Elemental Mercury Removal over CeO2/TiO2 Catalyst Prepared by Sol–Gel Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Performance Test and Characterization
3. Results and Discussion
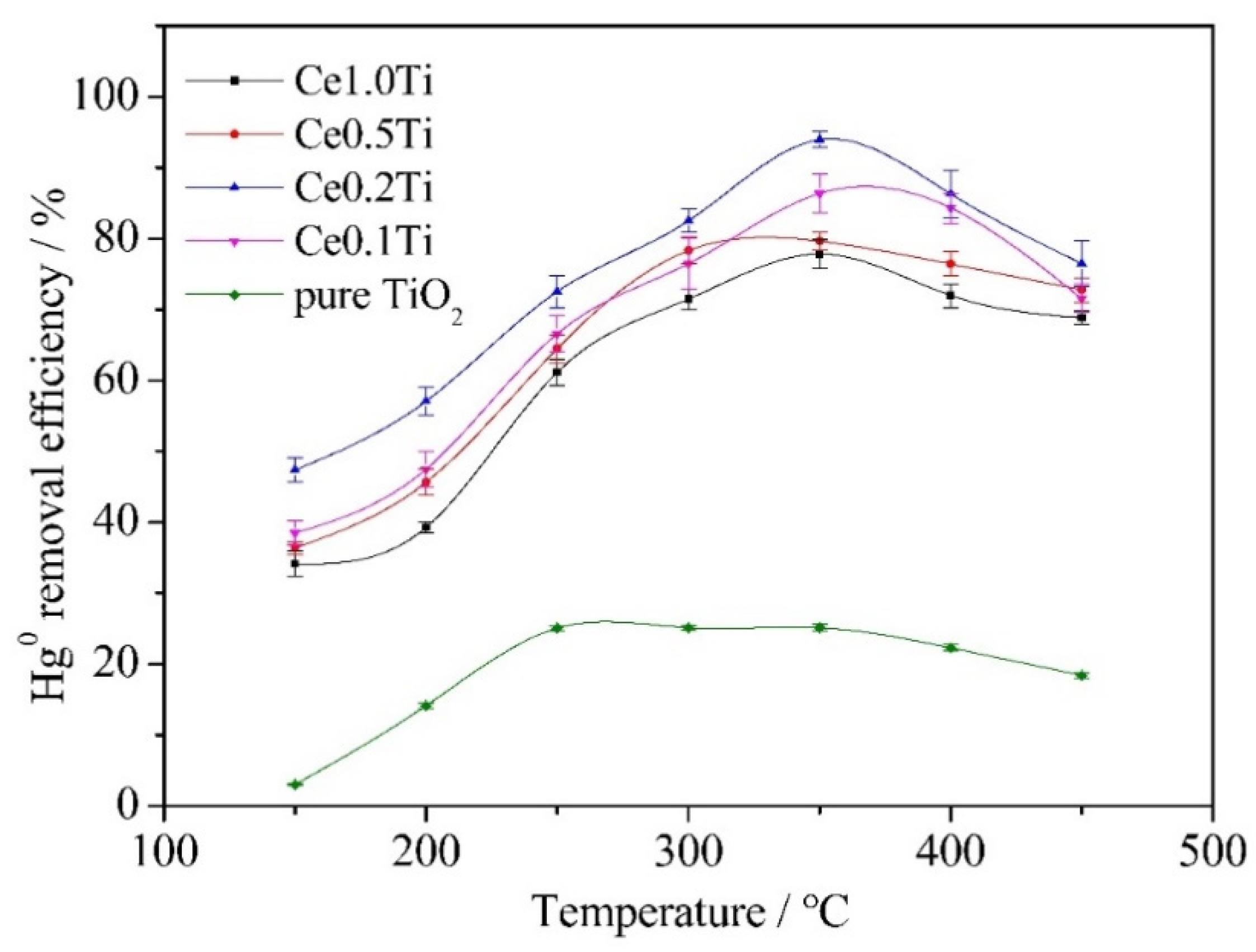
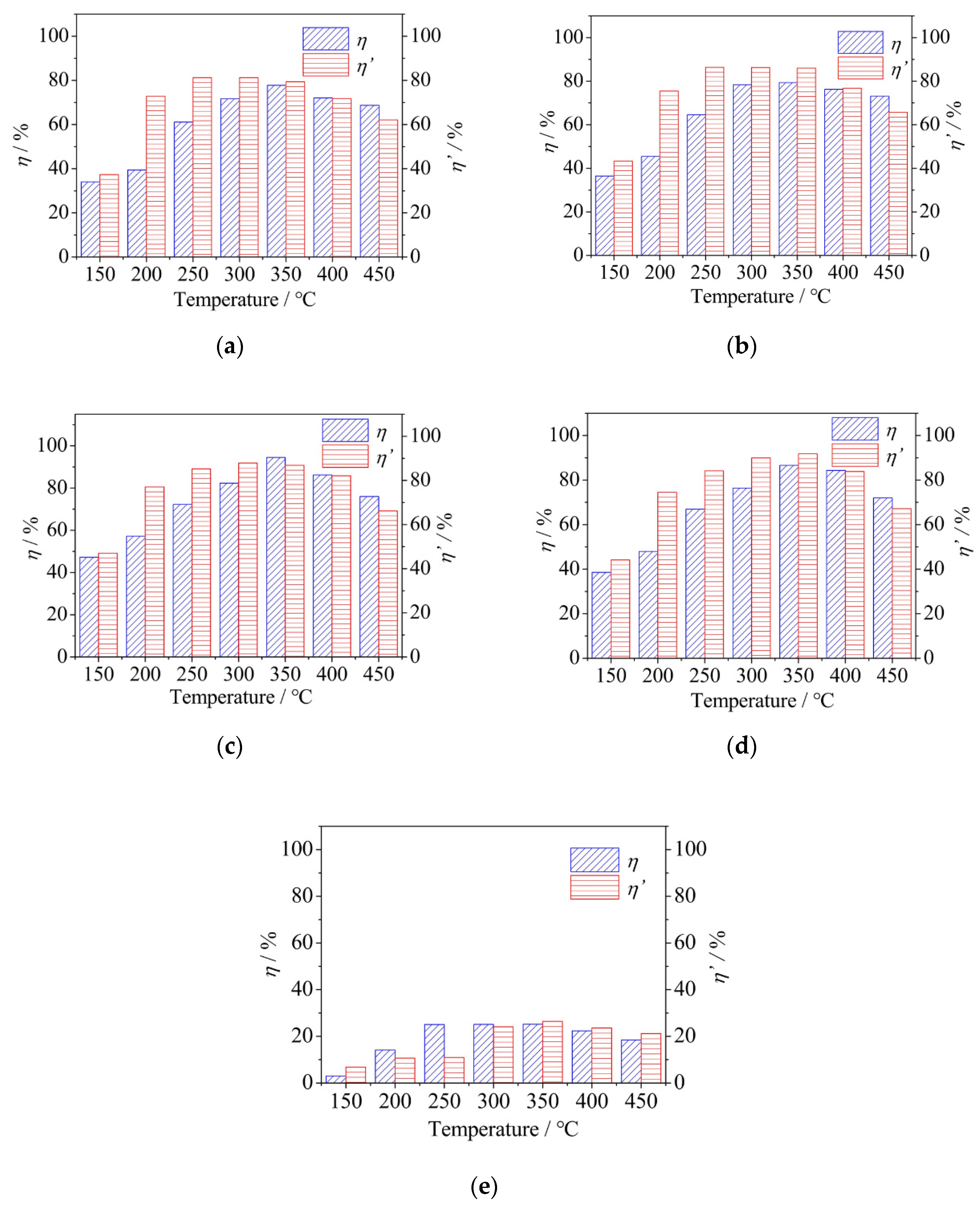
3.1. The Effect of Reaction Temperature on Catalytic Performance
