Evaluation of the Corrosion Resistance of Phosphate Coatings Deposited on the Surface of the Carbon Steel Used for Carabiners Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
- C45—C45 steel sample;
- I-Zn—The C45 steel phosphate in zinc-based solution sample;
- II-Zn/Fe—The C45 steel phosphate in zinc/iron-based solution sample;
- III-Mn—The C45 steel phosphate in manganese-based solution sample;
- OPS—The C45 steel phosphate in zinc-based solution and immersed in MoS2 oil sample;
- PPS—The C45 steel phosphate in zinc-based solution and painted sample.
- RW—Rainwater, pH = 6.5;
- BSW—Black Sea water, pH = 6.15;
- FES—Fire extinguishing solution, pH = 6.41.
3. Results and Discussion
3.1. Structural Analysis
3.2. Corrosion Resistance Evaluation
3.2.1. The Equivalent Circuit R(QR(LR))
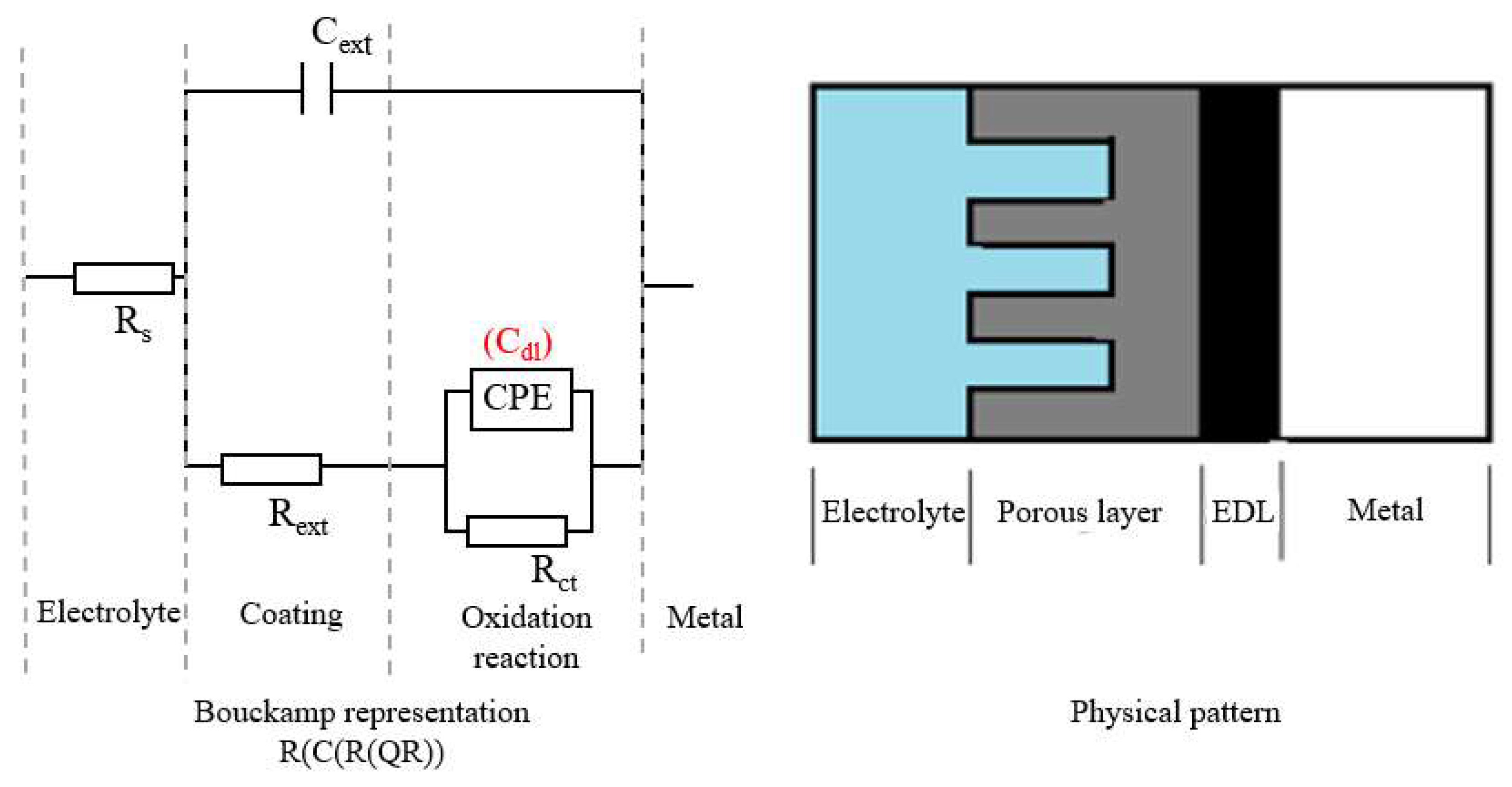
3.2.2. The Equivalent Circuit R(C(R(QR)))
3.2.3. The Equivalent Circuit R(Q(R(QR)))
3.2.4. The Equivalent Circuit R(C(R(Q(RW))))
3.2.5. The Equivalent Circuit R(Q(R(Q(RW))))
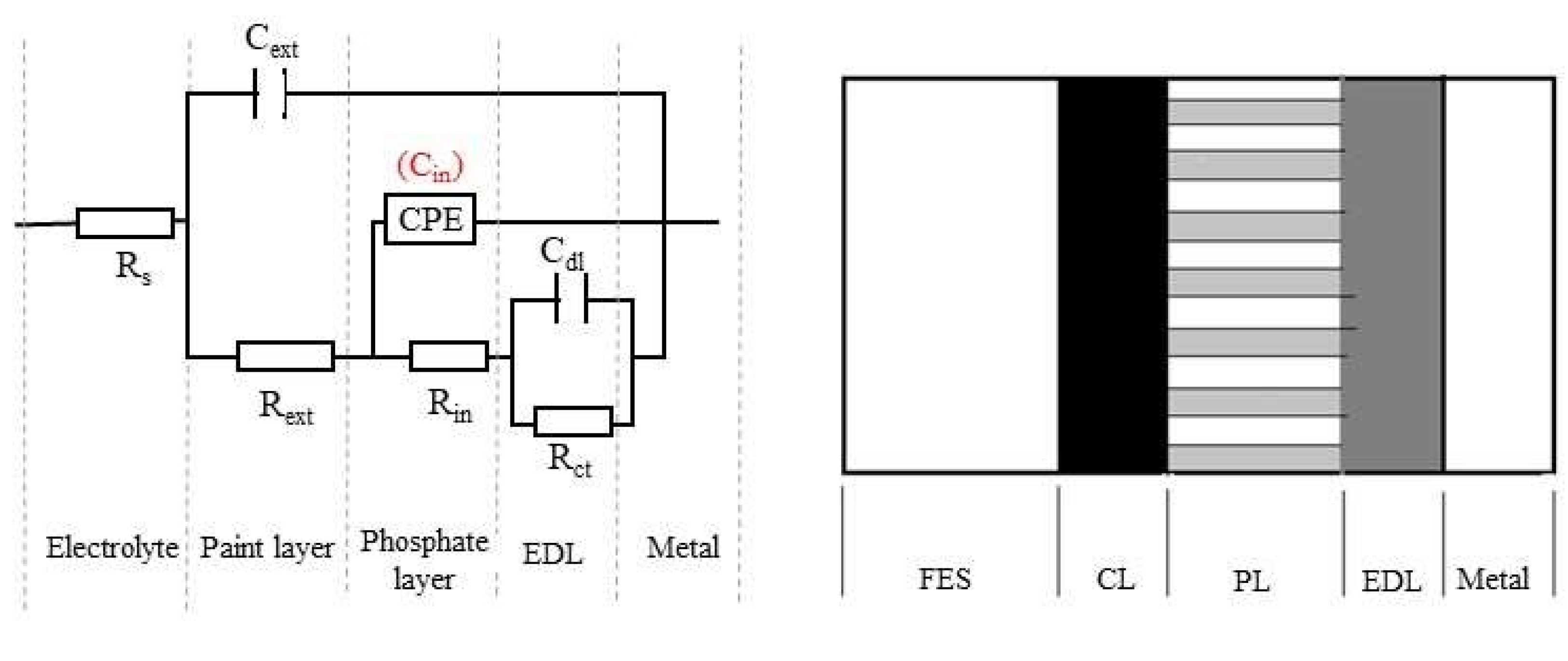
3.2.6. The Equivalent Circuit R(C(R(Q(R(CR)))))
4. Conclusions
- -
- the C45 samples exhibit a double layer structure with high polarization resistance and low corrosion rate when exposed to rainwater (RW). When exposed to Black Sea water (BSW), due to the aggressiveness of the chlorine ions, the steel, especially the iron, is rapidly attacked, consequently, the polarization resistance is 3.3 times lower and the corrosion rate is higher. In the fire extinguishing solution (FES), the equivalent circuit used is characteristic to a corrosive metal coated with a porous non-conducting layer in which the corrosion process is carried out under a mixed, kinetic and diffusion control. The estimated corrosion rate of the C45 samples exposed to FES is close to the value which corresponds to BSW;
- -
- the phosphate samples (I-ZN, II-Zn/Fe and III-Mn) exhibit a double-layer structure when exposed to RW, where the I-ZN sample (based on Zn3 (PO4)2) exhibits the best corrosion resistance - more than 10 times higher than that presented by the II-ZN/Fe and III-Mn samples. The sample with the lowest corrosion resistance is the III-Mn sample which was coated with an Mn3 (PO4)2 + Fe + Ni-based solution. The corrosion rates of phosphate samples are higher when exposed to BSW, compared to RW, also, the I-Zn phosphate sample provides the best corrosion protection, while the III-Mn phosphate sample the lowest. In FES, the II-Zn/Fe sample appears as the sample with the highest corrosion resistance, while the I-Zn sample is the second.
