One-Pot Synthesis and Characterization of VO2(B) with a Large Voltage Window Electrochemical Performance in Aqueous Solution
Abstract
:1. Introduction
2. Experiment and Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bi, W.C.; Wang, J.C.; Jahrman, E.P.; Seidler, G.T.; Gao, G.H.; Wu, G.M.; Cao, G.Z. Interface Engineering V2O5 Nanofibers for High-Energy and Durable Supercapacitors. Small. 2019, 15, 1901747. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Duan, H.; Li, L.C. High-energy flexible solid-state supercapacitors based on O, N, S-tridoped carbon electrodes and a 3.5 V gel-type electrolyte. Chem. Eng. J. 2019, 372, 1216–1225. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.M.; Sun, S.; Wang, Y. Low-cost birnessite as a promising cathode for high-performance aqueous rechargeable batteries. Electrochim. Acta 2018, 272, 154–160. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xiong, X.H.; Fan, M.N.; Xie, D.; Wang, G.; Yang, C.H.; Liu, M.L. Scalable synthesis of FeS2 nanoparticles encapsulated into N-doped carbon nanosheets as a high-performance sodium-ion battery anode. Nanoscale 2018, 10, 22329–22334. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Xiong, F.Y.; Meng, J.S.; Wang, X.P.; Niu, C.J.; An, Q.Y.; Mai, L.P. Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Wang, X.D.; Song, J.H.; Liu, J.; Wang, Z.L. Direct-current nanogenerator driven by ultrasonic waves. Science 2007, 316, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhao, L.; Wang, Q.F.; Ma, Y.; Zhang, D.H. A dynamic stretchable and self-healable supercapacitor with a CNT/graphene/PANI composite film. Nanoscale 2019, 11, 3773–3779. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Ding, C.H.; Hao, Y.N.; Zhai, X.M.; Wang, C.Z.; Li, Y.T.; Li, J.B.; Jin, H.B. Neat Design for the Structure of Electrode To Optimize the Lithium-Ion Battery Performance. ACS Appl. Mater. Interfaces 2018, 10, 27106–27115. [Google Scholar] [CrossRef]
- Balogun, M.S.; Yang, H.; Luo, Y.; Qiu, W.T.; Huang, Y.C.; Liu, Z.Q.; Tong, Y.X. Achieving high gravimetric energy density for flexible lithium-ion batteries facilitated by core–double-shell electrodes. Energy Environ. Sci. 2018, 11, 1859–1869. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.Z.; Shen, S.H.; Zhong, Y.; Liu, S.; Xia, X.H.; Tong, Y.X.; Lu, X.H. Enhancing the Capacitive Storage Performance of Carbon Fiber Textile by Surface and Structural Modulation for Advanced Flexible Asymmetric Supercapacitors. Adv. Funct. Mater. 2019, 29, 1806329. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Polyoxometalate-based materials for sustainable and clean energy conversion and storage. Energy Chem. 2019, 1, 100021. [Google Scholar] [CrossRef]
- Althumairy, D.; Murakami, H.A.; Zhang, D.M.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Effects of vanadium(IV) compounds on plasma membrane lipids lead to G protein-coupled receptor signal transduction. J. Inorg. Biochem. 2020, 203, 110873. [Google Scholar] [CrossRef] [PubMed]
