Sci. 2017, 492, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, L.; Lou, X.W.D. Embedding CoS2 nanoparticles in N-doped carbon nanotube hollow frameworks for enhanced lithium storage properties. Nano Res. 2017, 10, 4298–4304. [Google Scholar] [CrossRef]
- Tao, S.; Huang, W.; Xie, H.; Zhang, J.; Wang, Z.; Chu, W.; Qian, B.; Song, L. Formation of graphene-encapsulated CoS2 hybrid composites with hierarchical structures for high-performance lithium-ion batteries. RSC Adv. 2017, 7, 39427–39433. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, X.; Huang, Y.; Gong, L.; Zhang, H. CoS2 Nanoparticles Wrapping on Flexible Freestanding Multichannel Carbon Nanofibers with High Performance for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 35820–35828. [Google Scholar] [CrossRef]
- Liu, X.; Zou, F.; Liu, K.; Qiang, Z.; Taubert, C.J.; Ustriyana, P.; Vogt, B.D.; Zhu, Y. A binary metal organic framework derived hierarchical hollow Ni3S2/Co9S8/N-doped carbon composite with superior sodium storage performance. J. Mater. Chem. A 2017, 5, 11781–11787. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, R.; Duan, H.; Wang, K.; Guo, Y.; Li, H.; Liu, H. CoSe/Co nanoparticles wrapped by in situ grown N-doped graphitic carbon nanosheets as anode material for advanced lithium ion batteries. J. Power Sources 2018, 399, 223–230. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, K.; Liu, S.; Song, H.; Zhou, J. Flexible Co0.85Se nanosheets/graphene composite film as binder-free anode with high Li- and Na-Ion storage performance. J. Alloy. Compd. 2018, 731, 714–722. [Google Scholar] [CrossRef]
- Sun, W.; Cai, C.; Tang, X.; Lv, L.-P.; Wang, Y. Carbon coated mixed-metal selenide microrod: Bimetal-organic-framework derivation approach and applications for lithium-ion batteries. Chem. Eng. J. 2018, 351, 169–176. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, Y.C. MOF-Templated N-Doped Carbon-Coated CoSe2 Nanorods Supported on Porous CNT Microspheres with Excellent Sodium-Ion Storage and Electrocatalytic Properties. ACS Appl. Mater. Interfaces 2018, 10, 17203–17213. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. Formation of Hierarchical Cu-Doped CoSe2 Microboxes via Sequential Ion Exchange for High-Performance Sodium-Ion Batteries. Adv. Mater. 2018, 30, e1706668. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Zhao, L.; Hu, H.; Li, T.; Li, X.; Guo, S.; Li, Y.; Xue, Q.; Xing, W.; Yan, Z.; et al. Stable CoSe2/carbon nanodice@reduced graphene oxide composites for high-performance rechargeable aluminum-ion batteries. Energy Environ. Sci. 2018, 11, 2341–2347. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Chen, H.; Ji, X.; Liu, Y. 3D Nanosheet-Assembled CoSe Quasi-Microspheres as Advanced Electrode Materials for Electrochemical Energy Storage. J. Electrochem. Soc. 2017, 164, A2341–A2347. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Ding, L.; Zhou, Z.; Wang, Y.; Niu, S.; Liang, S.; Cao, G. Nitrogen-Doped Yolk-Shell-Structured CoSe/C Dodecahedra for High-Performance Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 3624–3633. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.K.; Kang, Y.C. Excellent sodium-ion storage performances of CoSe2 nanoparticles embedded within N-doped porous graphitic carbon nanocube/carbon nanotube composite. Chem. Eng. J. 2017, 328, 546–555. [Google Scholar] [CrossRef]