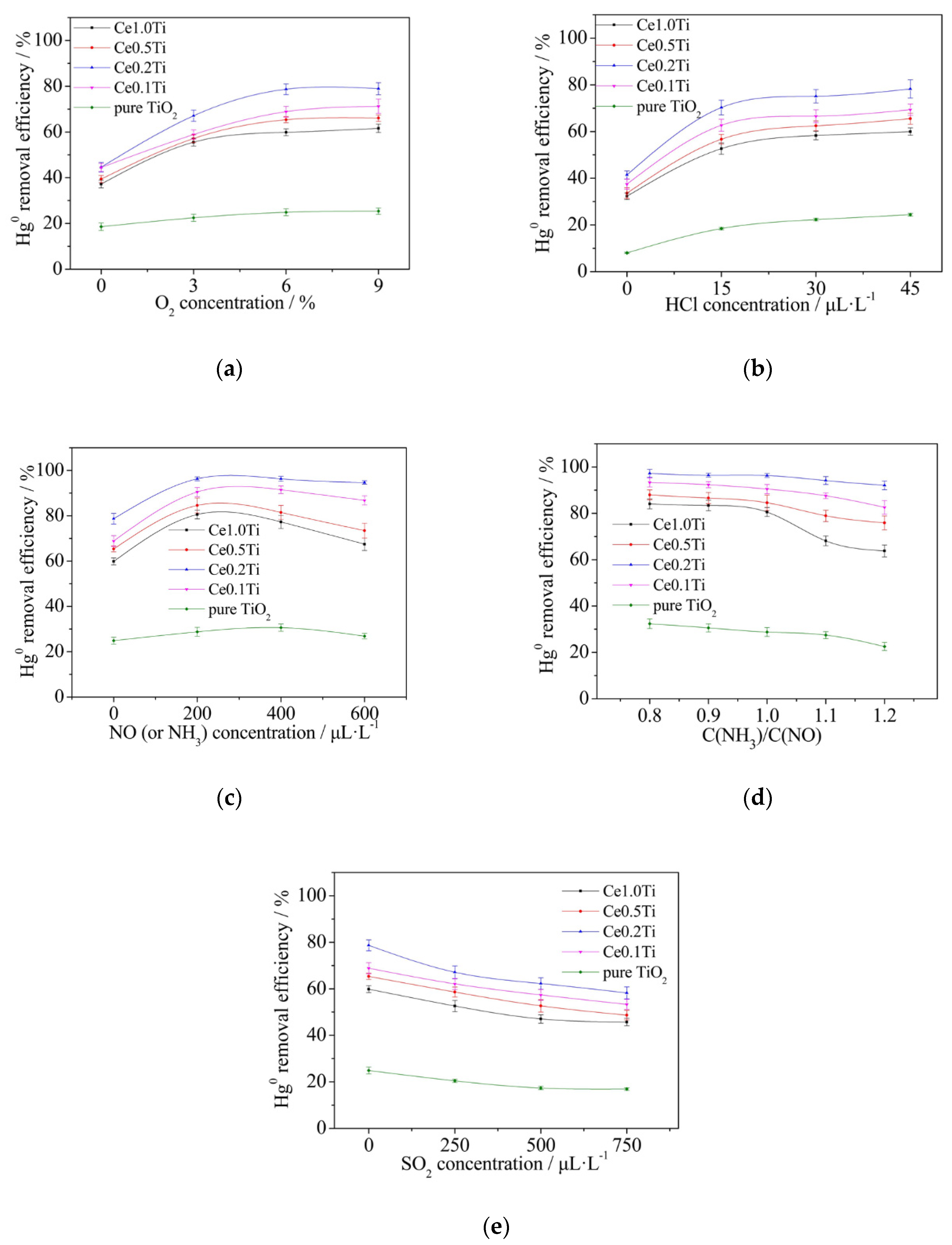
3.2. The Effects of flue Gas Components on Catalysts Performance
3.2.1. The Effect of O2 on Catalytic Performance
3.2.2. The Effect of HCl on Catalytic Performance
3.2.3. The Effects of NO and NH3 on Catalytic Performance
