Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review
Abstract
1. Introduction
2. Methodologies for the Synthesis and Characterization of Hyaluronic Acid–Catechol
3. General Properties of Hyaluronic Acid–Catechol
3.1. Biocompatibility
3.2. Tissue Adhesive Properties
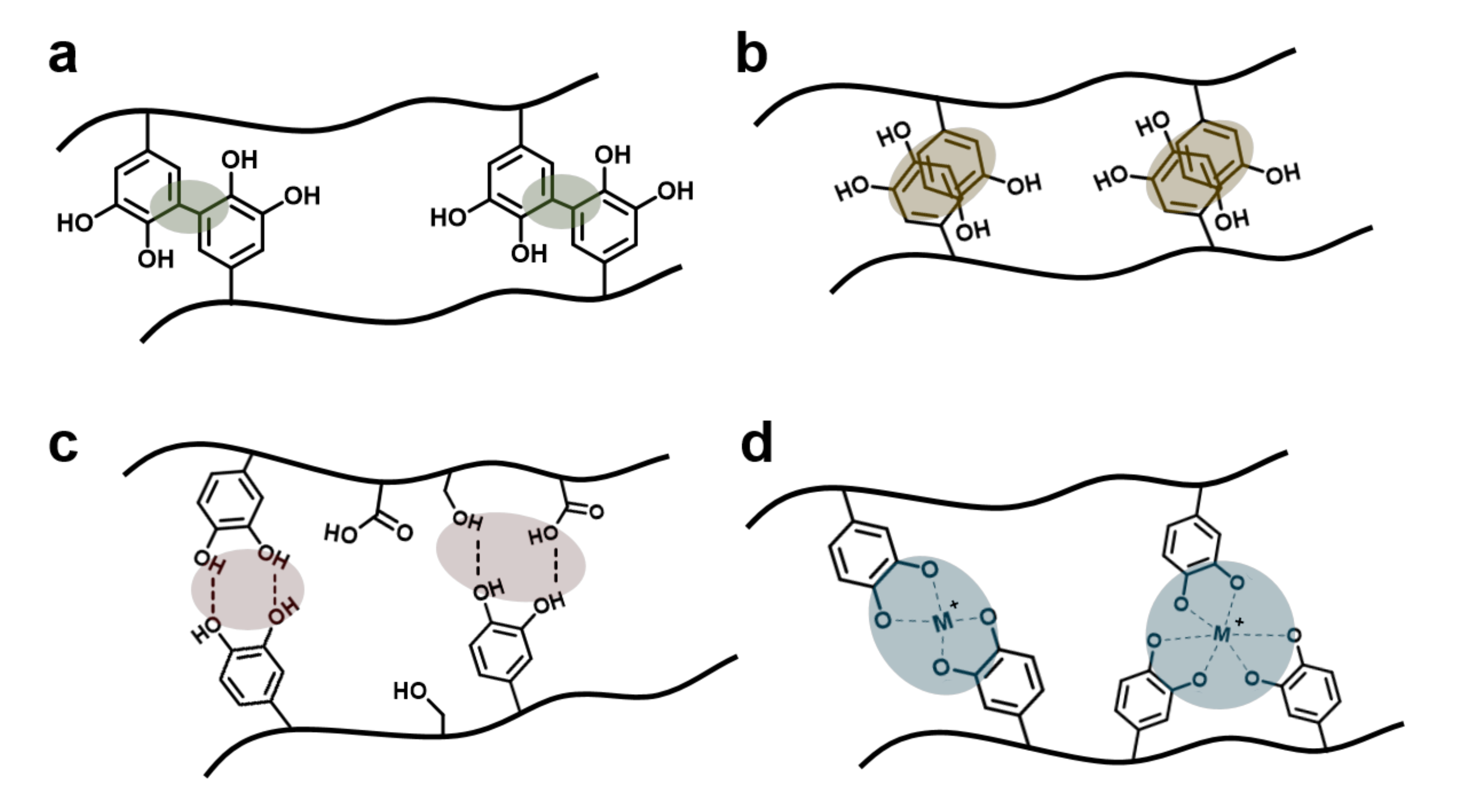
3.3. Catechol-Involving Reactions
4. Biomedical Applications
4.1. Antifouling Materials
4.2. Wound-Healing Materials
4.3. Drug/Gene Delivery Carriers
4.4. Tissue Engineering Scaffolds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moon, M.J.; Poilil Surendran, S.; Jeong, Y.Y. Biomedical applications of hyaluronic acid-based nanomaterials in hyperthermic cancer therapy. Pharmaceutics 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef]
- Monheit, G.D.; Coleman, K.M. Hyaluronic acid fillers. Dermatol. Ther. 2006, 19, 141–150. [Google Scholar] [CrossRef]
- Yeo, Y.; Highley, C.B.; Bellas, E.; Ito, T.; Marini, R.; Langer, R.; Kohane, D.S. In Situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials 2006, 27, 4698–4705. [Google Scholar] [CrossRef]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films new biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Lee, E.A.; Yoon, J.J.; Park, T.G. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 2005, 26, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Swann, D.A.; Radin, E.L.; Nazimiec, M.; Weisser, P.A.; Curran, N.; Lewinnek, G. Role of hyaluronic acid in joint lubrication. Ann. Rheum. Dis. 1974, 33, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Meyer, K. The biological significance of hyaluronic acid and hyaluronidase. Phys. Rev. 1947, 27, 335–359. [Google Scholar] [CrossRef]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L. Polyphenolic substance of Mytilus edulis: Novel adhesive containing L-dopa and hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Waite, J.H. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Hwang, J.; Deming, T.J. Role of L-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, H. Marine mussel adhesion and bio-inspired wet adhesives. Biotribology 2016, 5, 44–51. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Jho, Y.; Hwang, D.S. Cation-pi interactions and their contribution to mussel underwater adhesion studied using a surface forces apparatus: A mini-review. Langmuir ACS J. Surf. Colloids 2019, 35, 16002–16012. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Newland, B.; Liu, W.; Wang, W.; Wang, W. Catechol functionalized hyperbranched polymers as biomedical materials. Prog. Polym. Sci. 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Lee, S.B.; Gonzalez-Cabezas, C.; Kim, K.M.; Kim, K.N.; Kuroda, K. Catechol-functionalized synthetic polymer as a dental adhesive to contaminated dentin surface for a composite restoration. Biomacromolecules 2015, 16, 2265–2275. [Google Scholar] [CrossRef]