- Ou, X.; Liang, X.; Zheng, F.; Wu, P.; Pan, Q.; Xiong, X.; Yang, C.; Liu, M. In situ X-ray diffraction investigation of CoSe2 anode for Na-ion storage: Effect of cut-off voltage on cycling stability. Electrochim. Acta 2017, 258, 1387–1396. [Google Scholar] [CrossRef]
- Lu, W. CoSe2 Nanoparticles as Anode for Lithium Ion Battery. Int. J. Electrochem. Sci. 2017, 12, 1118–1129. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Fan, L.-Z. MOF-derived CoSe2 microspheres with hollow interiors as high-performance electrocatalysts for the enhanced oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 15310–15314. [Google Scholar] [CrossRef]
- Qiu, S.; Huang, J.; Chu, H.; Zou, Y.; Xiang, C.; Yan, E.; Xu, F.; Sun, L. The Co-B Amorphous Alloy: A High Capacity Anode Material for an Alkaline Rechargeable Battery. Metals 2016, 6, 269. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, M.; Wang, H.; Xu, H.; Guo, Y.; Song, Y.; Fang, F.; Yu, X.; Sun, D. General Synthesis of Dual Carbon-Confined Metal Sulfides Quantum Dots Toward High-Performance Anodes for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702046. [Google Scholar] [CrossRef]
- Peng, S.; Han, X.; Li, L.; Zhu, Z.; Cheng, F.; Srinivansan, M.; Adams, S.; Ramakrishna, S. Unique Cobalt Sulfide/Reduced Graphene Oxide Composite as an Anode for Sodium-Ion Batteries with Superior Rate Capability and Long Cycling Stability. Small 2016, 12, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.F.; Lou, X.W. Formation of CoS2 Nanobubble Hollow Prisms for Highly Reversible Lithium Storage. Angew. Chem. Int. Ed. Engl. 2016, 55, 13422–13426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Feng, J.; Jiang, X.; Yue, J.; Yang, J.; Qian, Y. Hollow nanospheres of mesoporous Co9S8 as a high-capacity and long-life anode for advanced lithium ion batteries. Nano Energy 2015, 12, 528–537. [Google Scholar] [CrossRef]
- Su, Q.; Du, G.; Zhang, J.; Zhong, Y.; Xu, B.; Yang, Y.; Li, W. In Situ Transmission Electron Microscopy Observation of Electrochemical Sodiation of Individual Co9S8-Filled Carbon Nanotubes. ACS Nano 2014, 8, 3620–3627. [Google Scholar] [CrossRef]
- Jin, R.; Liu, J.; Xu, Y.; Li, G.; Chen, G. Solvothermal synthesis and excellent electrochemical performance of polycrystalline rose-like Co9S8 hierarchical architectures. J. Mater. Chem. A 2013, 1, 7995. [Google Scholar] [CrossRef]
- Qiu, B.; Zhao, X.; Xia, D. In situ synthesis of CoS2/RGO nanocomposites with enhanced electrode performance for lithium-ion batteries. J. Alloy. Compd. 2013, 579, 372–376. [Google Scholar] [CrossRef]
- Su, Q.; Xie, J.; Zhang, J.; Zhong, Y.; Du, G.; Xu, B. In situ transmission electron microscopy observation of electrochemical behavior of CoS2 in lithium-ion battery. ACS Appl. Mater. Interfaces 2014, 6, 3016–3022. [Google Scholar] [CrossRef]
- Jin, R.; Yang, L.; Li, G.; Chen, G. Hierarchical worm-like CoS2 composed of ultrathin nanosheets as an anode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 10677–10680. [Google Scholar] [CrossRef]