3.2.4. The Effect of the Ratio of NH3/NO on Catalytic Performance
3.2.5. The Effect of SO2 on Catalytic Performance
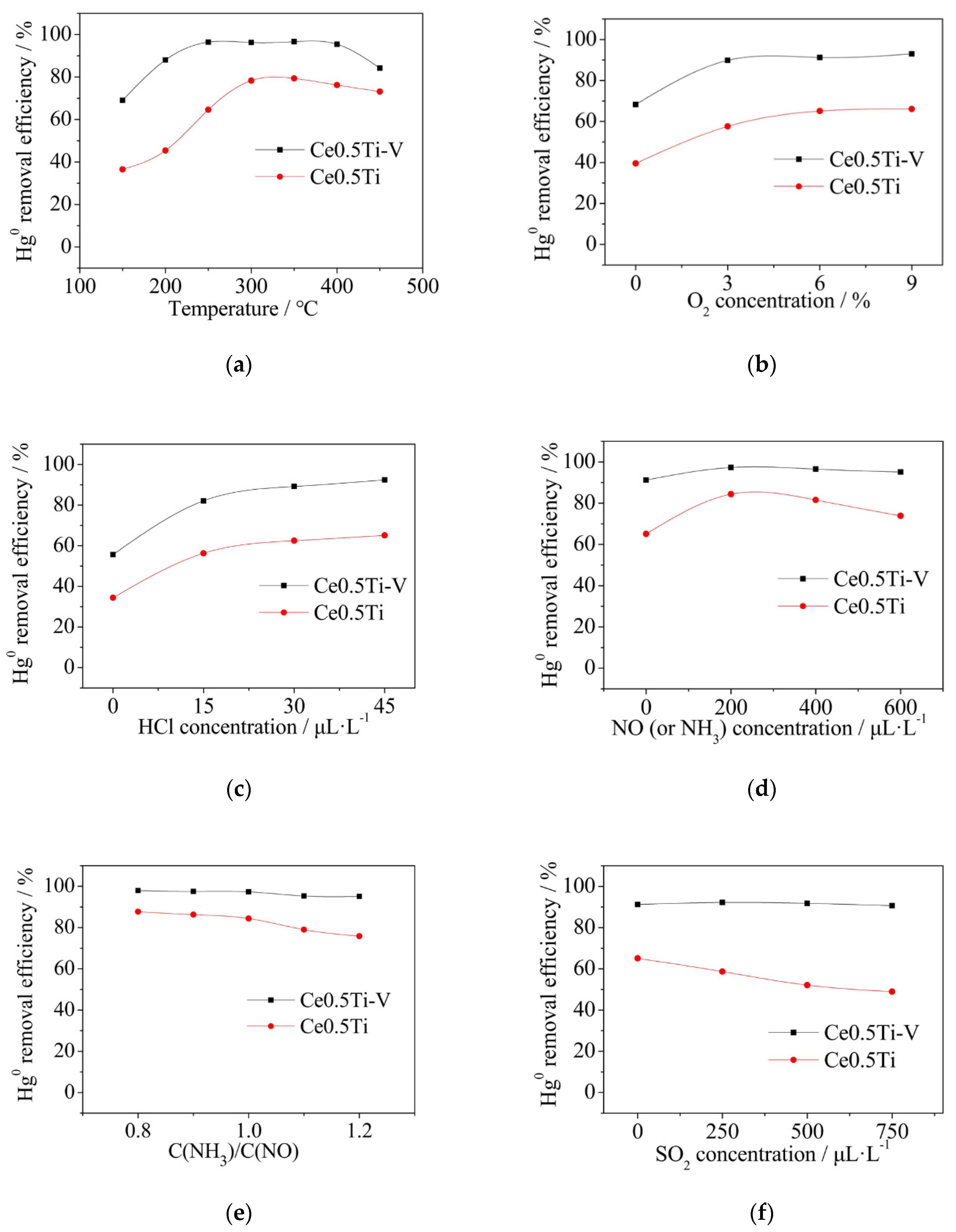
3.3. The Effect of Vanadium on Catalytic Performance
3.4. Simultaneous Removal of Hg0 and NO
3.5. Characterizations
3.5.1. Main Compositions of the Catalysts