- -
- the phosphate and oil immersed samples (OPS) show a very low corrosion rate in OPS/RW system. In the case of OPS/RW and OPS/FES systems, the circuit used describes a double-layer physical system with kinetic and diffusion mixed corrosion rate control. The capacities (expressed by the constant phase elements CPE1 and CPE2), the resistances (Rext and Rct) and the Warburg constant (W) obtained for OPS samples in both corrosion environments are in the same range. Given the very low values of the constant W, the diffusion impedance (ZW) is very high. The sum of kinetic (Rct + Rext) and diffusional (ZW) components leads to a very high polarization resistance and a significant reduction in corrosion rate, moreover, the instantaneous diffusion rate is 114 µm/year in seawater, while in FES it is of 243 µm/year.
- -
- the phosphate and rubber painted samples (PPS) were analyzed with a circuit that describes a three-layer physical state, with kinetic control of the corrosion rate. This type of sample shows high corrosion resistance in RW and FES, yet, in BSW the rubber layer is rapidly dissolved.
Author Contributions
Funding
Conflicts of Interest
References
- Samet, M. The Climbing Dictionary: Mountaineering Slang, Terms, Neologisms & Lingo: An Illustrated Reference to More Than 650 Words; Mountaineers Books: Seattle, WA, USA, 2011; ISBN 9781594855023. [Google Scholar]
- Bright, C.M. A History of Rock Climbing Gear Technology and Standards; ScholarWorks@UARK: Fayetteville, NC, USA, 2014. [Google Scholar]
- Burduhos-Nergis, D.P.; Baciu, C.; Vizureanu, P.; Lohan, N.M.; Bejinariu, C. Materials types and selection for carabiners manufacturing: A review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012027. [Google Scholar] [CrossRef]
- Aliha, M.R.M.; Bahmani, A.; Akhondi, S. Fracture and fatigue analysis for a cracked carabiner using 3D finite element simulations. Strength Mater. 2015, 47, 890–902. [Google Scholar] [CrossRef]