- Zhang, Z.; Gan, Y.; Lai, Y.; Shi, X.; Chen, W.; Li, J. Cobalt sulfides/dodecahedral porous carbon as anode materials for Na-ion batteries. RSC Adv. 2015, 5, 103410–103413. [Google Scholar] [CrossRef]
- Liu, J.; Wu, C.; Xiao, D.; Kopold, P.; Gu, L.; van Aken, P.A.; Maier, J.; Yu, Y. MOF-Derived Hollow Co9S8 Nanoparticles Embedded in Graphitic Carbon Nanocages with Superior Li-Ion Storage. Small 2016, 12, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Zhao, Y.; Dong, Y.; Fan, Q.; Kuang, Q. Synthesis of the Carbon-Coated Nanoparticle Co9S8 and Its Electrochemical Performance as an Anode Material for Sodium-Ion Batteries. Langmuir 2016, 32, 12593–12602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, K.; Lei, K.; Li, F.; Tao, Z.; Chen, J. Facile synthesis and electrochemical sodium storage of CoS2 micro/nano-structures. Nano Res. 2016, 9, 198–206. [Google Scholar] [CrossRef]
- Meng, X.; Deng, D. Trash to Treasure: Waste Eggshells Used as Reactor and Template for Synthesis of Co9S8 Nanorod Arrays on Carbon Fibers for Energy Storage. Chem. Mater. 2016, 28, 3897–3904. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Y.; Chen, T.; Xia, Q.; Yang, M.; Xia, H.; Yu, Y. Cobalt Sulfide Quantum Dot Embedded N/S-Doped Carbon Nanosheets with Superior Reversibility and Rate Capability for Sodium-Ion Batteries. ACS Nano 2017, 11, 12658–12667. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, P.; Fu, F.; Wang, Z.; Zhang, W. Self-assembled CoS2 nanoparticles wrapped by CoS2-quantum-dots-anchored graphene nanosheets as superior-capability anode for lithium-ion batteries. Electrochim. Acta 2015, 182, 424–429. [Google Scholar] [CrossRef]
- Khatib, R.; Dalverny, A.L.; Saubanère, M.; Gaberscek, M.; Doublet, M.L. Origin of the Voltage Hysteresis in the CoP Conversion Material for Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 837–849. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Wang, Z.; Yan, J.; Chen, Y. Progress of Metal-Phosphide Electrodes for Advanced Sodium-Ion Batteries. Funct. Mater. Lett. 2018, 11, 1830001. [Google Scholar] [CrossRef]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.-H.; Li, W.; Kang, Y.-M.; Chen, J. Urchin-Like CoSe2 as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 6728–6735. [Google Scholar] [CrossRef]
- Cho, J.S.; Won, J.M.; Lee, J.-K.; Kang, Y.C. Design and synthesis of multiroom-structured metal compounds–carbon hybrid microspheres as anode materials for rechargeable batteries. Nano Energy 2016, 26, 466–478. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Guan, B.; Lou, X.W. Unusual Formation of CoSe@carbon Nanoboxes, which have an Inhomogeneous Shell, for Efficient Lithium Storage. Angew. Chem. Int. Ed. Engl. 2016, 55, 9514–9518. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.N.; Choi, S.H.; Kang, Y.C. Hollow Cobalt Selenide Microspheres: Synthesis and Application as Anode Materials for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6449–6456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Zhang, J.; Chen, T.; Song, H.; Yang, H.Y. Two dimensional layered Co0.85Se nanosheets as a high-capacity anode for lithium-ion batteries. Nanoscale 2016, 8, 14992–15000. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, G.; Pei, J.; Hu, Y.; Qin, Z.; Wang, J.; Wu, F. Formation of Porous Cu-Doped CoSe2 Connected by Nanoparticles for Efficient Lithium Storage. ChemElectroChem 2017, 4, 2158–2163. [Google Scholar] [CrossRef]