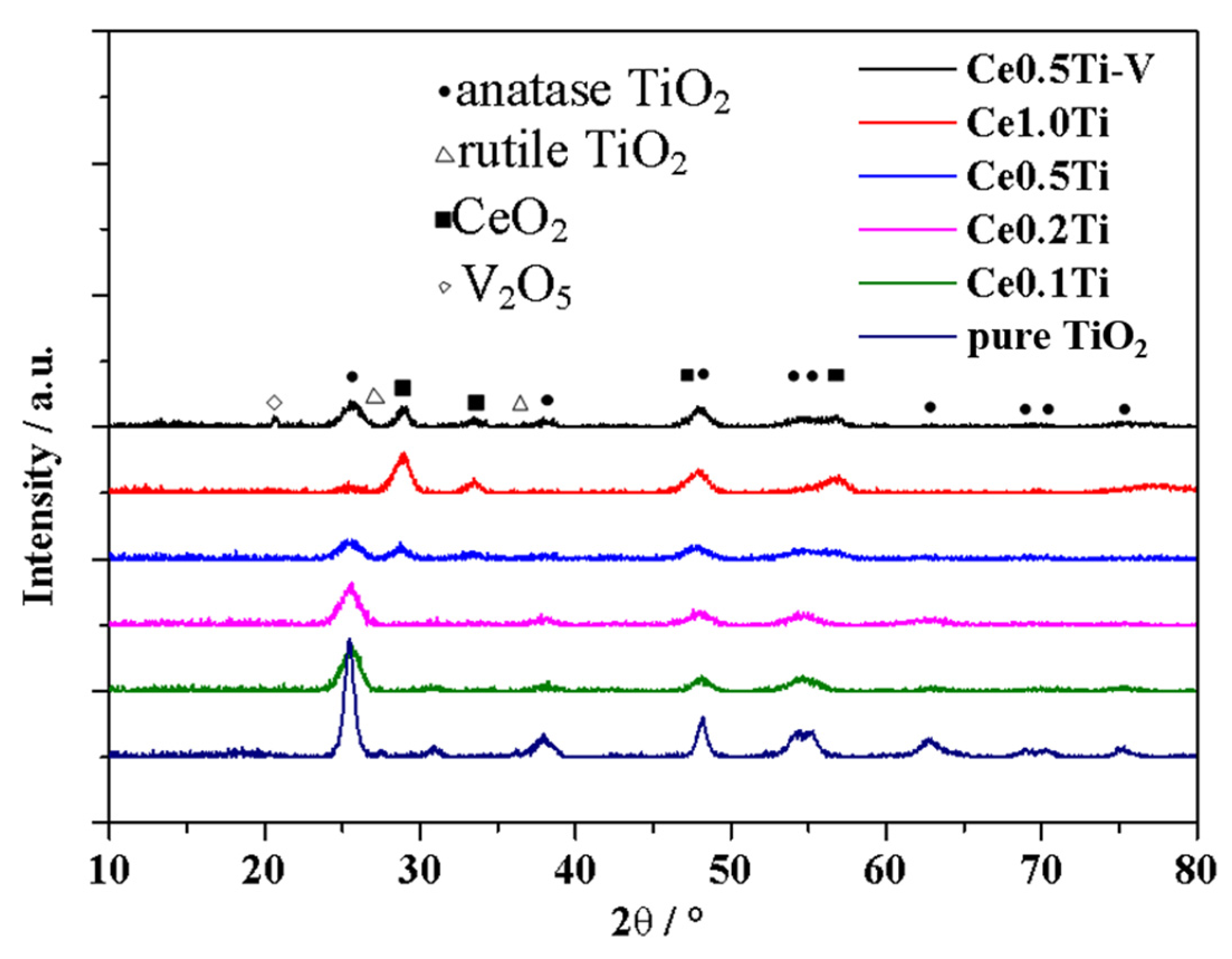
3.5.2. Crystalline Structures of the Catalysts
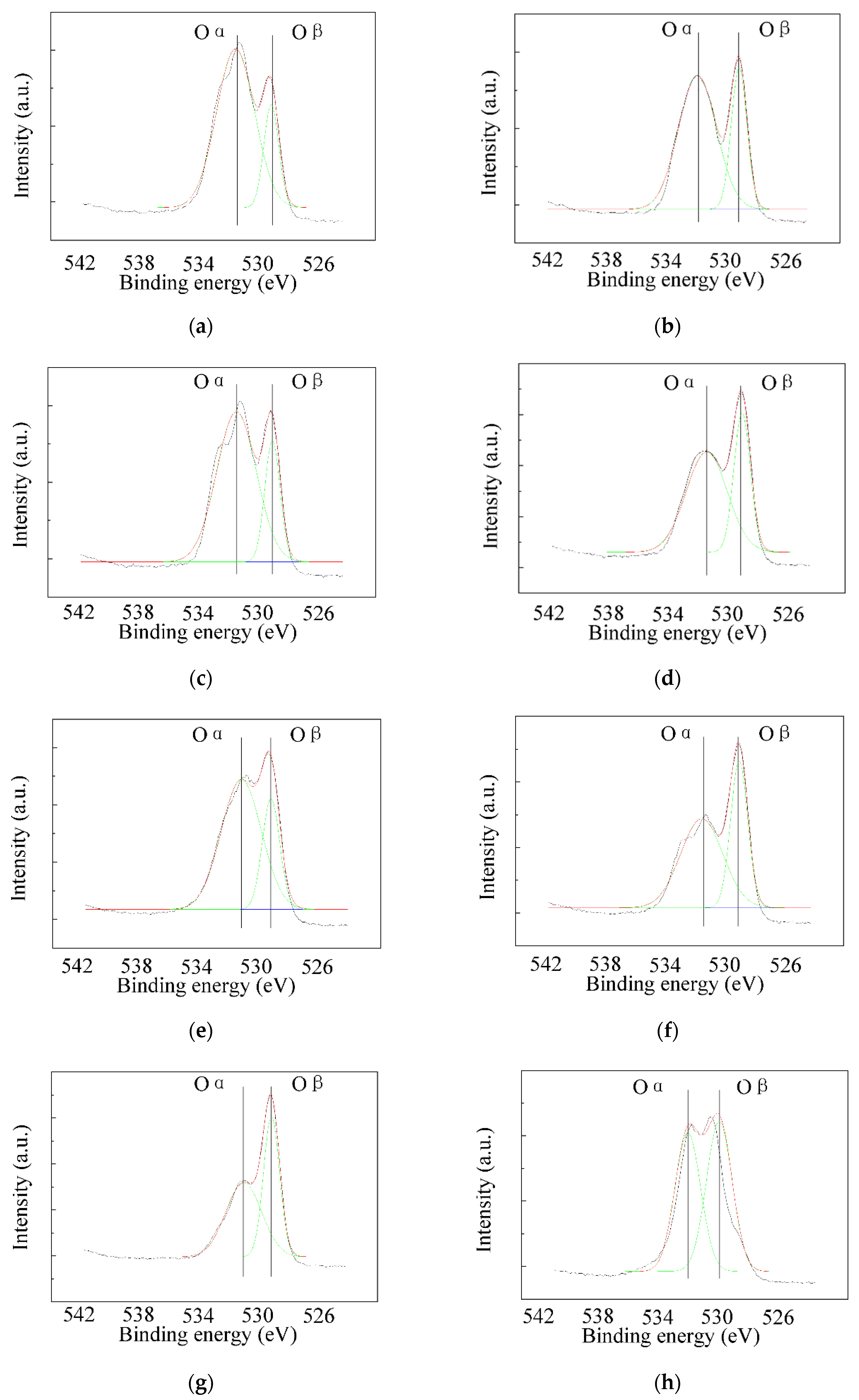
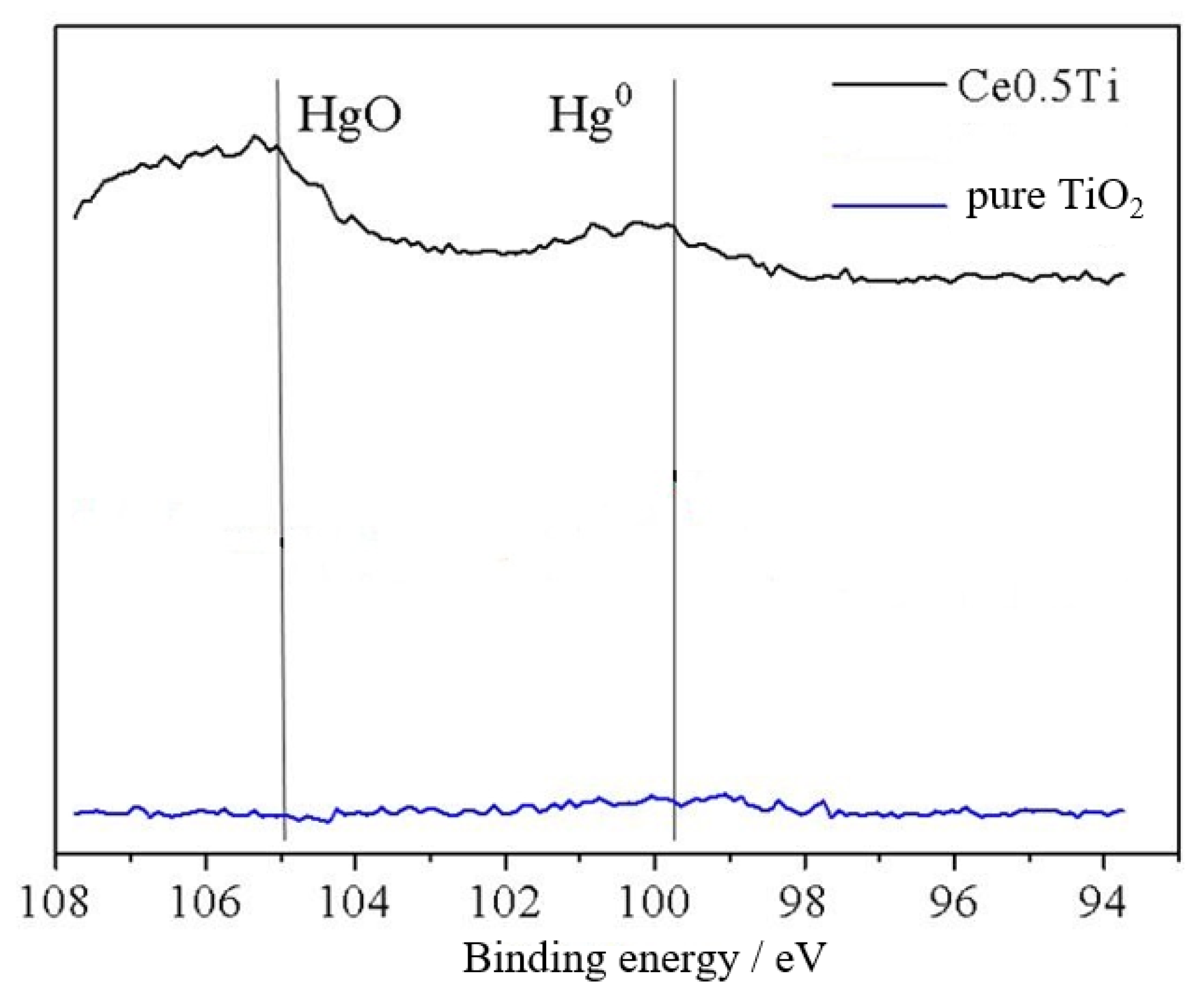
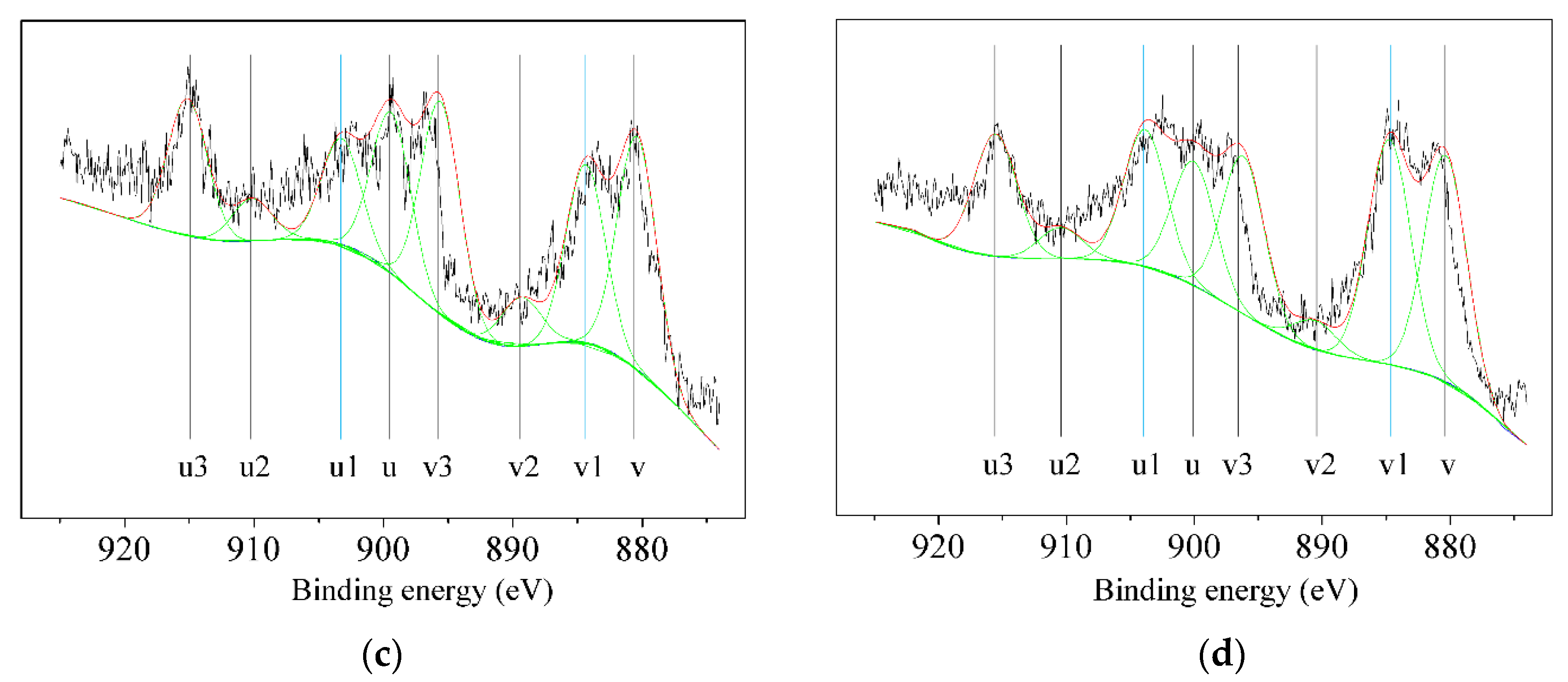
3.5.3. Valence of Selected Elements
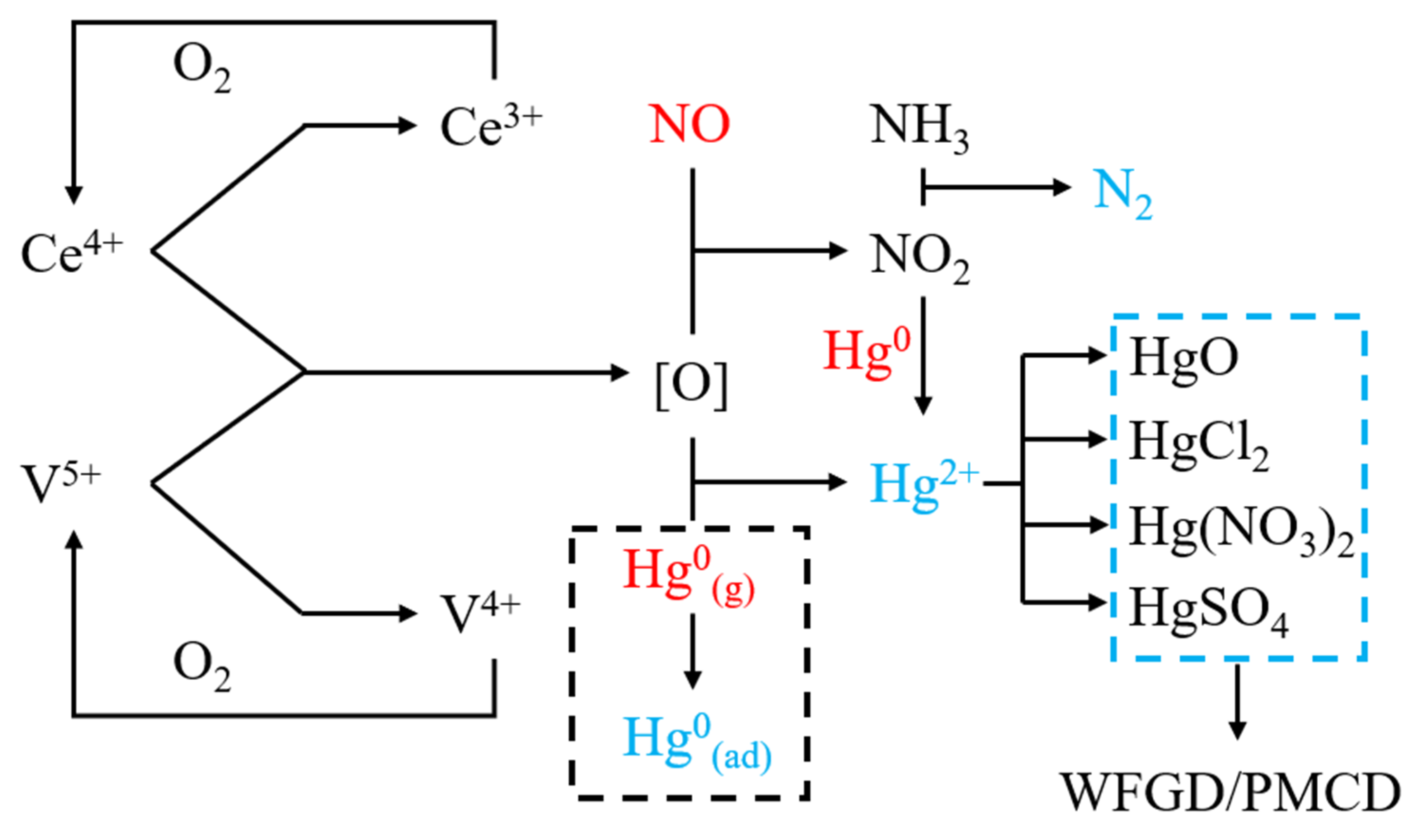
3.6. Discussion on Mechanisms
4. Conclusions
- (1)
- The Hg0 removal efficiency of CeO2/TiO2 catalyst was much higher than that of pure TiO2 catalyst. The optimal reaction temperature was 350 °C. Ce0.2Ti catalyst showed the best performance among all samples, since an excessive Ce loading value would cause the blockage of the catalyst.