- Blair, K.B.; Custer, D.R.; Graham, J.M.; Okal, M.H. Analysis of fatigue failure in D-shaped karabiners. Sports Eng. 2005, 8, 107–113. [Google Scholar] [CrossRef]
- Burduhos Nergiş, D.P.; Nejneru, C.; Achiţei, D.C.; Cimpoieşu, N.; Bejinariu, C. Structural Analysis of Carabiners Materials Used at Personal Protective Equipments. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012040. [Google Scholar] [CrossRef]
- Zafren, K.; Durrer, B.; Herry, J.P.; Brugger, H. Lightning injuries: Prevention and on-site treatment in mountains and remote areas: Official guidelines of the International Commission for Mountain Emergency Medicine and the Medical Commission of the International Mountaineering and Climbing Federation (ICAR and UIAA MEDCOM). Resuscitation 2005, 65, 369–372. [Google Scholar]
- Scott, V. Design of a Composite Carabiner for Rock Climbing; Final Report; Allen Institute for AI: London, UK, 2008. [Google Scholar]
- Smith, R.A. The development of equipment to reduce risk in rock climbing. Sports Eng. 1998, 1, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Harutyunyan, D.; Milton, G.W.; Dick, T.J.; Boyer, J. On ideal dynamic climbing ropes. Proc. Inst. Mech. Eng. Part P J. Sports Eng. Technol. 2017, 231, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Nergis, D.P.B.; Cimpoesu, N.; Vizureanu, P.; Baciu, C.; Bejinariu, C. Tribological characterization of phosphate conversion coating and rubber paint coating deposited on carbon steel carabiners surfaces. Mater. Today Proc. 2019, 19, 969–978. [Google Scholar] [CrossRef]
- Bejinariu, C.; Burduhos-Nergiș, D.P.; Cimpoeșu, N.; Bernevig-Sava, M.A.; Toma Ștefan, L.; Darabont, D.C.; Baciu, C. Study on the anticorrosive phosphated steel carabiners used at personal protective. Calitatea 2019, 20, 71. [Google Scholar]
- Burduhos-Nergiș, D.P.; Nejneru, C.; Cimpoeșu, R.; Cazac, A.M.; Baciu, C.; Darabont, D.C.; Bejinariu, C. Analysis of chemically deposited phosphate layer on the carabiners steel surface used at personal protective equipments. Qual. Access Success 2019, 20, 77–82. [Google Scholar]
- Etteyeb, N.; Sanchez, M.; Dhouibi, L.; Alonso, C.; Andrade, C.; Triki, E. Corrosion protection of steel reinforcement by a pretreatment in phosphate solutions: Assessment of passivity by electrochemical techniques. Corros. Eng. Sci. Technol. 2006, 41, 336–341. [Google Scholar] [CrossRef]