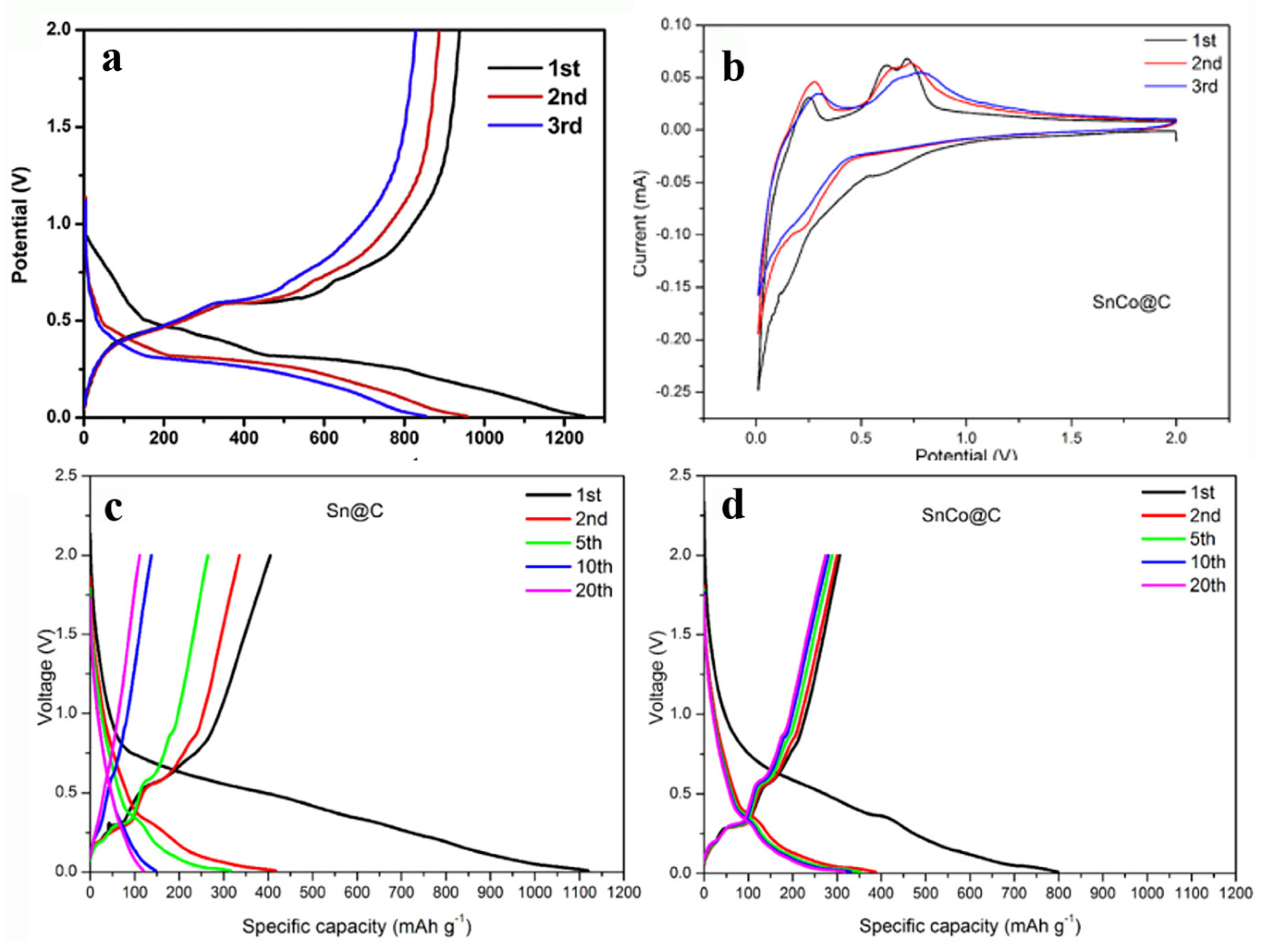
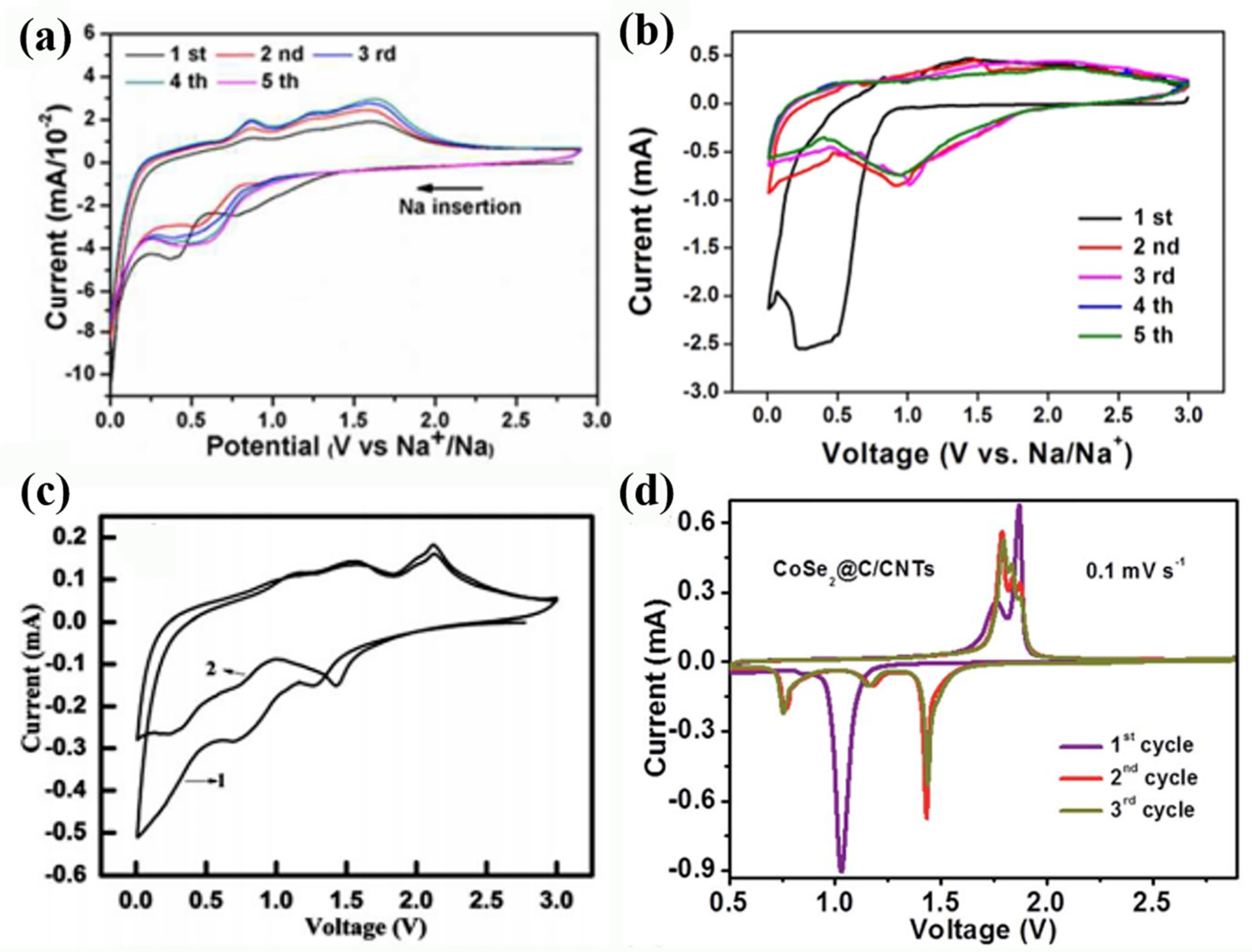
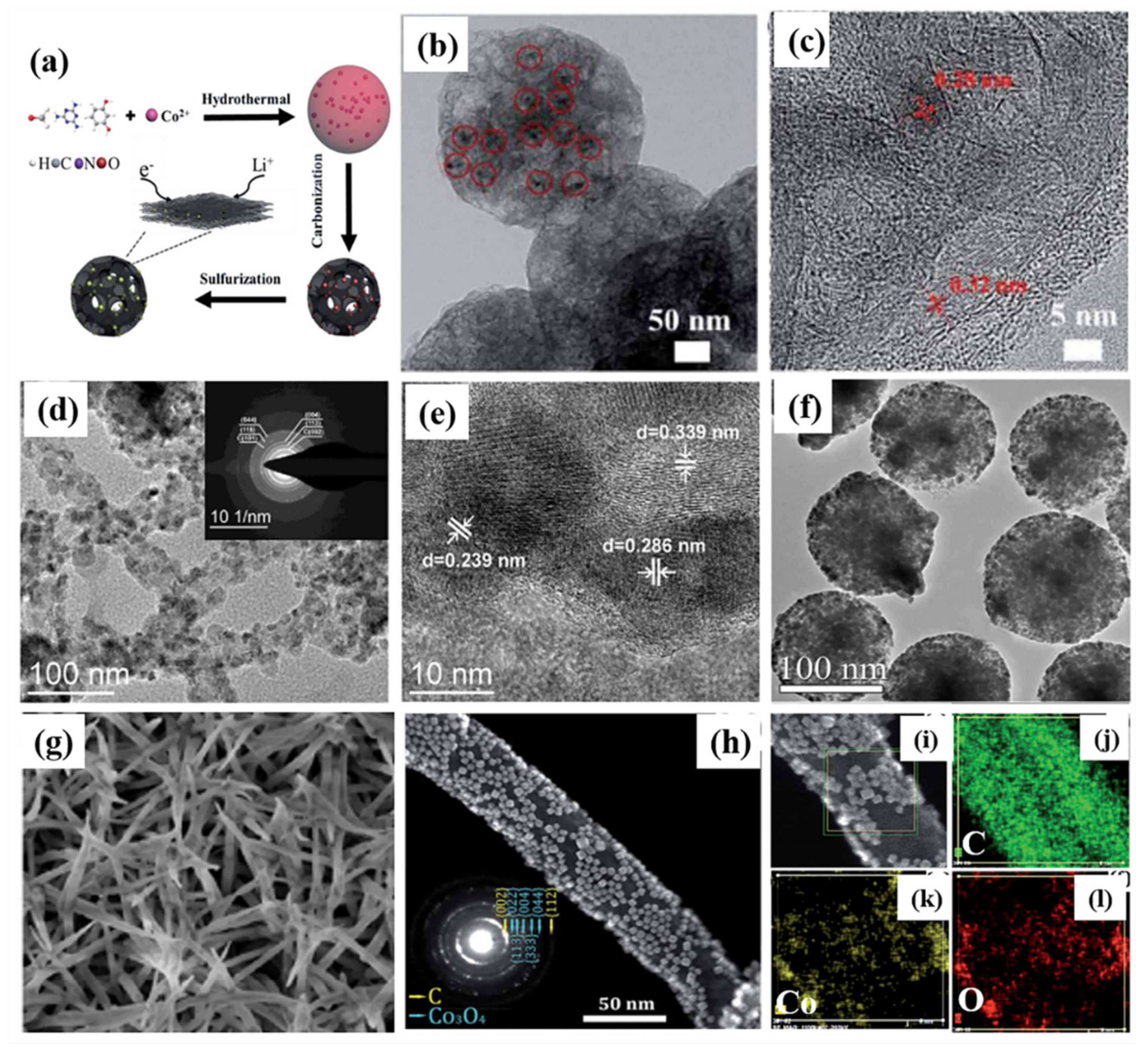
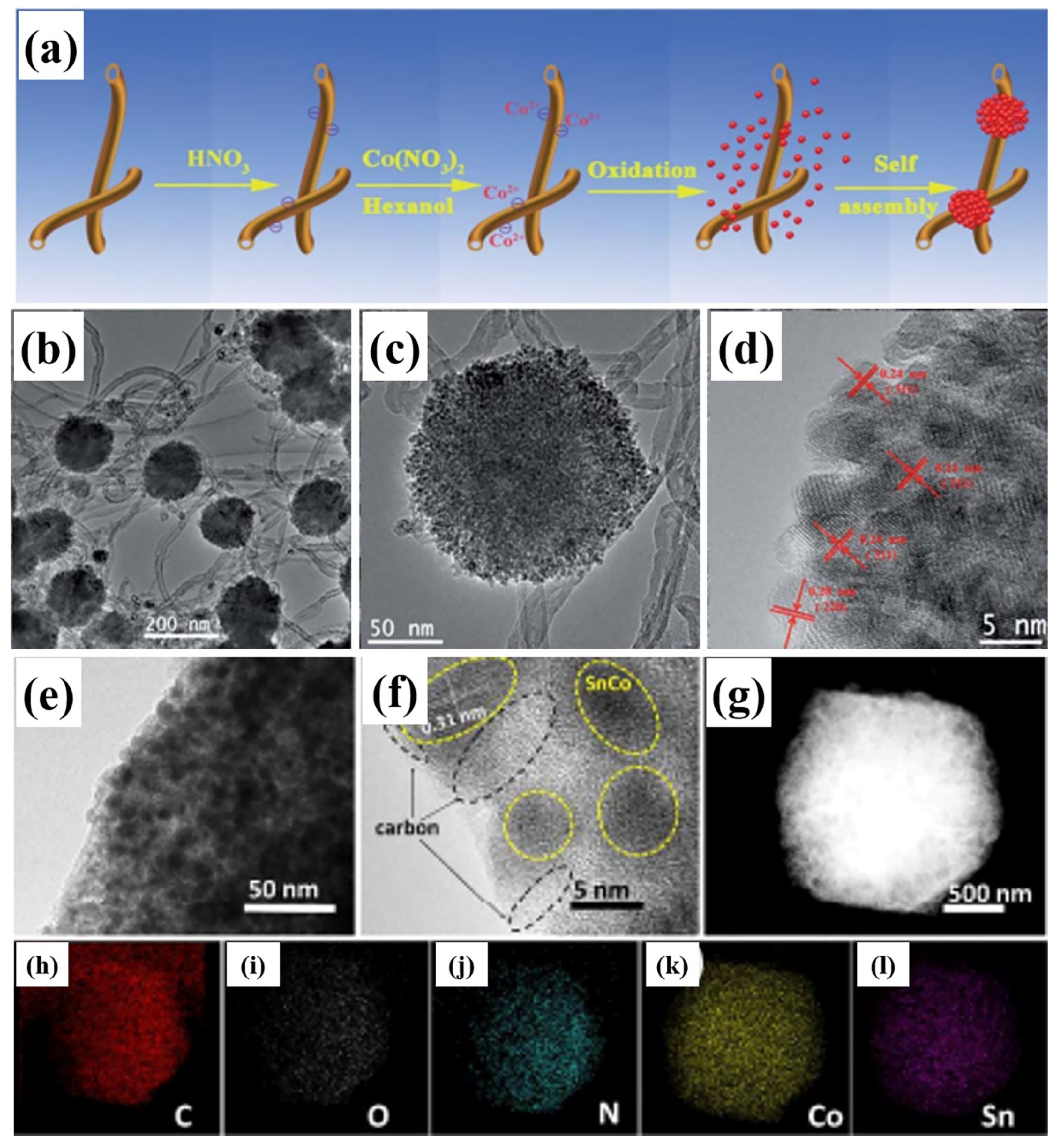
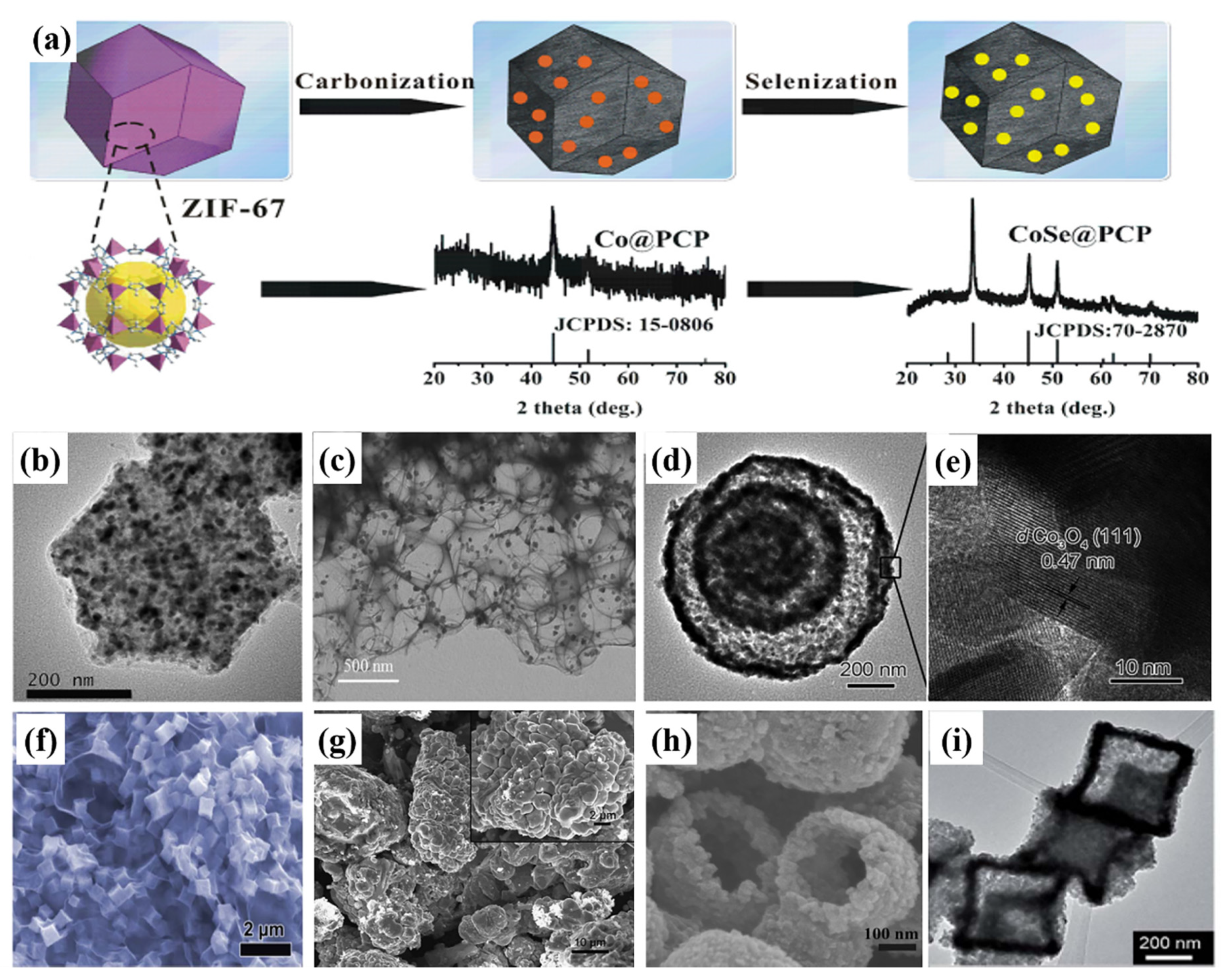

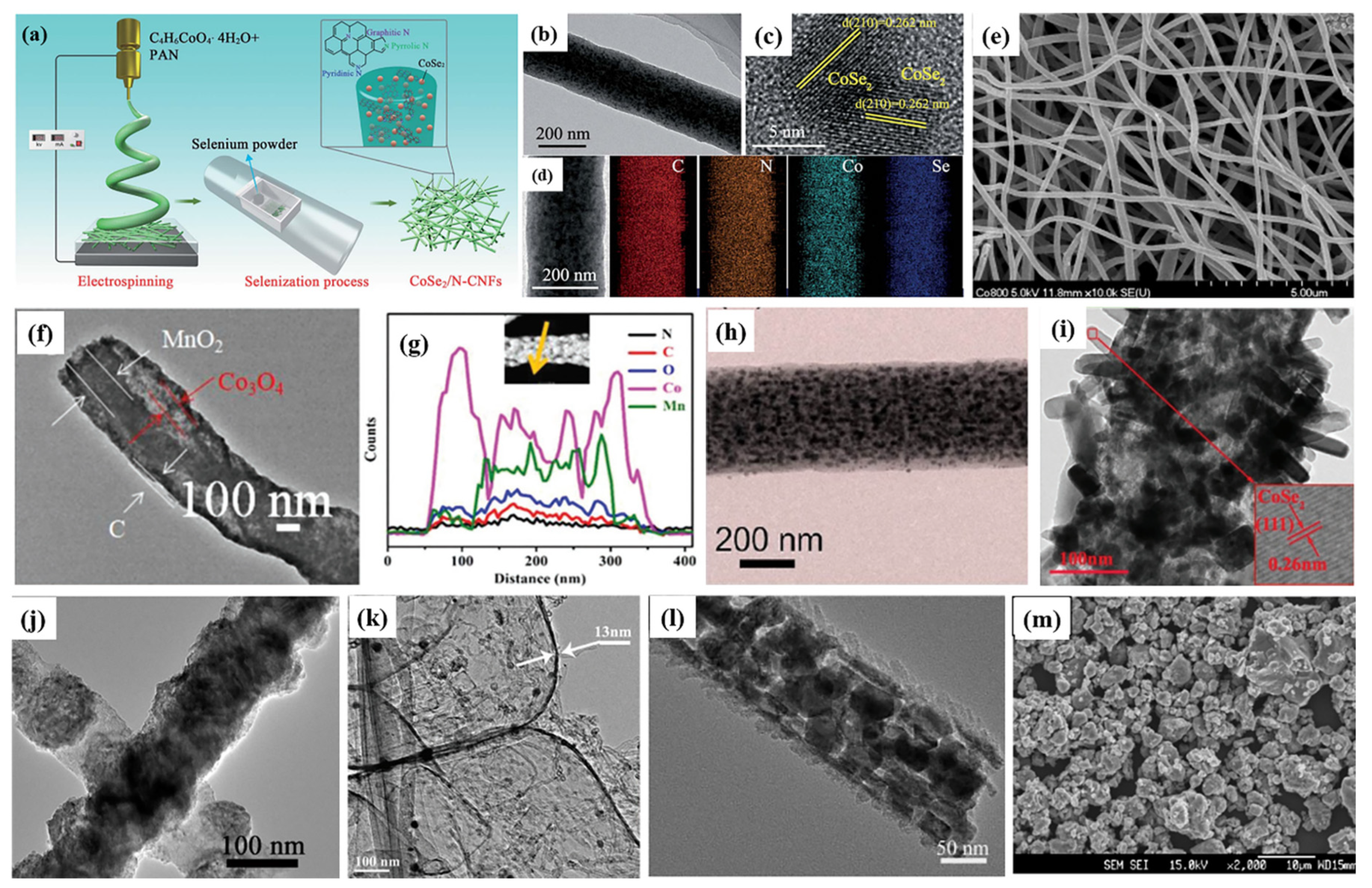
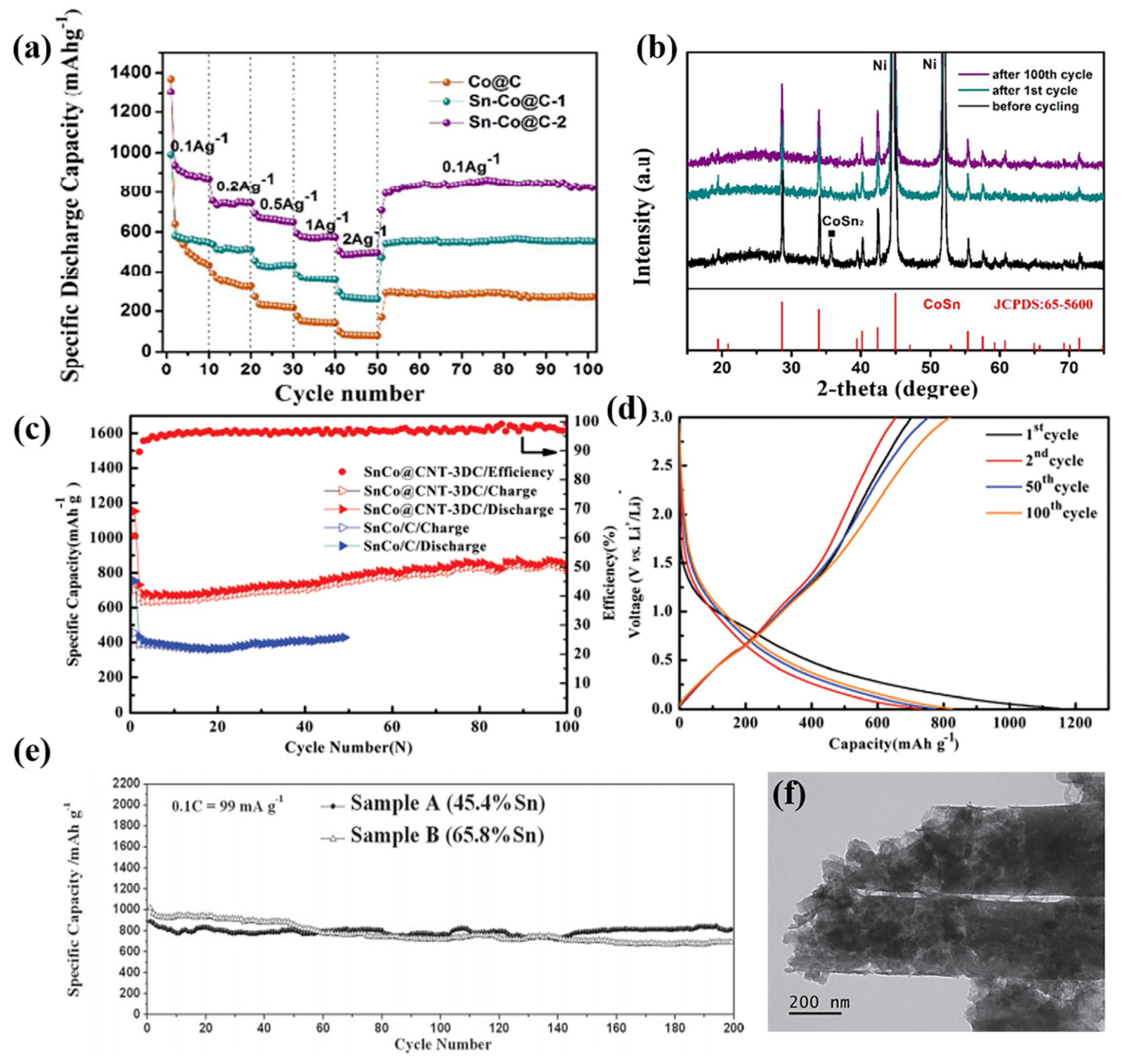

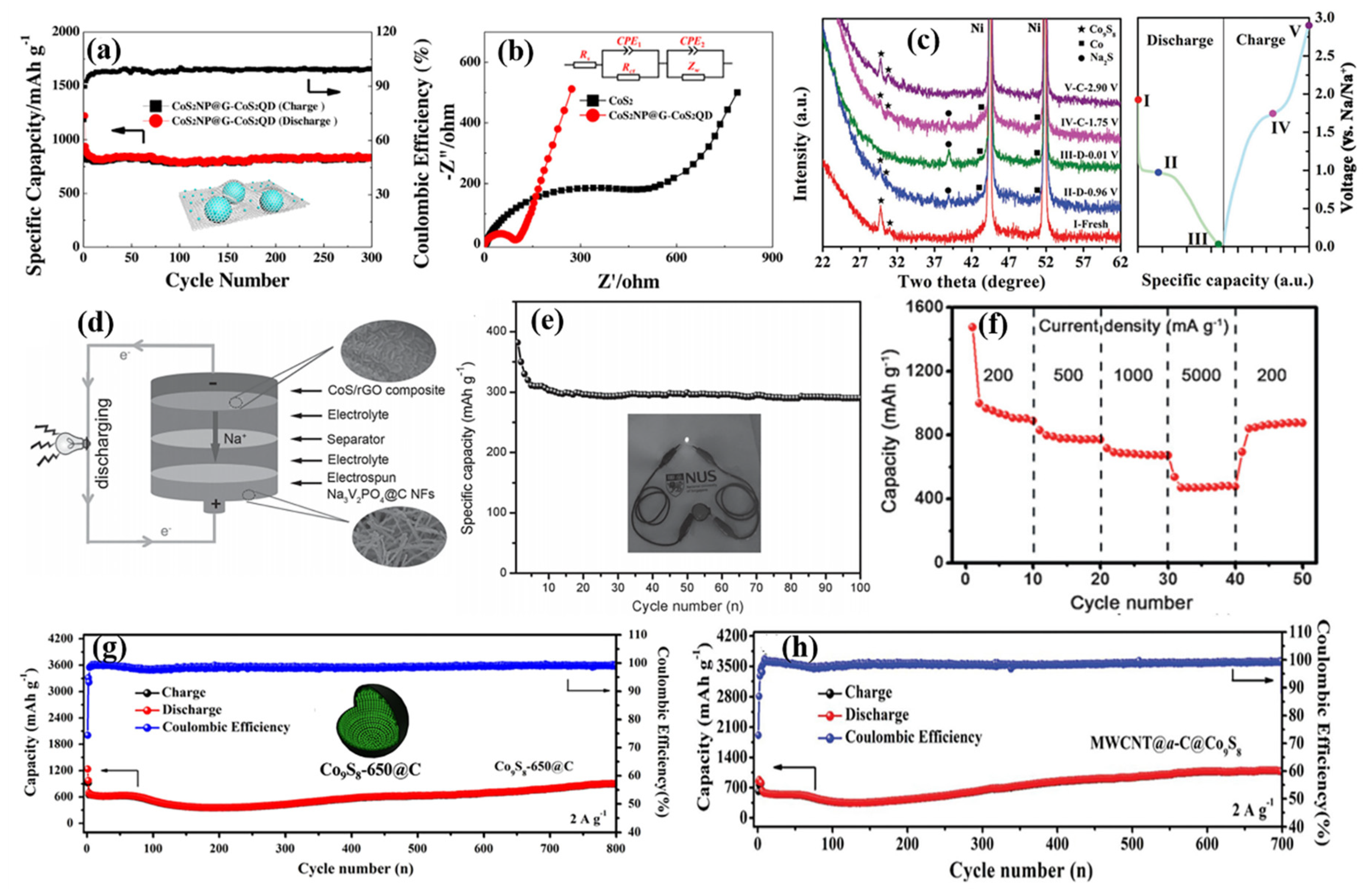
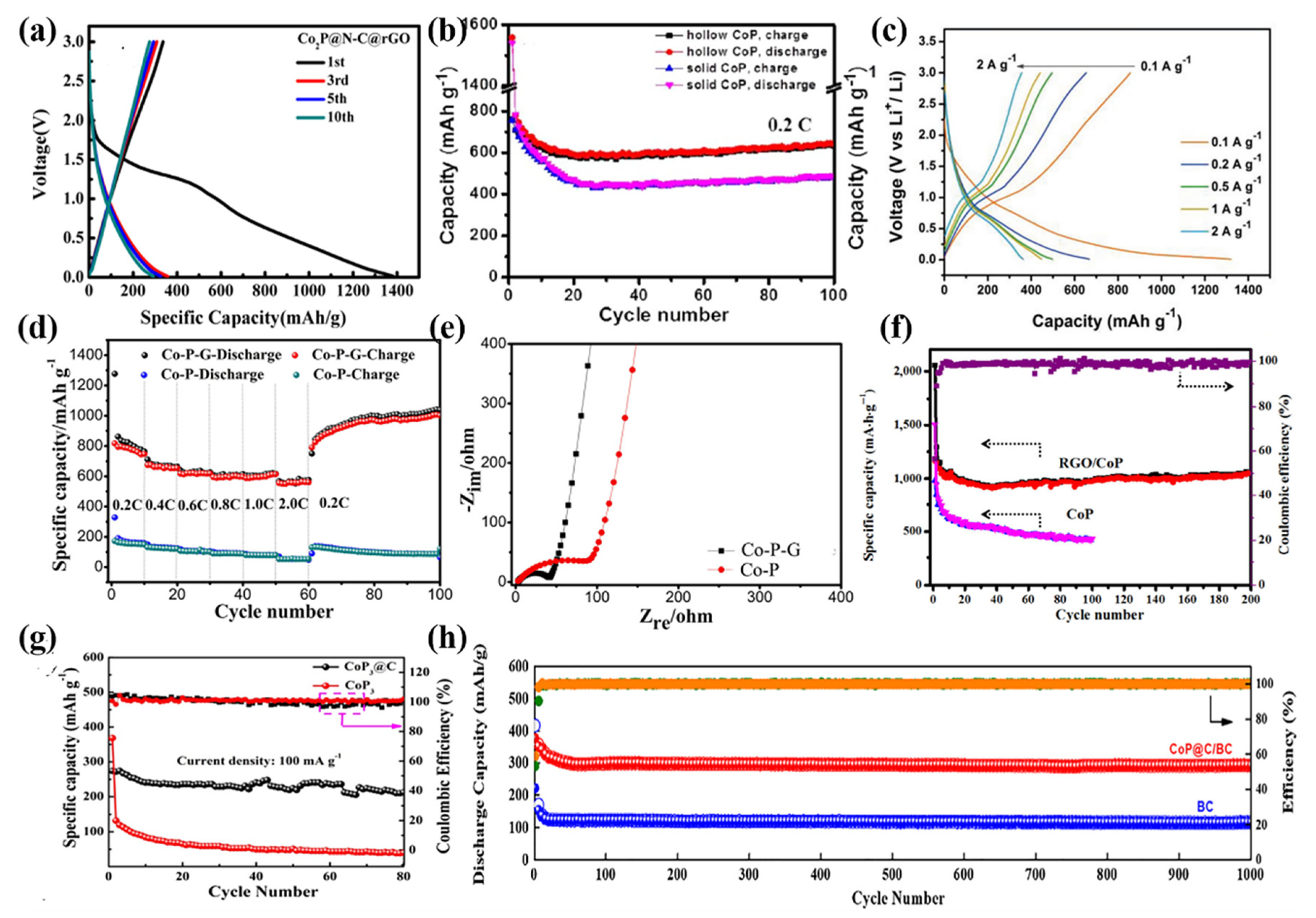


| Active Materials | Li+ Storage Mechanism | Reference |
|---|---|---|
| Co3O4 | [15] | |
| Co2P | [46] | |
| CoS2 | Co | [14] |
| CoSe2 | [20] |
| Types of Materials | Synthetic Method | Co Source | Reference |
|---|---|---|---|
| Co3Sn2@Co-NG | Hydrothermal (250 °C, 24 h) | CoCl2•6H2O | [53] |
| Special CoSn2/Sn alloy | Heat treatment (800 °C, Ar) | Co3O4 | [22] |
| Sn-Co-C ternary alloy | HEMM | Co powder | [34] |
| Sn36Co41C23 alloy | Magnetron sputtering | Co powder | [19] |
| Sn40Co40C20 | HEMM | Co powder | [45] |
| Sn-Co-C composite | HEMM | Co(C5H7O2)3 | [42] |
| Sn-Co alloy | Electrodepositing | CoCl2•6H2O | [43] |
| Sn-Co/CNTs | Galvanic replacement | CoCl2•6H2O | [102] |
| Sn-Co-C composite | HEMM | CoO | [83] |
| Nano-sized Co-Sn alloy | Hydrothermal (160 °C,48 h) | CoCl2•6H2O | [55] |
| Sn30Co30C40 alloy | Ball-milling | Co powder | [81] |
| CoSn3 | Hydrothermal (195 °C, 1.5 h) | CoCl2•6H2O | [56] |
| Sn-Co alloy | Electrodepositing | CoCl2•6H2O | [38] |
| CoSn2 | Electrodepositing | Co electrode | [89] |
| Nanostructure Sn-Co-C composites | Galvanic replacement | Co(CH3COO)2•4H2O | [103] |
| porous Sn-Co alloy | Electrodepositing | CoCl2•6H2O | [86] |
| CoSn3-MWCNTs | Galvanic replacement | CoCl2•6H2O | [104] |
| Sn54Sb41Co5 alloy | Electrodepositing | CoCl2•6H2O | [85] |
| CoSn5 nanospheres | Galvanic replacement | CoCl2•6H2O | [105] |
| Grapheme wrapped-SnCo nanoparticles | Galvanic replacement | CoCl2•6H2O | [106] |
| CoSn2 alloy | Electrodepositing | CoCl2•6H2O | [84] |
| Sn-Co-CNT@CNT | Galvanic replacement | CoCl2•6H2O | [67] |
| Co-Sn/C nanofiber | Electrodepositing | C4H6CoO4 | [90] |
| SnCo/C nanofibers | Electrospinning | C4H6CoO4 | [92] |
| Sn-Co@PMMA nanospheres | Galvanic replacement | C4H6CoO4 | [40] |
| Amorphous Co3Sn2 | Hydrothermal (180 °C, 24 h) | CoCl2•6H2O | [107] |
| SnCo@CNT-3DC | CVD | CoCl2•6H2O | [44] |
| Sn-Co@C | Galvanic replacement | Co(NO3)2 •6H2O | [64] |
| carbon encapsulated Sn-Co alloy | Heat treatment (800 °C, Ar/H2) | Co3O4 | [41] |
| NiCo2O4 powders | Heat treatment (320 °C, air) | CoC2O4 | [72] |
| Co2MnSi | Arc-melting | Co powder | [96] |
| Sb-Co-P | Electroplating | CoCl2•6H2O | [50] |
| CoSnC alloy | Galvanic replacement | CoSO4•6H2O | [108] |
| Co2SnO4 HC@rGO | Heat treatment (900 °C, Ar) | CoCl2•6H2O | [39] |
| CoSnO3/GN/CNTs | Galvanic replacement | CoCl2•6H2O | [109] |
| CoMn2O4 | Hydrothermal (180 °C,10 h) | Co(CH3COO)2 •4H2O | [51] |
| CoSnO3 | Galvanic replacement | CoCl2•6H2O | [52] |