- (2)
- Both O2 and HCl would benefit the removal of elemental mercury. However, higher NH3/NO ratio and SO2 concentration could bring negative effects on the Hg0 removal process. When the NH3/NO ratio was set as 1:1, the Hg0 removal efficiency increased firstly and then decreased as the NO and NH3 concentration rose from 0 to 600 μL·L−1.
- (3)
- The Hg0 removal efficiency increased remarkably after the addition of vanadium. Elemental mercury could be oxidized by V5+, which is a strong oxidant. Besides, V2O5-CeO2/TiO2 catalyst showed good resistance to NH3 and SO2.
- (4)
- Excellent simultaneous removal performance of NO and Hg0 could be obtained within the temperature window from 300 to 350 °C. The SCR process was reinforced, since NO could be oxidized into NO2 by Ce4+. NO2 also has a synergistic effect on the Hg0 removal over the catalysts.
- (5)
- The content of each component in the catalyst can be controlled accurately by the sol–gel method. Cerium oxides distributed evenly on the catalyst’s surface unless the Ce loading value was high enough. The proportion of adsorbed oxygen in the catalyst increased significantly after Ce was added. Chemical reaction, instead of physical adsorption, would be the dominant mechanism of Hg0 removal for the catalyst with high Ce content. Ce4+/Ce3+ pair and V5+/V4+ pair were of great importance in the removal mechanisms of NO and Hg0.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuzen, M.; Uluozlu, O.D.; Karaman, I.; Soylak, M. Mercury(II) and methyl mercury speciation on Streptococcus pyrogenes loaded Dowex Optipore SD-2. J. Hazard. Mater. 2009, 169, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.Y.; Zhang, A.C.; Zhang, D.; Feng, C.X.; Su, S.; Zhang, X.M.; Xiang, J.; Chen, G.Y.; Wang, Y. Efficient removal of Hg-0 from simulated flue gas by novel magnetic Ag2WO4/BiOI/CoFe2O4 photocatalysts. Chem. Eng. J. 2019, 373, 780–791. [Google Scholar] [CrossRef]
- Lv, Q.; Cai, M.; Wang, C.A.; He, Y.; Che, D.F. Investigation on elemental mercury removal by cerium modified semi-coke. J. Energy Inst. 2020, 93, 666–678. [Google Scholar] [CrossRef]
- Global Mercury Assessment 2018. Available online: https://www.unenvironment.org/resources/publication/global-mercury-assessment-2018 (accessed on 4 March 2019).
- Wang, P.Y.; Su, S.; Xiang, J.; You, H.W.; Cao, F.; Sun, L.S.; Hu, S.; Zhang, Y. Catalytic oxidation of Hg-0 by MnOx-CeO2/gamma-Al2O3 catalyst at low temperatures. Chemosphere 2014, 101, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.J.; Liao, Z.Q.; Zheng, Y.; Zhou, Z.J. Highly dispersed Mn–Ce binary metal oxides supported on carbon nanofibers for Hg0 removal from coal-fired flue gas. Appl. Sci. 2018, 8, 2501. [Google Scholar] [CrossRef]
- Liu, H.W.; Diao, X.; Yu, B.; Shi, J.B.; Liu, Q.; Yin, Y.G.; Hu, L.G.; Yuan, C.G.; Jiang, G.B. Effect of air pollution control devices on mercury isotopic fractionation in coal-fired power plants. Chem. Geol. 2019, 517, 1–6. [Google Scholar] [CrossRef]
- Lv, Q.; Cai, M.; Wang, C.A.; Zhang, Z.H.; Che, D.F. Investigation on elemental mercury removal and antideactivation performance of modified SCR catalysts. Asia-Pac. J. Chem. Eng. 2018, 13, 14. [Google Scholar] [CrossRef]
- Xu, W.; Hussain, A.; Liu, Y.X. A review on modification methods of adsorbents for elemental mercury from flue gas. Chem. Eng. J. 2018, 346, 692–711. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.C. Structural characterization of CeO2-TiO2 and V2O5/CeO2-TiO2 catalysts by Raman and XPS techniques. J. Phys. Chem. B 2003, 107, 5162–5167. [Google Scholar] [CrossRef]