- Jegdić, B.V.; Bajat, J.B.; Popić, J.P.; Stevanović, S.I.; Mišković-Stanković, V.B. The EIS investigation of powder polyester coatings on phosphated low carbon steel: The effect of NaNO2 in the phosphating bath. Corros. Sci. 2011, 53, 2872–2880. [Google Scholar] [CrossRef]
- Manna, M. Characterisation of phosphate coatings obtained using nitric acid free phosphate solution on three steel substrates: An option to simulate TMT rebars surfaces. Surf. Coat. Technol. 2009, 203, 1913–1918. [Google Scholar] [CrossRef]
- Dhouibi, L.; Triki, E.; Salta, M.; Rodrigues, P.; Raharinaivo, A. Studies on corrosion inhibition of steel reinforcement by phosphate and nitrite. Mater. Struct. 2003, 36, 530–540. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Bejinariu, C.; Toma, S.-L.; Tugui, A.-C.; Baciu, E.-R. Carbon steel carabiners improvements for use in potentially explosive atmospheres. MATEC Web Conf. 2020, 305, 00015. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Sandu, A.-V.; Burduhos-Nergis, D.-D.; Darabont, D.-C.; Comaneci, R.-I.; Bejinariu, C. Shock Resistance Improvement of Carbon Steel Carabiners Used at PPE. MATEC Web Conf. 2019, 290, 12004. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.P.; Nejneru, C.; Burduhos Nergis, D.D.; Savin, C.; Sandu, A.V.; Toma, S.L.; Bejinariu, C. The galvanic corrosion behavior of phosphated carbon steel used at carabiners manufacturing. Revista de Chimie 2019, 70, 215–219. [Google Scholar] [CrossRef]
- Nejneru, C.; Perju, M.C.; Nergis, D.D.B.; Sandu, A.V.; Bejinariu, C. Galvanic Corrosion Behaviour of Phosphate Nodular Cast Iron in Different Types of Residual Waters and Couplings. Revista de Chimie 2019, 70, 3597–3602. [Google Scholar] [CrossRef]
- Cottis, R.; Turgoose, S. Electrochemical Impedance and Noise; Syrett, B.C., Ed.; NACE International: Houston, TX, USA, 1999; ISBN 157-590-0939. [Google Scholar]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J. Graphical analysis of electrochemical impedance spectroscopy data in Bode and Nyquist representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Teixeira, C.H.S.B.; Alvarenga, E.A.; Vasconcelos, W.L.; Lins, V.F.C. Effect of porosity of phosphate coating on corrosion resistance of galvanized and phosphated steels Part I: Measurement of porosity of phosphate. Mater. Corros. 2011, 62, 771–777. [Google Scholar] [CrossRef]