| Co3O4 | Heat treatment (800 °C, Air) | CoCO3 | [15] |
| Co3O4 nanotubes | Heat treatment (500 °C, Oxygen) | Co4(CO)12 | [73] |
| Co3O4 thin films | Electrodepositing | Co(NO3)2 | [88] |
| porous Co3O4 thin films | Electrodepositing | Co(NO3)2 | [87] |
| Co3O4 microspheres | Hydrothermal (200 °C,3 h) | Co(NO3)2•6H2O | [21] |
| needlelike Co3O4 nanotubes | Galvanic replacement | Co(NO3)2•6H2O | [65] |
| Co3O4 nanosheets | Hydrothermal (140 °C,20 h) | CoCl2•6H2O | [54] |
| agglomerated Co3O4 | Heat treatment (600 °C, Air) | Co3(NDC)3(DMF)4 | [74] |
| macroporous Co3O4 platelets | Heat treatment (450 °C, Air) | CoCl2•6H2O | [75] |
| Co3O4/graphene | Galvanic replacement | (C2H3O2)2Co •4H2O | [110] |
| Co3O4/graphene | Galvanic replacement | CoCl2•6H2O | [111] |
| Co3O4 porous nanocages | Heat treatment (400 °C, Air) | (C2H3O2)2Co •4H2O | [76] |
| single-crystalline Co3O4 nanobelts | Hydrothermal (120 °C, 24 h) | Co(NO3)2•6H2O | [57] |
| C@Co3O4 | Hydrothermal (180 °C, 8 h) | Co(NO3)2•6H2O | [112] |
| Co3O4 nanorods/graphene nanosheets | Hydrothermal (120 °C, 12 h) | CoSO4•7H2O | [113] |
| mesoporous Co3O4 | Galvanic replacement | Co(NO3)2•6H2O | [114] |
| Co3O4 nanocages | Heat treatment (550 °C, Air) | (C2H3O2)2Co•nH2O | [77] |
| graphene/Co3O4 | Hydrothermal (80 °C, 4 h) | CoCl2•6H2O | [115] |
| CoO/graphene | Heat treatment (350 °C, Ar/H2) | Co(NO3)2•6H2O | [78] |
| Co3O4/graphene | Galvanic replacement | (C2H3O2)2Co•4H2O | [116] |
| multi-shelled Co3O4 hollow microspheres | Heat treatment (500 °C, Air) | Co(Ac)2•4H2O | [69] |
| shale-like Co3O4 | Heat treatment (400 °C, Air) | (C2H3O2)2Co•4H2O | [35] |
| hierarchical Co3O4/CNTs | Galvanic replacement | Co(NO3)2•6H2O | [63] |
| bowl-like hollow Co3O4 microspheres | Heat treatment (400 °C, Air) | (C2H3O2)2Co•4H2O | [117] |
| mesoporous Co3O4 nanoflakes | Heat treatment (250 °C, Air) | CoCO3 | [118] |
| hollow structured Co3O4 nanoparticles | Heat treatment (400 °C, Air) | CoCl2•6H2O | [119] |
| Co3O4/MCNTs | Heat treatment (500 °C, Air) | CoCO3 | [120] |
| peapod-like Co3O4@carbon nanotube | Heat treatment (450 °C, Ar) | Co(NO3)2•6H2O | [121] |
| Co3O4/CNT nanocomposites | Heat treatment (300 °C, Air) | CoCl2•6H2O | [122] |
| layer-by-layer Co3O4/graphene | Hydrothermal (170 °C, 15 h) | (C2H3O2)2Co•4H2O | [123] |
| Co3O4/CNTs nanotubes | Hydrothermal (120 °C, 2 h) | (C2H3O2)2Co•4H2O | [62] |
| Co/Co3O4 nanoparticles | Electrospinning | CoCl2•6H2O | [94] |
| Co3O4@NC | Heat treatment (550 °C, Ar) | ZIF-67 | [124] |
| carbon doped Co3O4 hollow nanofibers | Hydrothermal (180 °C, 12 h) | Co(NO3)2•6H2O | [125] |
| Ni-doped Co/CoO/NC hybrid | Heat treatment (500 °C, Ar) | Co(NO3)2•6H2O | [126] |
| hollow Co3O4/NGC | Heat treatment | Co(NO3)2•6H2O | [127] |
| starfish-like Co3O4@nitrogen-doped carbon | Heat treatment | Co(NO3)2•6H2O | [128] |
| yolk-shell Co3O4/C dodecahedrons | Heat treatment (350 °C, Air) | Co(NO3)2•6H2O | [129] |
| hexagonal Co3O4 nanosheets | Hydrothermal (120 °C, 10 h) | Co(NO3)2•6H2O | [130] |
| ultrathin mesoporous Co3O4 nanosheet | Heat treatment (450 °C, Air) | Co(NO3)2•6H2O | [131] |
| flower-like Co3O4/C nanosheets | Heat treatment (500 °C, Air) | Co(NO3)2•6H2O | [132] |