- Xu, Y.H.; Chen, H.R.; Zeng, Z.X.; Lei, B. Investigation on mechanism of photocatalytic activity enhancement of nanometer cerium-doped titania. Appl. Surf. Sci. 2006, 252, 8565–8570. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.Y.; Cen, K.F. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.T.; Zeng, Q.; Du, X.Y.; Gao, L.; Li, S.H.; Zhai, Y.B. Study on removal of elemental mercury over MoO3-CeO2/cylindrical activated coke in the presence of SO2 by Hg-temperature-programmed desorption. Chem. Eng. J. 2019, 371, 666–678. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Li, S.; Wang, Y.; Zhang, J.; Wang, T.; Zeng, G. Simultaneous removal of elemental mercury and NO in simulated flue gas over V2O5/ZrO2-CeO2 catalyst. Appl. Catal. B-Environ. 2016, 198, 420–430. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Zhang, J.; Zhang, X.; Zhan, F.; Ma, J.; Xie, Y.E.; Zeng, G. Promotional effect of CeO2 modified support on V2O5-WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 2015, 153, 361–369. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Zhu, J.; Ma, L. Novel V2O5-CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NOx by NH3. Appl. Catal. B-Environ. 2014, 158, 11–19. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.T.; Li, S.H.; Zhang, W.; Du, X.Y.; Huang, L.; Zhu, Y.C.; Zhai, Y.B.; Zeng, G.M. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg-0 and NO removal. Chem. Eng. J. 2019, 371, 781–795. [Google Scholar] [CrossRef]
- Kamata, H.; Ueno, S.; Sato, N.; Naito, T. Mercury oxidation by hydrochloric acid over TiO2 supported metal oxide catalysts in coal combustion flue gas. Fuel Process. Technol. 2009, 90, 947–951. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, C.Y.; Li, Y.; Zhang, J.Y. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas. Environ. Sci. Technol. 2011, 45, 7394–7400. [Google Scholar] [CrossRef]
- He, C.; Shen, B.; Li, F. Effects of flue gas components on removal of elemental mercury over Ce-MnO(x)/Ti-PILCs. J. Hazard. Mater. 2016, 304, 10–17. [Google Scholar] [CrossRef]
- He, C.; Shen, B.; Chi, G.; Li, F. Elemental mercury removal by CeO2/TiO2-PILCs under simulated coal-fired flue gas. Chem. Eng. J. 2016, 300, 1–8. [Google Scholar] [CrossRef]
- Qiao, S.; Chen, J.; Li, J.; Qu, Z.; Liu, P.; Yan, N.; Jia, J. Adsorption and catalytic oxidation of gaseous elemental mercury in flue gas over MnOx/alumina. Ind. Eng. Chem. Res. 2009, 48, 3317–3322. [Google Scholar] [CrossRef]
- Kellie, S.; Cao, Y.; Duan, Y.F.; Li, L.C.; Chu, P.; Mehta, A.; Carty, R.; Riley, J.T.; Pan, W.P. Factors affecting mercury speciation in a 100-MW coal-fired boiler with low-NOx burners. Energy Fuels 2005, 19, 800–806. [Google Scholar] [CrossRef]
- Olson, E.S.; Sharma, R.K.; Pavlish, J.H. On the analysis of mercuric nitrate in flue gas by GC-MS. Anal. Bioanal. Chem. 2002, 374, 1045–1049. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, W.; Qi, P.; Gao, X.; Luo, Z.; Cen, K. CeO2-TiO2 sorbents for the removal of elemental mercury from syngas. Environ. Sci. Technol. 2013, 47, 10056–10062. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Stenger, H.G. Understanding mercury conversion in selective catalytic reduction (SCR) catalysts. Energy Fuels 2005, 19, 2328–2334. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, C.Y.; Li, Y.; Li, L.Q.; Zhao, Y.C.; Zhang, J.Y. Impact of SO2 on elemental mercury oxidation over CeO2-TiO2 catalyst. Chem. Eng. J. 2013, 219, 319–326. [Google Scholar] [CrossRef]
- Kim, M.H.; Ham, S.-W.; Lee, J.-B. Oxidation of gaseous elemental mercury by hydrochloric acid over CuCl2/TiO2-based catalysts in SCR process. Appl. Catal. B-Environ. 2010, 99, 272–278. [Google Scholar] [CrossRef]
- Ding, S.; Liu, F.; Shi, X.; He, H. Promotional effect of Nb additive on the activity and hydrothermal stability for the selective catalytic reduction of NOx with NH3 over CeZrOx catalyst. Appl. Catal. B-Environ. 2016, 180, 766–774. [Google Scholar] [CrossRef]