- Bolokang, A.S.; Motaung, D.E. Reduction-oxidation of V2O5-WO3 nanostructured by ball milling and annealing: Their improved H2S gas sensing performance. Appl. Surf. Sci. 2019, 43, 164–173. [Google Scholar] [CrossRef]
- Liu, K.; Lee, S.; Delaire, O.; Wu, J.Q.; Yang, S. Recent progresses on physics and applications of vanadium dioxide. Mater. Today. 2018, 21, 875–896. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.P.; Zhang, P.F.; Zhang, Q.H.; Qiao, R.M.; Wu, J.; Tokura, Y.; Yu, P. Electric-field control of tri-state phase transformation with a selective dual-ion switch. Nature 2017, 546, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théobald, F.; Cabala, R.; Bernard, J. Essai sur la structure de VO2(B). J. Solid State Chem. 1976, 17, 431–438. [Google Scholar] [CrossRef]
- Georg, A. Studies on Vanadium Oxides. II. The Crystal Structure of Vanadium Dioxide. Acta Chem. Scand. 1956, 10, 623–628. [Google Scholar]
- Sven, W. Note on a Phase Transition in VO2. Acta Chem. Scand. 1961, 15, 217. [Google Scholar]
- Oka, Y.; Sato, S.; Yao, T.; Yamamoto, N. Crystal Structures and Transition Mechanism of VO2(A). J. Solid State Chem. 1998, 141, 594–598. [Google Scholar] [CrossRef]