| Co3O4/GF | Heat treatment (300 °C, Air) | Co(NO3)2•6H2O | [133] |
| ES-CNCo3O4 fibers | Heat treatment (800 °C, Ar/H2) | Co(NO3)2•6H2O | [11] |
| Co3O4/MnO2@C | Electrospinning | (C2H3O2)2Co•4H2O | [91] |
| CoP3 | Electrodeposition | CoO | [18] |
| CoPx | Ball milling | Co powder | [82] |
| Co2P | Electrodeposition | CoCl2•6H2O | [46] |
| CoxP | Heat treatment (320 °C, Ar) | (C2H3O2)2Co•4H2O | [79] |
| CoxP | HEMM | Co powder | [134] |
| CoP/RGO | Hydrothermal (180 °C, 16 h) | CoCl2•6H2O | [135] |
| CoP microflake | Heat treatment (350 °C, Ar) | Co(NO3)2•6H2O | [136] |
| CoP nanorod | Hydrothermal (100 °C, 12 h) | Co(NO3)2•6H2O | [61] |
| honeycomb-like CoP/Co2P | Heat treatment (600 °C, Ar) | CoCl2•6H2O | [137] |
| core-shell CoP/FeP porous microcubes | Heat treatment (300 °C, Ar) | (C2H3O2)2Co•4H2O | [70] |
| mesoporous CoP nanorods | Hydrothermal (120 °C, 6 h) | Co(NO3)2•6H2O | [138] |
| Co2P-Co/graphene | Galvanic replacement | (C2H3O2)2Co•4H2O | [139] |
| A-Co2P/CxNyBz-650 | Heat treatment (650 °C, Ar) | Co(NO3)2•6H2O | [140] |
| CoP/CNS | Hydrothermal (120 °C, 6 h) | Co(NO3)2•6H2O | [13] |
| CoP@NPPCS | Heat treatment (900 °C, Ar) | (C2H3O2)2Co•4H2O | [141] |
| CoP hollow nanorods/graphene | Hydrothermal (140 °C, 10 h) | CoCl2•6H2O | [25] |
| carbon-encapsulated CoP nanoparticles | Heat treatment (850 °C, Ar) | Co(NO3)2•6H2O | [142] |
| Co2P@N-C@rGO | Heat treatment (900 °C, Ar) | Co(NO3)2•6H2O | [143] |
| CoP3@Ppy microcubes | Coprecipitation& Parkerizing | (C2H3O2)2Co•4H2O | [144] |
| carbon coated CoP3 | HEMM | Co powder | [80] |
| Co9S8@C nanoparticles | Hydrothermal & heat treatment | Co(NO3)2•6H2O | [23] |
| 3D spongy CoS2 nanoparticles/carbon | Freeze-dry &heat treatment &hydrothermal | Co(NO3)2•6H2O | [12] |
| hollow Co9S8@C | Heat treatment & sulfuration | (C2H3O2)2Co•4H2O | [145] |
| CoS@S-doped OLC | Hydrothermal & heat treatment | Co(NO3)2•6H2O | [146] |
| SnS2@CoS2-rGO | Hydrothermal (180 °C, 24 h) | CoCl2•6H2O | [147] |
| CoS2@NCH | Solvothermal & heat treatment | Co(NO3)2•6H2O | [148] |
| CoS2/C micropolyhedron | Heat treatment (900 °C, N2) | Co(NO3)2•6H2O | [33] |
| CoS-24 | Solvothermal (180 °C 24 h) | CoCl2•6H2O | [149] |
| CNT@NC@CuCo2S4 | Solvothermal (200 °C 12 h) | (C2H3O2)2Co•4H2O | [59] |
| Co9S8-QDs@NC | Heat treatment (750 °C, Ar) | CoCl2•6H2O | [30] |
| TiO2 nanobelts@Co9S8 | Hydrothermal & heat treatment | (C2H3O2)2Co•4H2O | [150] |
| 7-CoS/C | Heat treatment & sulfur | Co(NO3)2•6H2O | [151] |
| MWCNTs/Co9S8 composites | Solvothermal & heat treatment | Co(NO3)2•6H2O | [152] |
| Co9S8/Co | Ball-milling | Co powder | [153] |
| Co9S8@CNNs | Freeze-drying & heat treatment | CoCl2•6H2O | [154] |
| CoS2/NCNTF | Heat treatment & sulfur | Co(NO3)2•6H2O | [155] |
| Co9S8/N-C hollow nanospheres | Heat treatment & sulfur | CoSO4•7H2O | [28] |
| Co9S8/RGO | Hydrothermal (180 °C, 12 h) | (C2H3O2)2Co•4H2O | [97] |
| CoS2/G composite | Hydrothermal (200 °C, 12 h) | (C2H3O2)2Co•4H2O | [156] |
| CoS2@MCNF | Hydrothermal & heat treatment | CoCl2•6H2O | [157] |
| Ni3S2/Co9S8/N-doped carbon composite | Hydrothermal & heat treatment | Co(NO3)2•6H2O | [158] |