- Itagaki, M.; Taya, A.; Watanabe, K.; Noda, K. Deviations of capacitive and inductive loops in the electrochemical impedance of a dissolving iron electrode. Anal. Sci. 2002, 18, 641–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Zhang, J. Introduction of Electrochemical Impedance Spectroscopy; Science Press of China: Beijing, China, 2002; ISBN 7-03-009854-4. [Google Scholar]
- Chen, M.; Du, C.Y.; Yin, G.P.; Shi, P.F.; Zhao, T.S. Numerical analysis of the electrochemical impedance spectra of the cathode of direct methanol fuel cells. Int. J. Hydrogen Energy 2009, 34, 1522–1530. [Google Scholar] [CrossRef]
- Nakayama, S.; Kaji, T.; Notoya, T.; Osakai, T. Mechanistic study of the reduction of copper oxides in alkaline solutions by electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 3493–3499. [Google Scholar] [CrossRef]






| Element | C | Si | Mn | P | Cu | Cr | Fe |
|---|---|---|---|---|---|---|---|
| wt, % | 0.45 | 0.22 | 0.98 | 0.02 | 0.15 | 0.17 | balance |
| Rs, Ω cm2 | CPE | Rct, Ω cm2 | RL, Ω cm2 | L, H cm2 | 103.χ2 | εz * | ||
|---|---|---|---|---|---|---|---|---|
| Q, Ssn/cm2 | n | |||||||
| 39.6 | 3.418 × 10−4 | 0.780 | 588 | 1007 | 496 | 5.92 | 6.34 | |
| εEC**, % | 1.22 | 5.6 | 1.59 | 3.95 | 5.4 | 2.87 | - | - |
| System | Rs, Ω·cm2 | Cext, µF/cm2 | Rext, Ω·cm2 | CPE | Rct, Ω·cm2 | 103χ2 | εZ | |
|---|---|---|---|---|---|---|---|---|
| Q S·sn/cm2 | n | |||||||
| C45/RW | 1386 | 2.54 × 10−3 | 1014 | 1.00 × 10−4 | 0.65 | 3596 | 0.932 | 3.05 |
| I-Zn/BSW | 36.3 | 0.106 | 30.82 | 6.20 × 10−5 | 0.51 | 1982 | 5.77 | 7.59 |
| II-Zn/Fe/BSW | 40.4 | 2.870 | 11.04 | 2.51 × 10−4 | 0.61 | 883 | 0.694 | 2.44 |
| III-Mn/BSW | 37.9 | 4.46 | 2.76 | 5.31 × 10−2 | 0.73 | 463 | 0.109 | 1.02 |
| OPS/RW | 1969 | 2.06 × 10−6 | 1393 | 1.48 × 10−5 | 0.49 | 2.19 × 104 | 1.89 | 4.68 |
| System | Rs, Ω cm2 | CPE 1 | Rext Ω cm2 | CPE 2 | Rct Ω cm2 | 103χ2 | εZ | ||
|---|---|---|---|---|---|---|---|---|---|
| Q1 Ssn/cm2 | n1 | Q2 Ssn/cm2 | n2 | ||||||
| I-Zn/RW | 2913 | 7.78 × 10−10 | 0.90 | 4894 | 9.53 × 10−6 | 0.52 | 3.91 × 104 | 0.25 | 1.57 |
| II-Zn/Fe/RW | 2328 | 1.63 × 10−9 | 0.95 | 1987 | 1.58 × 10−4 | 0.68 | 1858 | 0.21 | 1.44 |
| III-Mn/RW | 2159 | 2.45 × 10−9 | 0.95 | 1507 | 1.51 × 10−3 | 0.72 | 1357 | 5.52 | 7.5 |