- Li, R.X.; Yu, X.; Bian, X.F.; Hu, F. Preparation and electrochemical performance of VO2(A) hollow spheres as a cathode for aqueous zinc ion batteries. RSC Adv. 2019, 9, 35117–35123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.F.; Zhang, H.Z.; Huang, Y.; Huang, C.; Niu, F.; Meng, C.G.; Tan, X.Y. One-step hydrothermal conversion of VO2(B) into W-doped VO2(M) and its phase transition and optical switching properties. Solid State Comm. 2014, 180, 24–27. [Google Scholar] [CrossRef]
- Li, L.Y.; Liu, P.C.; Zhu, K.J.; Wang, J.; Liu, J.S.; Qiu, J.H. A general and simple method to synthesize well-crystallized nanostructured vanadium oxides for high performance Li-ion batteries. J. Mater. Chem. A 2015, 3, 9385–9389. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, K.M.; Xu, W.W.; Meng, J.S.; He, L.; An, Q.Y.; Xu, X.; Luo, Y.Z.; Zhao, T.W.; Mai, L.Q. Mesoporous VO2 nanowires with excellent cycling stability and enhanced rate capability for lithium batteries. RSC Adv. 2014, 4, 33332–33337. [Google Scholar] [CrossRef]
- Xiao, X.X.; Li, S.; Wei, H.; Sun, D.; Wu, Y.Z.; Jin, G.Z.; Wang, F.; Zou, Y.P. Synthesis and characterization of VO2(B)/graphene nanocomposite for supercapacitors. J. Mater. Sci.: Mater. Electron. 2015, 26, 4226–4233. [Google Scholar] [CrossRef]
- Gu, L.P.; Wang, J.; Ding, J.W.; Li, B.; Yang, S.B. W-doped VO2(B) nanosheets-built 3D networks for fast lithium storage at high temperatures. Electrochim. Acta 2019, 295, 393–400. [Google Scholar] [CrossRef]