- Li, G.; Cheng, G.; Xue, H.; Chen, S.; Zhang, F.; Jiang, S. Ultra low fouling zwitterionic polymers with a biomimetic adhesive group. Biomaterials 2008, 29, 4592–4597. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, H.J.; Kim, K.; Yoon, G.; Wang, Y.; Choi, G.-S.; Lee, H.; Park, J.S. Multipurpose intraperitoneal adhesive patches. Adv. Funct. Mater. 2019, 29, 1900495. [Google Scholar] [CrossRef]
- Shin, M.; Ryu, J.H.; Kim, K.; Kim, M.J.; Jo, S.; Lee, M.S.; Lee, D.Y.; Lee, H. Hemostatic swabs containing polydopamine-like catecholamine chitosan-catechol for normal and coagulopathic animal models. ACS Biomater. Sci. Eng. 2018, 4, 2314–2318. [Google Scholar] [CrossRef]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.-W.; Lee, H. Hyaluronic acid-catechol: A biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Lih, E.; Choi, S.G.; Ahn, D.J.; Joung, Y.K.; Han, D.K. Optimal conjugation of catechol group onto hyaluronic acid in coronary stent substrate coating for the prevention of restenosis. J. Tissue Eng. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Zhou, Y.; Kang, L.; Yue, Z.; Liu, X.; Wallace, G.G. Composite tissue adhesive containing catechol-modified hyaluronic acid and poly-l-lysine. ACS Appl. Bio Mater. 2020, 3, 628–638. [Google Scholar] [CrossRef]
- Ye, H.; Xia, Y.; Liu, Z.; Huang, R.; Su, R.; Qi, W.; Wang, L.; He, Z. Dopamine-assisted deposition and zwitteration of hyaluronic acid for the nanoscale fabrication of low-fouling surfaces. J. Mater. Chem. B 2016, 4, 4084–4091. [Google Scholar] [CrossRef]
- Lee, J.E.; Yin, Y.; Lim, S.Y.; Kim, E.S.; Jung, J.; Kim, D.; Park, J.W.; Lee, M.S.; Jeong, J.H. Enhanced transfection of human mesenchymal stem cells using a hyaluronic acid/calcium phosphate hybrid gene delivery system. Polymers 2019, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.I.; Cibrao, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small 2014, 10, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chang, K.; Kim, S.; Gite, V.; Chung, H.; Sohn, D. Phase controllable hyaluronic acid hydrogel with iron(III) Ion–catechol induced dual cross-linking by utilizing the gap of gelation kinetics. Macromolecules 2016, 49, 7450–7459. [Google Scholar] [CrossRef]
- Lee, S.W.; Ryu, J.H.; Do, M.J.; Namkoong, E.; Lee, H.; Park, K. NiCHE Platform: Nature-inspired catechol-conjugated hyaluronic acid environment platform for salivary gland tissue engineering. ACS Appl. Mater. Interfaces 2020, 12, 4285–4294. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.E.; Byun, E.; Kim, N.W.; Lee, K.; Lee, H.; Sim, S.J.; Lee, D.S.; Jeong, J.H. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J. Control. Release 2014, 192, 122–130. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.-H.; Chung, H.-M.; et al. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Hong, S.H.; Ryu, J.H.; Lee, H. Effect of charge on In Vivo adhesion stability of catechol-conjugated polysaccharides. J. Ind. Eng. Chem. 2019, 79, 425–430. [Google Scholar] [CrossRef]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.-H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol–gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Clotrimazole nanosuspensions-loaded hyaluronic acid-catechol/polyvinyl alcohol mucoadhesive films for oral candidiasis treatment. J. Drug Deliv. Sci. Technol. 2020, 60, 101927. [Google Scholar] [CrossRef]