| CoSe/Co@NC | Heat treatment (800 °C, Ar) | Co(NO3)2•6H2O | [159] |
| Co0.85Se NSs/G | Hydrothermal (180 °C, 16 h) | (C2H3O2)2Co•4H2O | [160] |
| Co-Zn-Se@C | Hydrothermal & heat treatment | Co(NO3)2•6H2O | [161] |
| CoSe2@NC-NR/CNT | Selenization | Co(NO3)2•6H2O | [162] |
| cobblestone-like CoSe2@C nanospheres | Heat treatment (500 °C, Ar/H2) | Co(NO3)2•6H2O | [32] |
| Cu-doped CoSe2 microboxes | Hydrothermal (160 °C, 8 h) | (C2H3O2)2Co•4H2O | [163] |
| CoSe2/N-CNFs | Electrospinning & heat treatment | (C2H3O2)2Co•4H2O | [24] |
| CoSe2/C-ND@RGO | Heat treatment (600 °C, Air) | Co(NO3)2•6H2O | [164] |
| CoSe@CSs | Hydrothermal & heat treatment | Co(NO3)2•6H2O | [60] |
| CoSe quasi-microspheres | Hydrothermal (180 °C, 2 h) | Co(NO3)2•6H2O | [165] |
| yolk-shell structured CoSe/C | Heat treatment (800 °C, Ar) | Co(NO3)2•6H2O | [166] |
| Co9Se8/RGO hybrid nanosheet | Hydrothermal (180 °C, 12 h) | (C2H3O2)2Co•4H2O | [97] |
| urchin-like CoSe2 nanorods | Electrospinning & heat treatment | (C2H3O2)2Co•4H2O | [93] |
| CoSe2@C/CNTs | Heat treatment & selenization | Co(NO3)2•6H2O | [48] |
| CoSe2@N-PGC/CNTs | Heat treatment & selenization | Co(NO3)2•6H2O | [167] |
| Co/(NiCo)Se2 box in box structure | Selenization (270 °C 6 h) | Co(NO3)2•6H2O | [71] |
| CoSe2 powders | Hydrothermal (180 °C, 18 h) | Co(NO3)2•6H2O | [168] |
| CoSe2 nanoparticles | Hydrothermal (180 °C, 24 h) | CoCl2•6H2O | [169] |
| CoSe2 microspheres | Heat treatment | Co(NO3)2•6H2O | [170] |
| CoSe@PCP | Heat treatment & selenization | Co(NO3)2•6H2O | [68] |
| Types of Materials | Current Density (A g−1) | Cut-off Voltage (V) | Cycle Number | Specific Capacity (mA h g−1) | Reference |
|---|---|---|---|---|---|
| For LIBs | |||||
| Sn-Co@C-2 | 0.1 | 0.01–3 | 100 | 818 | [64] |
| SnCo@CNT-3DC | 0.1 | 0.005–3 | 100 | 826 | [44] |
| Sn-Co@PMMA | 0.1 | 0.001–2 | 100 | 594 | [40] |
| Co3Sn2@Co-NG | 0.25 | 0.005–3 | 100 | 1615 | [53] |
| SnCo/PAN-CNFs | 0.267 | 0.005–2.5 | 100 | 548 | [92] |
| Co-Sn/CNF-800 | 0.161 | 0.02–2.8 | 80 | 560 | [90] |
| Sn-Co-CNT@CNT | 0.099 | 0.005–3 | 200 | 811 | [67] |
| GNS-SnCo | 0.072 | 0.005–3 | 60 | 571 | [106] |
| Sb-Co-P | 0.1 | 0.02–1.5 | 50 | 539 | [50] |
| CoSnC | 0.1 | 0–2 | 50 | 450 | [108] |
| Co2SnO4 | 0.1 | 0.01–2.5 | 100 | 1000 | [39] |
| CoSnO3/GN/CNTs | 0.1 | 0–3 | 150 | 1098.7 | [109] |
| CoMn2O4 | 0.1 | 0.001–3 | 500 | 772 | [51] |
| CoOx/MCS | 0.07 | 0.01–3 | 30 | 703 | [100] |
| Co3O4/Graphene | 0.2 | 0.001–3 | 42 | 800 | [110] |
| Co3O4 nanobelts | 1 | 0–3 | 60 | 614 | [57] |
| Co3O4 nanorods/GNS | 1 | 0.01–3 | 40 | 1090 | [113] |
| Shale-like Co3O4 | 0.2 | 0.005–2.9 | 100 | 1045.3 | [35] |
| Co3O4 nanoflakes | 0.089 | 0.01–3 | 300 | 806 | [118] |
| Peapod-like Co3O4@CNT | 0.1 | 0–3 | 60 | 318 | [121] |
| CoP/C | 0.1 | 0–2 | 200 | 407 | [134] |
| CoP microflake | 1 | 0.01–3 | 800 | 619.2 | [136] |
| CoP/Co2P | 0.2 | 0.01–3 | 450 | 851.2 | [137] |
| CoP nanorods | 0.5 | 0.01–3 | 300 | 894 | [138] |
| Co2P-Co | 0.1 | 0.01–3 | 200 | 929 | [139] |
| CoP nanorod arrays | 0.4 | 0.01–3 | 900 | 390 | [61] |
| CoP HR@rGO | 0.1 | 0.005–3 | 100 | 714.7 | [25] |
| CoP@C/BC | 1 | 0.01–3 | 1000 | 351 | [142] |