- Cui, F.H.; Zhao, J.; Zhang, D.X.; Fang, Y.Z.; Zhu, K. VO2(B) nanobelts and reduced graphene oxides composites as cathode materials for low-cost rechargeable aqueous zinc ion batteries. Chem. Eng. J. 2020, 390, 124118. [Google Scholar] [CrossRef]
- Kong, D.B.; Li, X.G.; Zhang, W.B.; Hai, X.; Wang, B.; Qiu, X.Y.; Song, Q.; Yang, Q.H.; Zhi, L.J. Encapsulating V2O5 into carbon nanotubes enables the synthesis of flexible high-performance lithium ion batteries. Energy Environ. Sci. 2016, 9, 906–911. [Google Scholar] [CrossRef]
- Gilson, T.R.; Bizri, O.F.; Cheetham, N. Single-crystal Raman and infrared spectra of vanadium (V) oxide. J. Chem. Soc. Dalton Trans. 1973, 291–294. [Google Scholar] [CrossRef]
- Faggio, G.; Modafferi, V.; Panzera, G.; Alfieri, D.; Santangelo, S. Micro-Raman and photoluminescence analysis of composite vanadium oxide/poly-vinyl acetate fibres synthesised by electro-spinning. J. Raman Spectrosc. 2012, 43, 761–768. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Marzouk, A.; Pereira-Ramos, J.P. Structural modifications of LixV2O5 in a composite cathode (0 ≤ x < 2) investigated by Raman microspectrometry. J. Raman Spectrosc. 2012, 43, 153–160. [Google Scholar]
- Sing, K.; Everett, D.H.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Baudrin, E.; Sudant, G.; Larcher, D.; Dunn, B.; Tarascon, J.M. Preparation of Nanotextured VO2[B] from Vanadium Oxide Aerogels. Chem. Mater. 2006, 18, 4369–4374. [Google Scholar] [CrossRef]