- Hu, X.; Neoh, K.G.; Shi, Z.; Kang, E.T.; Poh, C.; Wang, W. An In Vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials 2010, 31, 8854–8863. [Google Scholar] [CrossRef]
- Park, H.J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.W. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wei, Z.; Xu, X.; Feng, Q.; Xu, J.; Bian, L. Efficient catechol functionalization of biopolymeric hydrogels for effective multiscale bioadhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109835. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.; Lee, H. Enhanced loading efficiency and sustained release of doxorubicin from hyaluronic acid/graphene oxide composite hydrogels by a mussel-inspired catecholamine. J. Nanosci. Nanotechnol. 2014, 14, 7395–7401. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.W. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef]
- Li, X.; Cui, T.; Zhang, W.; Zhai, Z.; Wu, F.; Zhang, Y.; Yang, M.; Zhong, W.; Yue, W. Dopamine-functionalized hyaluronic acid microspheres for effective capture of CD44-overexpressing circulating tumor cells. Colloids Surf. B Biointerfaces 2020, 196, 111281. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, S.; Oh, I.; Kato, S.; Kosuge, T.; Sokolova, A.V.; Lee, J.; Otsuka, H.; Sohn, D. Internal structure of hyaluronic acid hydrogels controlled by iron(III) ion–catechol complexation. Macromolecules 2019, 52, 6502–6513. [Google Scholar] [CrossRef]
- Wang, D.; Xu, P.; Wang, S.; Li, W.; Liu, W. Rapidly curable hyaluronic acid-catechol hydrogels inspired by scallops as tissue adhesives for hemostasis and wound healing. Eur. Polym. J. 2020, 134, 109763. [Google Scholar] [CrossRef]
- Guo, Z.; Mi, S.; Sun, W. The multifaceted nature of catechol chemistry: Bioinspired pH-initiated hyaluronic acid hydrogels with tunable cohesive and adhesive properties. J. Mater. Chem. B 2018, 6, 6234–6244. [Google Scholar] [CrossRef]
- Guo, Z.; Mi, S.; Sun, W. A facile strategy for preparing tough, self-healing double-network hyaluronic acid hydrogels inspired by mussel cuticles. Macromol. Mater. Eng. 2019, 304, 1800715. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, J.; Lee, J.Y. Universal surface modification using dopamine-hyaluronic acid conjugates for anti-biofouling. Int. J. Biol. Macromol. 2020, 151, 1314–1321. [Google Scholar] [CrossRef]