| CoP3@PPy | 0.5 | 0–2 | 220 | 650 | [144] |
| CoS2NP@G-CoS2QD | 1 | 0.05–3 | 300 | 831 | [187] |
| Worm-like CoS2 | 0.1 | 0.01–3 | 100 | 883 | [180] |
| CoS2@NG | 0.1 | 0.01–3 | 150 | 882 | [26] |
| NC/CoS2-650 | 0.1 | 0.1–3 | 50 | 560 | [66] |
| Mesoporous Co9S8 | 2 | 0.01–3 | 800 | 896 | [175] |
| MWCNT@a-C@Co9S8 | 2 | 0.01–3 | 700 | 1065 | [5] |
| CoS2 nanobubble | 1 | 0.05–3 | 200 | 737 | [174] |
| rGO/CoSe2 | 0.2 | 0.01–3 | 200 | 1577 | [20] |
| Co0.85Se nanosheets | 0.2 | 0.01–3 | 50 | 516 | [194] |
| Cu-doped CoSe2 | 1 | 0.01–3 | 200 | 807 | [195] |
| CoSe@PCP | 1 | 0.005–3 | 500 | 708.2 | [68] |
| CoSe2@C/CNTs | 1 | 0.5–2.9 | 1000 | 390 | [48] |
| CoSe2@CNFs | 0.2 | 0.01–3 | 300 | 1405 | [93] |
| For SIBs | |||||
| SnCo@C | 0.1 | 0.1–2 | 120 | 276.2 | [41] |
| CoSnO3-NCs | 1 | 0.01–3 | 1000 | 273.8 | [52] |
| Shale-like Co3O4 | 0.05 | 0.005–2.9 | 50 | 380 | [35] |
| Co3O4@CNT | 0.05 | 0.01–3 | 100 | 403 | [122] |
| Co3O4@NC | 1 | 0.01–3 | 1100 | 175 | [124] |
| Ni-doped Co/CoO/NC | 0.5 | 0.01–3 | 100 | 218.7 | [126] |
| Yolk-shell Co3O4/C | 1 | 0.01–3 | 200 | 240 | [129] |
| Co3O4/MnO2@C | 0.8 | 0.01–3 | 1000 | 126 | [91] |
| CoP@C-RGO-NF | 0.05 | 0.01–3 | 100 | 473.1 | [27] |
| RGO@CoP@C-FeP | 0.1 | 0.01–3 | 200 | 456.2 | [70] |
| A-Co2P/CxNyB2-650 | 0.2 | 0.005–2.5 | 100 | 251.2 | [140] |
| CoP nanorod arrays | 0.2 | 0.01–3 | 550 | 297 | [61] |
| CoP-O | 1 | 0.01–2.5 | 900 | 386 | [13] |
| Co2P@N-C@rGO | 0.05 | 0.01–3 | 100 | 225 | [143] |
| CoP3@C | 0.1 | 0–2.5 | 80 | 212 | [80] |
| CoS2-MWCNT | 0.1 | 1–2.9 | 100 | 568 | [47] |
| cs-CoxSy/DPC | 0.5 | 0.01–3 | 50 | 300 | [181] |
| CoS2/rGO | 1 | 0.01–3 | 1000 | 192 | [49] |
| CoS@rGO | 1 | 0.1–2.9 | 1000 | 420 | [173] |
| (Co9S8 QD@HCP)@rGO | 0.3 | 0.01–2.9 | 500 | 628 | [172] |
| CoS@rGO | 1 | 0.01–2.9 | 1000 | 420 | [173] |
| CoSx@NSC | 1 | 0.01–3 | 200 | 606 | [186] |
| Co9S8@C | 5 | 0.01–3 | 1000 | 305 | [23] |
| Urchin-like CoSe2 | 1 | 0.5–3 | 1800 | 410 | [190] |
| CoSe@PCP | 0.1 | 0.005–3 | 100 | 341 | [68] |
| CoSe2 nanorods | 5 | 0.4–3 | 2000 | 386 | [168] |
| CoSe2@N-PGC/CNTs | 0.2 | 0.001–3 | 100 | 424 | [48] |
| Co9Se8/rGO | 0.05 | 0.01–3 | 100 | 406 | [97] |
| CoSe/C | 0.5 | 0.01–3 | 50 | 552.5 | [166] |
| CoSe@100CSs | 4 | 0.5–2.8 | 10,000 | 260 | [60] |
| CoSe2/N-CNF | 2 | 0.5–3 | 1000 | 308.4 | [24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, N.; Bai, Z. The Progress of Cobalt-Based Anode Materials for Lithium Ion Batteries and Sodium Ion Batteries. Appl. Sci. 2020, 10, 3098. https://doi.org/10.3390/app10093098
Zhang Y, Wang N, Bai Z. The Progress of Cobalt-Based Anode Materials for Lithium Ion Batteries and Sodium Ion Batteries. Applied Sciences. 2020; 10(9):3098. https://doi.org/10.3390/app10093098
Chicago/Turabian StyleZhang, Yaohui, Nana Wang, and Zhongchao Bai. 2020. "The Progress of Cobalt-Based Anode Materials for Lithium Ion Batteries and Sodium Ion Batteries" Applied Sciences 10, no. 9: 3098. https://doi.org/10.3390/app10093098
APA StyleZhang, Y., Wang, N., & Bai, Z. (2020). The Progress of Cobalt-Based Anode Materials for Lithium Ion Batteries and Sodium Ion Batteries. Applied Sciences, 10(9), 3098. https://doi.org/10.3390/app10093098