- Zhang, C.F.; Chen, Z.X.; Guo, Z.P.; Lou, X.W. Additive-free synthesis of 3D porous V2O5 hierarchical microspheres with enhanced lithium storage properties. Energy Environ. Sci. 2013, 6, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruña, P.S.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Wang, H.W.; Yi, H.; Chen, X.; Wang, X.F. One-step strategy to three-dimensional graphene/VO2 nanobelt composite hydrogels for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 1165–1173. [Google Scholar] [CrossRef]
- Deng, L.J.; Zhang, G.N.; Kang, L.P.; Lei, Z.B.; Liu, C.L.; Liu, Z.H. Graphene/VO2 hybrid material for high performance electrochemical capacitor. Electrochim. Acta 2013, 112, 448–457. [Google Scholar] [CrossRef]
- Yu, Z.N.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.Y.; Liu, H.M.; Yang, W.S. Fabrication of VO2(B) hybrid with multiwalled carbon nanotubes to form a coaxial structure and its electrochemical capacitance performance. J. Alloy. Compd. 2013, 559, 167–173. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, C.; Wang, L.; Zuo, Q.S.; Li, X.L.; Xie, B. Hierarchical vanadium oxide microspheres forming from hyperbranched nanoribbons as remarkably high performance electrode materials for supercapacitors. J. Mater. Chem. A 2015, 3, 22892–22901. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zheng, J.Q.; Hu, T.; Tian, F.P.; Meng, C.G. Synthesis and supercapacitor electrode of VO2(B)/Core–shell composites with a pseudocapacitance in aqueous solution. Appl. Surf. Sci. 2016, 371, 189–195. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Chen, L.Z.; Wang, Y.X.; Cai, S.Y.; Yang, H.J.; Yu, H.; Ding, F.Y.; Huang, C.; Liu, X.H. VO2(B)/Graphene Composite-Based Symmetrical Supercapacitor Electrode via Screen Printing for Intelligent Packaging. Nanomaterials 2018, 8, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.F.; Huang, Y.T. A facile hydrothermal synthesis of tungsten doped monoclinic vanadium dioxide with B phase for supercapacitor electrode with pseudocapacitance. Mater. Lett. 2016, 182, 285–288. [Google Scholar] [CrossRef]

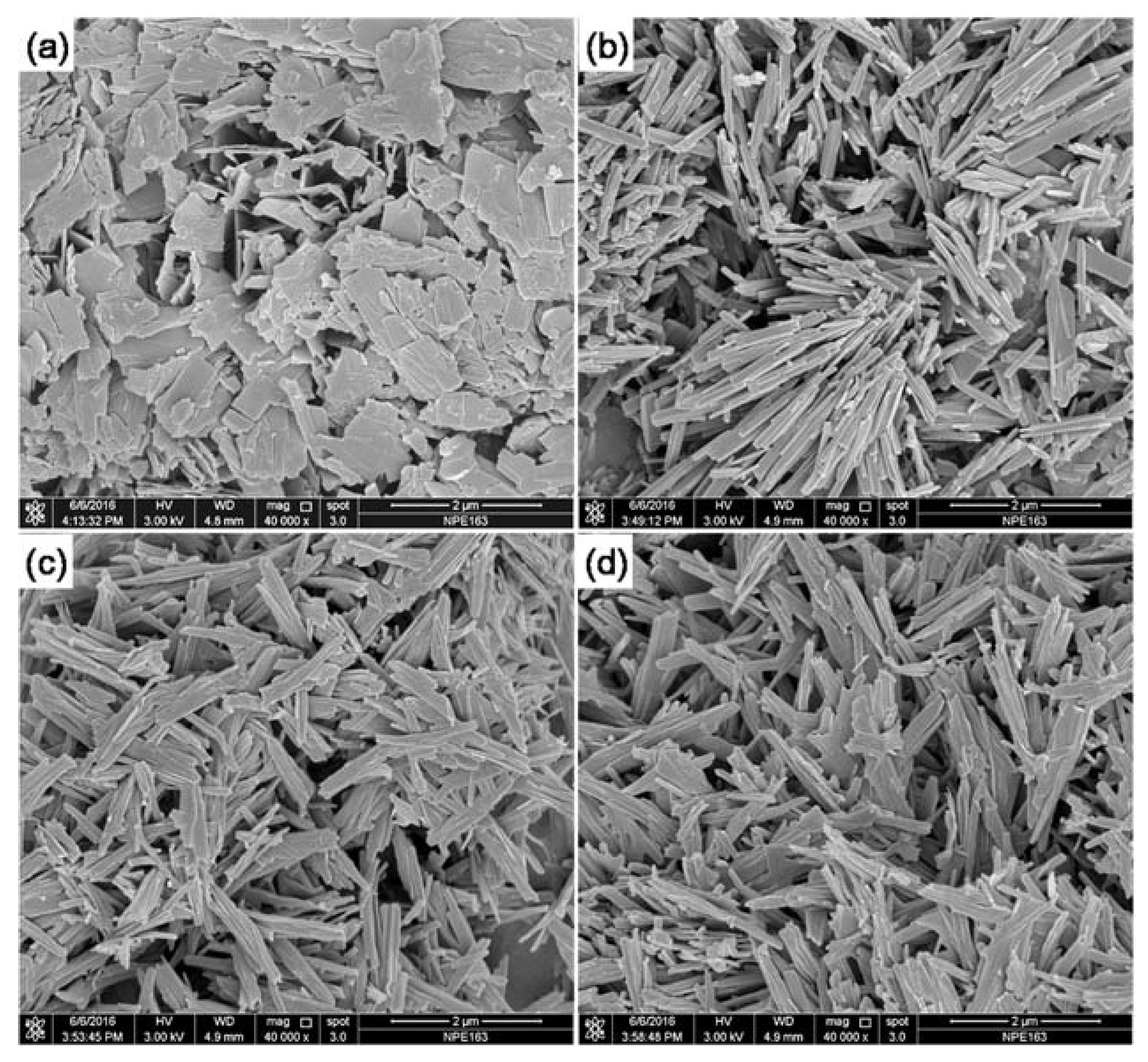
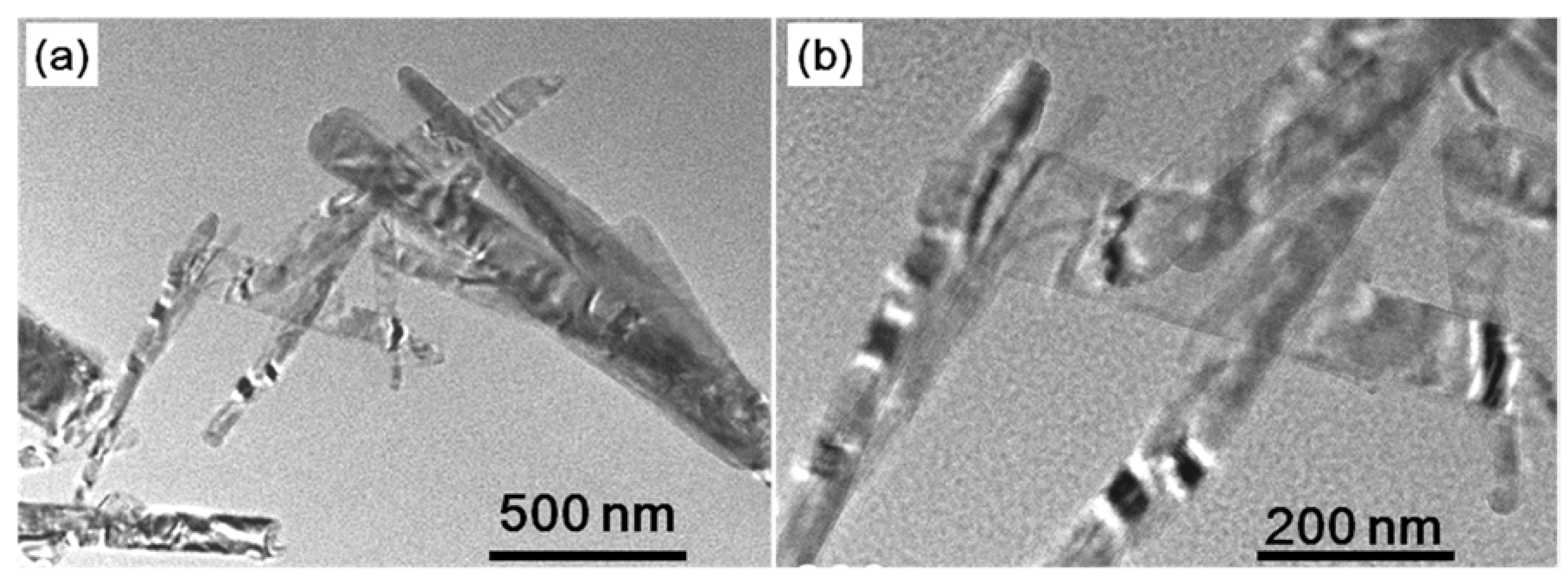
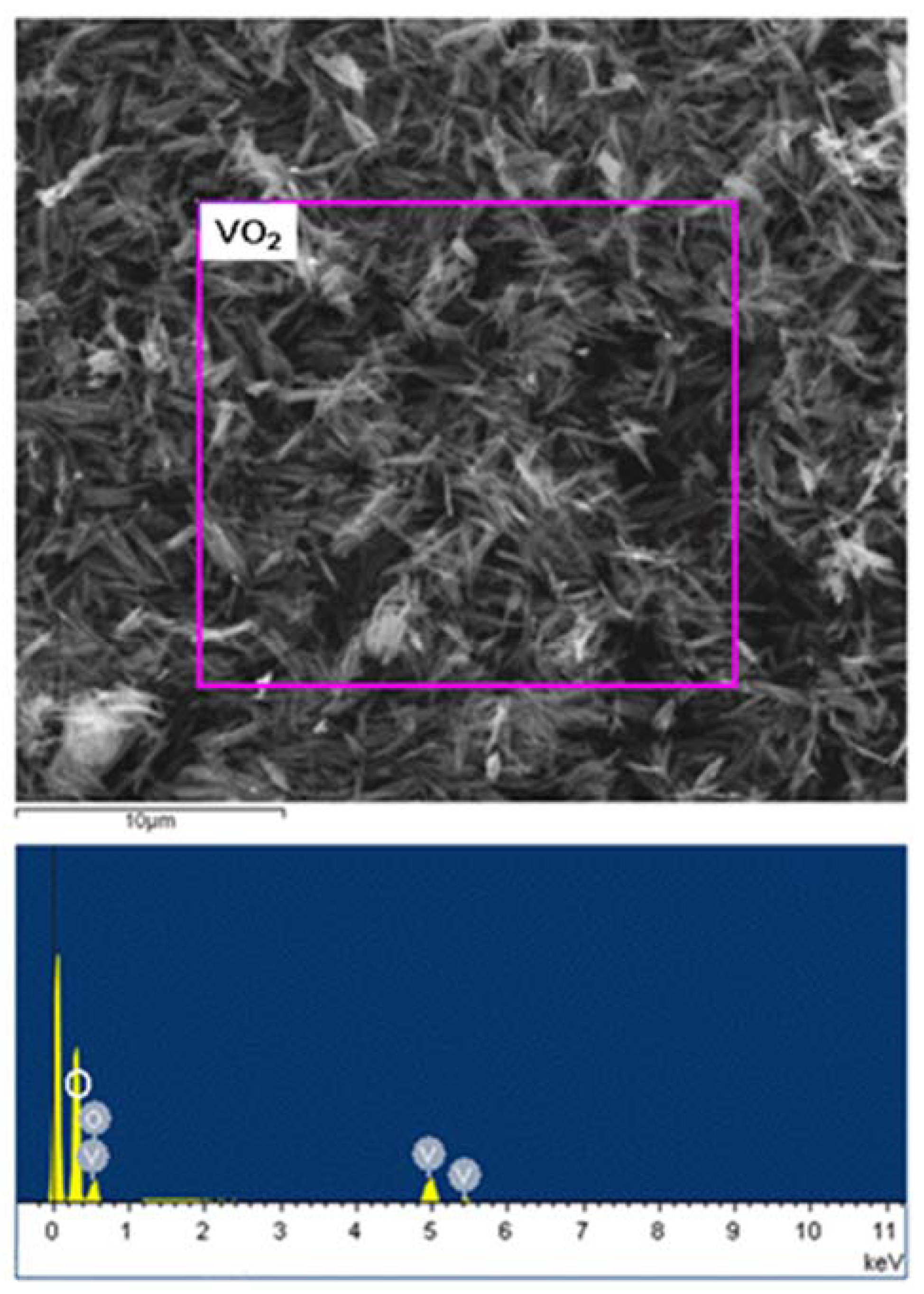
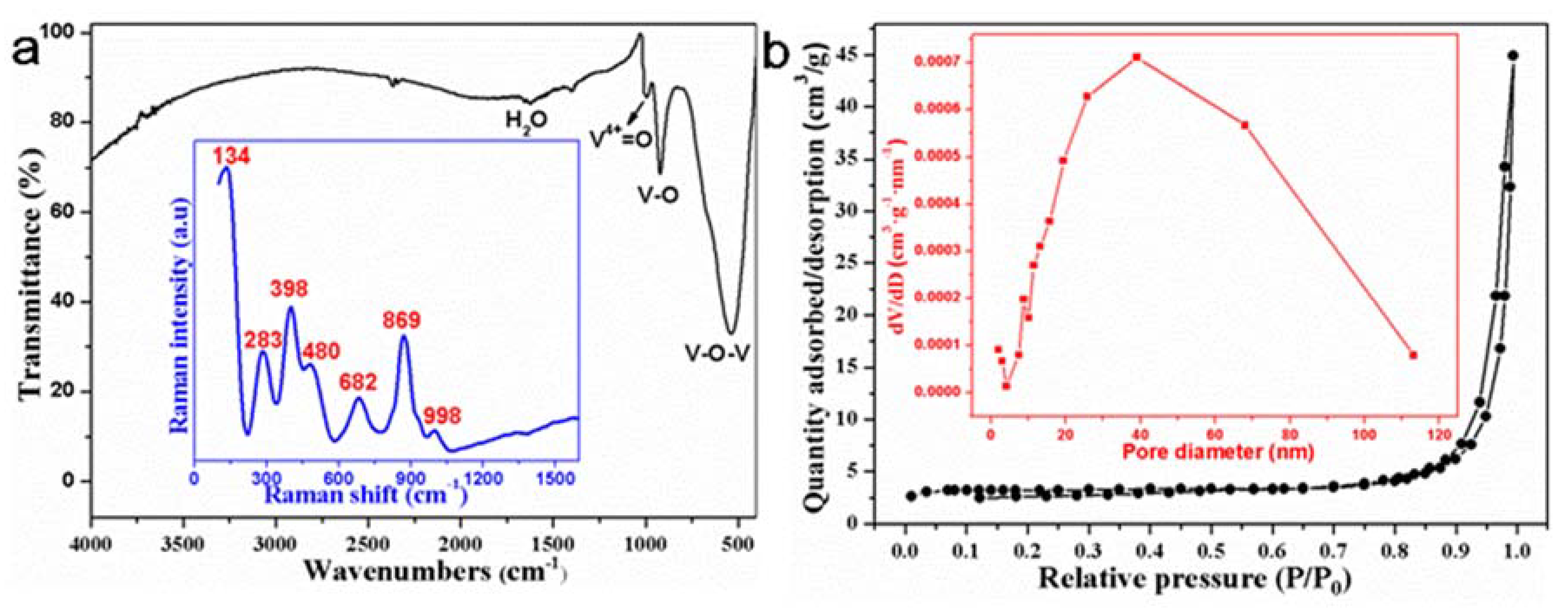
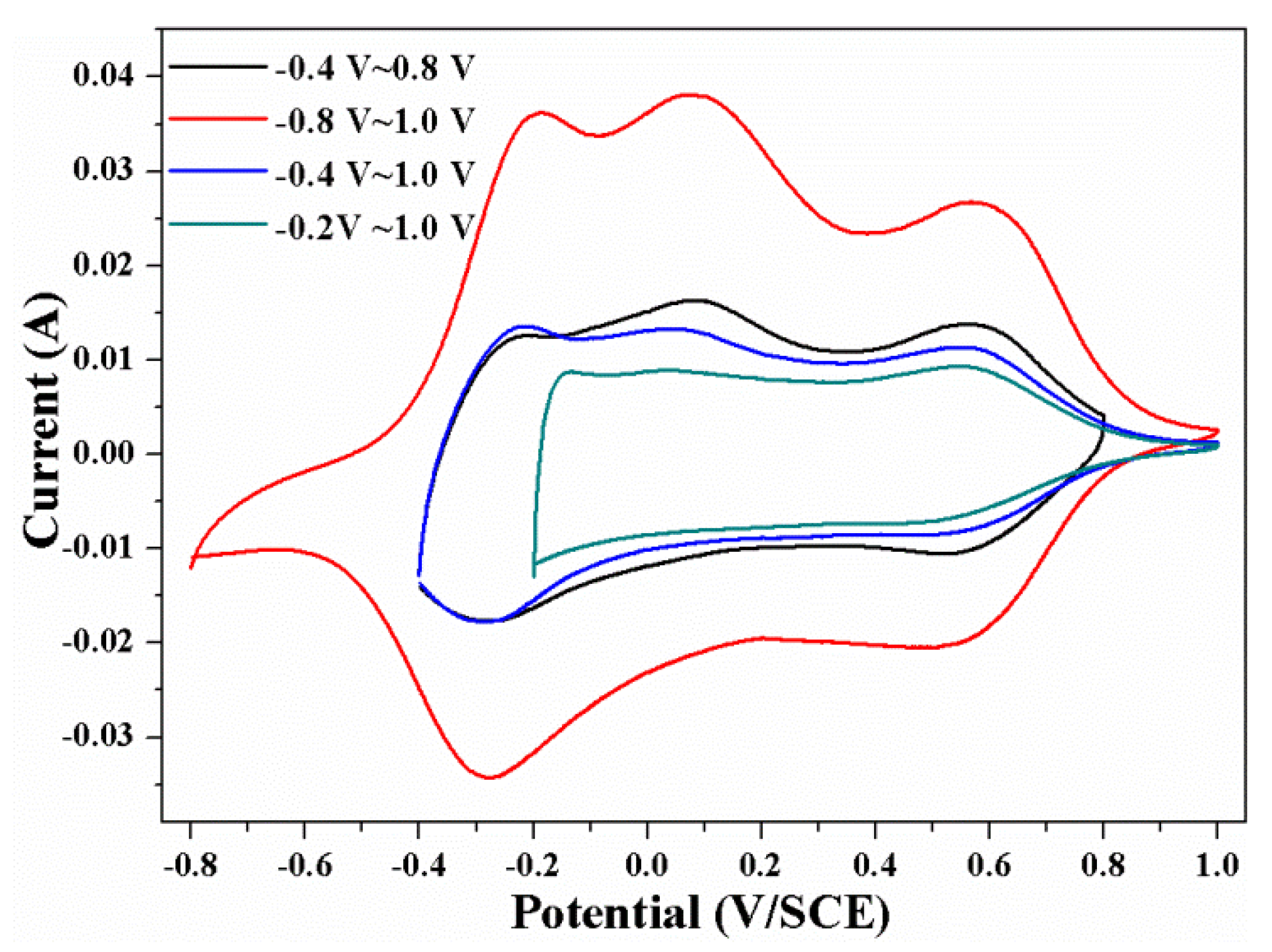
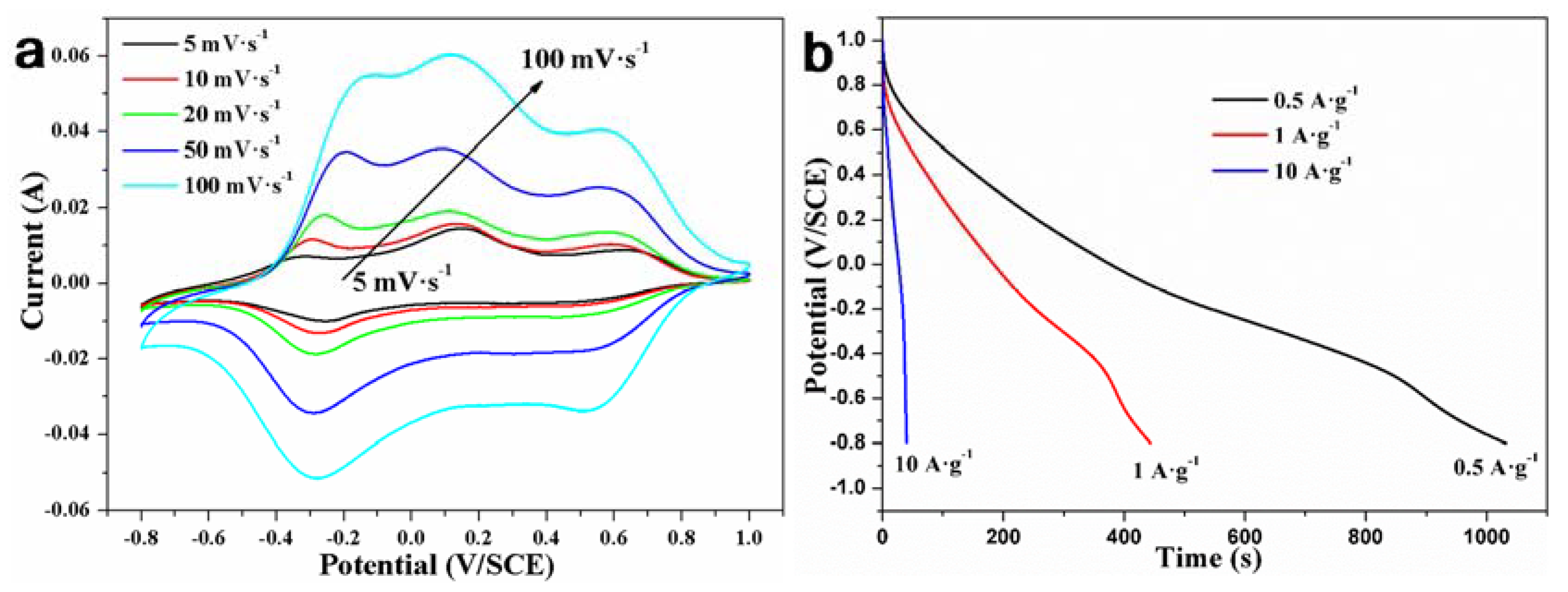
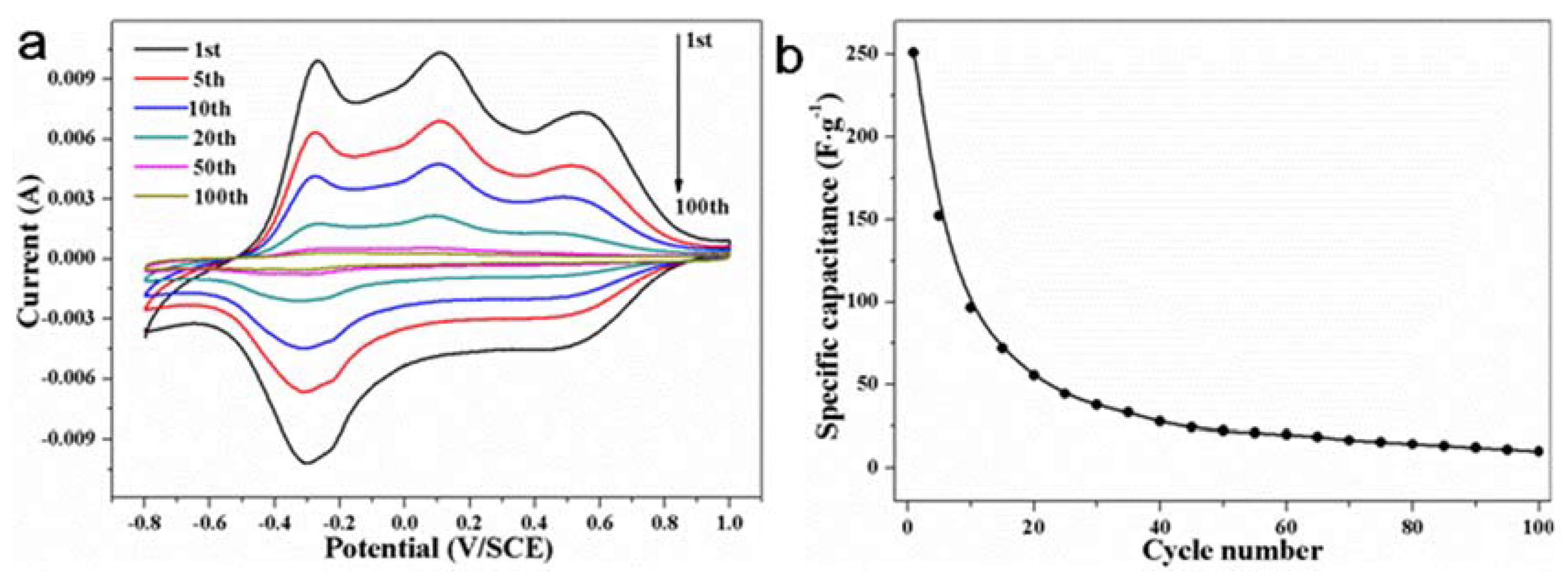
| Phase | Transition Temperature (K) | a(Å) | b(Å) | c(Å) | Crystal System |
|---|---|---|---|---|---|
| VO2(M) | 341 | 5.74 | 4.16 | 5.38 | Monoclinic |
| VO2(R) | 341 | 4.53 | 4.53 | 2.87 | Monoclinic |
| VO2(A) | 435 | 8.44 | 8.44 | 7.67 | Tetragonal |
| VO2(B) | — | 12.03 | 3.69 | 6.42 | Tetragonal |
| Materials | Current/A·g−1 | Potential/V | Capacitance/F·g−1 | Literatures |
|---|---|---|---|---|
| VO2(B)/RG(1.0) | 1 | −0.6~0.4 | 245 | [24] |
| VO2(B) particles | 0.25 | −0.2~0.8 | 136 | [36] |