- Yang, B.; Shen, Y.; Shen, S.; Zhu, S. Regeneration of the deactivated TiO2-ZrO2-CeO2/ATS catalyst for NH3-SCR of NOx in glass furnace. J. Rare Earth. 2013, 31, 130–136. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Raimondi, F.; Wokaun, A. Enhanced reoxidation of vanadia by NO2 in the fast SCR reaction. J. Catal. 2002, 209, 159–165. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. PCCP Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Romeo, M.; Bak, K.; Elfallah, J.; Lenormand, F.; Hilaire, L. XPS study of the reduction of cerium dioxide. Surf. Interface Anal. 1993, 20, 508–512. [Google Scholar] [CrossRef]
- Zou, Z.Q.; Meng, M.; Zha, Y.Q. Surfactant-Assisted Synthesis, Characterizations, and catalytic oxidation mechanisms of the mesoporous MnOx-CeO2 and Pd/MnOx-CeO2 catalysts used for CO and C3H8 oxidation. J. Phys. Chem. C 2010, 114, 468–477. [Google Scholar] [CrossRef]
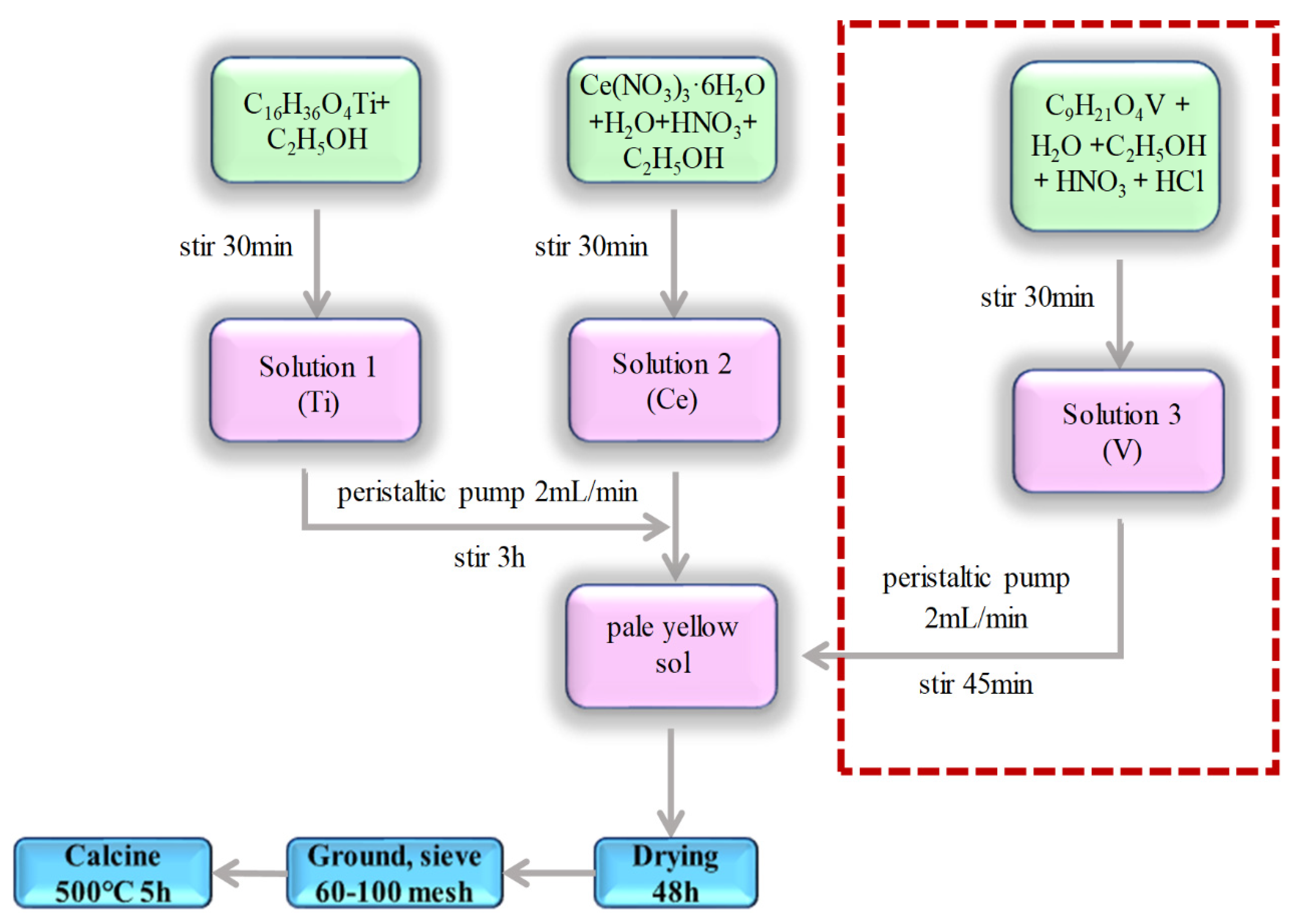



| Catalyst | C16H36O4Ti/mL | Ce(NO3)3·6H2O/g | C9H21O4V/g |
|---|---|---|---|
| pure TiO2 | 44.00 | - | - |
| Ce0.1Ti | 40.00 | 2.29 | - |
| Ce0.2Ti | 36.67 | 4.21 | - |
| Ce0.5Ti | 29.33 | 8.41 | - |
| Ce1.0Ti | 22.00 | 12.62 | - |
| Ce0.5Ti-V | 29.18 | 8.37 | 1.34 |
| Variable | Temperature/°C | Flue Gas Components |
|---|---|---|
| Temperature | 150, 200, 250, 300, 350, 400, 450 | BG * + 6% O2 + 45 μL·L−1 HCl + 200 μL·L−1 NO + 200 μL·L−1 NH3 + 500 μL·L−1 SO2 |
| O2 | 350 | BG + 45 μL·L−1 HCl + O2 (0, 3%, 6%, 9%) |
| HCl | 350 | BG + 6% O2 + HCl (0, 15, 30, 45 μL·L−1) |
| NO and NH3 | 350 | BG + 45 μL·L−1 HCl + 6% O2 + (NO + NH3) (0, 200, 400, 600 μL·L−1) |
| NH3/NO | 350 | BG + 45 μL·L−1 HCl + 6% O2 +200 μL·L−1 NO + NH3 (0, 160, 180, 200, 220, 240 μL·L−1) |
| SO2 | 350 | BG + 45 μL·L−1 HCl + 6% O2 + SO2 (0, 250, 500, 750 μL·L−1) |
| Catalyst | Ce1.0Ti | Ce0.5Ti | Ce0.2Ti | Ce0.1Ti | Pure TiO2 |
|---|---|---|---|---|---|
| NO | 175.3 | 184.0 | 190.8 | 194.7 | 198.3 |
| NO2 | 25.9 | 16.1 | 9.7 | 5.0 | 1.5 |
| Catalyst | Ti/% | O/% | Ce/% | V/% | TiO2/% | CeO2/% | V2O5/% |
|---|---|---|---|---|---|---|---|
| pure TiO2 | 76.2 | 23.6 | - | - | 99.8 | - | - |
| Ce0.1Ti | 64.6 | 25.9 | 8.53 | - | 90.2 | 8.52 | - |
| Ce0.2Ti | 56.0 | 26.4 | 15.9 | - | 81.4 | 16.7 | - |
| Ce0.5Ti | 33.9 | 38.4 | 22.3 | - | 63.1 | 31.4 | - |
| Ce1.0Ti | 21.2 | 41.9 | 28.7 | - | 45.9 | 44.9 | - |
| Ce0.5Ti-V | 44.0 | 25.9 | 26.2 | 0.161 | 65.0 | 31.1 | 0.483 |
| Catalyst | Fraction/% | ||
|---|---|---|---|
| Oα | Oβ | ||
| Ce1.0Ti | fresh | 79.7 | 20.3 |
| used | 69.4 | 30.6 | |
| Ce0.5Ti | fresh | 76.3 | 23.7 |
| used | 64.3 | 35.7 | |
| Ce0.2Ti | fresh | 73.0 | 27.0 |
| used | 60.0 | 40.0 | |
| Ce0.1Ti | fresh | 54.2 | 45.8 |
| used | 48.0 | 52.0 | |
| pure TiO2 | fresh | 45.6 | 54.4 |
| used | 27.8 | 72.2 | |
| Catalyst | Fraction/% | |
|---|---|---|
| Ce3+ | Ce4+ | |
| fresh Ce0.5Ti | 26.10 | 73.90 |
| used Ce0.5Ti | 34.35 | 65.65 |
| fresh Ce0.5Ti-V | 25.35 | 74.65 |
| used Ce0.5Ti-V | 34.09 | 65.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Q.; Wang, C.; He, Y.; Cai, M.; Che, D. Elemental Mercury Removal over CeO2/TiO2 Catalyst Prepared by Sol–Gel Method. Appl. Sci. 2020, 10, 2706. https://doi.org/10.3390/app10082706
Lv Q, Wang C, He Y, Cai M, Che D. Elemental Mercury Removal over CeO2/TiO2 Catalyst Prepared by Sol–Gel Method. Applied Sciences. 2020; 10(8):2706. https://doi.org/10.3390/app10082706
Chicago/Turabian StyleLv, Qiang, Chang’an Wang, Yang He, Ming Cai, and Defu Che. 2020. "Elemental Mercury Removal over CeO2/TiO2 Catalyst Prepared by Sol–Gel Method" Applied Sciences 10, no. 8: 2706. https://doi.org/10.3390/app10082706
APA StyleLv, Q., Wang, C., He, Y., Cai, M., & Che, D. (2020). Elemental Mercury Removal over CeO2/TiO2 Catalyst Prepared by Sol–Gel Method. Applied Sciences, 10(8), 2706. https://doi.org/10.3390/app10082706