| I-Zn/FES | 4.59 | 1.14 × 10−3 | 0.79 | 115 | 7.22 × 10−3 | 0.64 | 326 | 0.88 | 2.97 |
| II-Zn/Fe/FES | 4.64 | 3.80 × 10−4 | 0.63 | 283 | 2.03 × 10−3 | 0.53 | 989 | 2.26 | 4.75 |
| III-Mn/FES | 4.96 | 3.47 × 10−2 | 0.80 | 130 | 3.62 × 10−2 | 0.78 | 2.97 | 0.21 | 1.46 |
| System | Rs, Ω cm2 | Cext µF/cm2 | Rext Ω cm2 | CPE | Rct Ω cm2 | W S s½/c | 103χ2 | εZ | |
|---|---|---|---|---|---|---|---|---|---|
| Q S.sn/cm2 | n | ||||||||
| C45/FES | 4.64 | 1.71 | 6.42 | 1.43 × 10−3 | 0.65 | 1544 | 0.277 | 0.55 | 2.3 |
| System | Rs, Ω cm2 | CPE 1 | Rext, Ω cm2 | CPE 2 | Rct Ω cm2 | W Ss½/cm2 | 103χ2 | εZ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 Ssn/cm2 | n1 | Q2 Ssn/cm2 | n2 | |||||||
| OPS/RW | 28.83 | 3.16 × 10−5 | 0.54 | 4753 | 5.44 × 10−5 | 0.73 | 3893 | 5.00 × 10−4 | 7.8 | 8.8 |
| OPS/FES | 6.69 | 6.95 × 10−5 | 0.71 | 271 | 3.63 × 10−5 | 0.70 | 4246 | 8.30 × 10−4 | 8.8 | 9.4 |
| System | Rs Ω·cm2 | Cext F/cm2 | Rext Ω·cm2 | CPE | Rin Ω·cm2 | Cdl F/cm2 | Rct Ω·cm2 | 103χ2 | εZ | |
|---|---|---|---|---|---|---|---|---|---|---|
| Q S·sn/cm2 | n | |||||||||
| OFV/RW | 1499 | 0.73 × 10−12 | 4709 | 3.83 × 10−4 | 0.59 | 521 | 161.7 | 5208 | 0.86 | 2.95 |
| OFV/BSW | 107.1 | 0.175 | 19.12 | 6.51 × 10−4 | 0.63 | 16.5 | 20.4 | 1179 | 0.58 | 2.41 |
| OFV/FES | 16.8 | 52.8 | 119 | 1.80 × 10−4 | 0.76 | 576 | 175 | 1604 | 0.47 | 2.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burduhos-Nergis, D.-P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Evaluation of the Corrosion Resistance of Phosphate Coatings Deposited on the Surface of the Carbon Steel Used for Carabiners Manufacturing. Appl. Sci. 2020, 10, 2753. https://doi.org/10.3390/app10082753
Burduhos-Nergis D-P, Vizureanu P, Sandu AV, Bejinariu C. Evaluation of the Corrosion Resistance of Phosphate Coatings Deposited on the Surface of the Carbon Steel Used for Carabiners Manufacturing. Applied Sciences. 2020; 10(8):2753. https://doi.org/10.3390/app10082753
Chicago/Turabian StyleBurduhos-Nergis, Diana-Petronela, Petrica Vizureanu, Andrei Victor Sandu, and Costica Bejinariu. 2020. "Evaluation of the Corrosion Resistance of Phosphate Coatings Deposited on the Surface of the Carbon Steel Used for Carabiners Manufacturing" Applied Sciences 10, no. 8: 2753. https://doi.org/10.3390/app10082753