| RG(1.0)/VO2(B) | 0.25 | −0.2~0.8 | 225 | [36] |
| VO2(B) nanofibers | 0.5 | −0.6~0.4 | 174 | [38] |
| VO2(B)/CNTs | 0.5 | −0.6~0.4 | 229 | [38] |
| VO2(B)/GN (20%) | 0.5 | 0~0.6 | 197 | [41] |
| W-dopedVO2(B) nanobelts | 1 | −0.4~0.6 | 253 | [42] |
| Porous VO2(B) nanobelts | 0.5 | −0.8~1.0 | 287 | This work |
| Porous VO2(B) nanobelts | 1 | −0.8~1.0 | 246 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zheng, J.; Jing, X.; Cheng, Y.; Meng, C. One-Pot Synthesis and Characterization of VO2(B) with a Large Voltage Window Electrochemical Performance in Aqueous Solution. Appl. Sci. 2020, 10, 2742. https://doi.org/10.3390/app10082742
Liu X, Zheng J, Jing X, Cheng Y, Meng C. One-Pot Synthesis and Characterization of VO2(B) with a Large Voltage Window Electrochemical Performance in Aqueous Solution. Applied Sciences. 2020; 10(8):2742. https://doi.org/10.3390/app10082742
Chicago/Turabian StyleLiu, Xiaoyu, Jiqi Zheng, Xuyang Jing, Yan Cheng, and Changgong Meng. 2020. "One-Pot Synthesis and Characterization of VO2(B) with a Large Voltage Window Electrochemical Performance in Aqueous Solution" Applied Sciences 10, no. 8: 2742. https://doi.org/10.3390/app10082742
APA StyleLiu, X., Zheng, J., Jing, X., Cheng, Y., & Meng, C. (2020). One-Pot Synthesis and Characterization of VO2(B) with a Large Voltage Window Electrochemical Performance in Aqueous Solution. Applied Sciences, 10(8), 2742. https://doi.org/10.3390/app10082742