- Kim, T.G.; Lee, Y.; Park, T.G. Controlled gene-eluting metal stent fabricated by bio-inspired surface modification with hyaluronic acid and deposition of DNA/PEI polyplexes. Int. J. Pharm. 2010, 384, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hou, J.; Yuan, Q.; Xin, P.; Cheng, H.; Gu, Z.; Wu, J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2020, 392, 123775. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B Biointerfaces 2020, 196, 111279. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, H.; Messersmith, P.B.; Park, T.G. A bioinspired polymeric template for 1D assembly of metallic nanoparticles, semiconductor quantum dots, and magnetic nanoparticles. Macromol. Rapid. Commun. 2010, 31, 2109–2114. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, K.C.; Ko, J.; Yoo, B.; Shin, J.; Lee, S.J.; Sohn, D. Hollow hyaluronic acid particles by competition between adhesive and cohesive properties of catechol for anticancer drug carrier. Carbohydr. Polym. 2017, 164, 309–316. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Suo, A.; Wang, Y.; Wang, J.; Sun, T.; Yang, M.; Wan, X.; Yao, Y. Hyaluronic acid-functionalized gold nanorods with pH/NIR dual-responsive drug release for synergetic rargeted photothermal chemotherapy of breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 36533–36547. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yuan, X.; Cui, Z.; Yang, X. Fabrication of dopamine-modified hyaluronic acid/chitosan multilayers on titanium alloy by layer-by-layer self-assembly for promoting osteoblast growth. Appl. Surf. Sci. 2013, 284, 732–737. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.; Ren, J.; Qu, X. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and adhesive properties of multilayered coatings based on catechol-functionalized chitosan/hyaluronic acid and bioactive glass nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef]
- Joo, H.; Byun, E.; Lee, M.; Hong, Y.; Lee, H.; Kim, P. Biofunctionalization via flow shear stress resistant adhesive polysaccharide, hyaluronic acid-catechol, for enhanced in vitro endothelialization. J. Ind. Eng. Chem. 2016, 34, 14–20. [Google Scholar] [CrossRef]
- Oh, Y.J.; Cho, I.H.; Lee, H.; Park, K.J.; Lee, H.; Park, S.Y. Bio-inspired catechol chemistry: A new way to develop a re-moldable and injectable coacervate hydrogel. Chem. Commun. 2012, 48, 11895–11897. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Aoyagi, T.; Ebara, M.; Auzely-Velty, R. Catechol-modified hyaluronic acid in situ-forming hydrogels by auto-oxidation of catechol or photo-oxidation using visible light. Polym. Bull. 2017, 74, 4069–4085. [Google Scholar] [CrossRef]
- Lee, J.; Yang, S.H.; Hong, S.P.; Hong, D.; Lee, H.; Lee, H.Y.; Kim, Y.G.; Choi, I.S. Chemical control of yeast cell division by cross-linked shells of catechol-grafted polyelectrolyte multilayers. Macromol. Rapid. Commun. 2013, 34, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-D.; Deng, X.; Wang, Y.-F.; Wang, X.-T.; Zhang, X.; Chen, L.-L.; Cao, X.; Zhang, Y.-Z.; Zhang, C.-Y.; Zheng, X.; et al. Layer-by-layer coating of polyvinylamine and dopamine-modified hyaluronic acid inhibits the growth of bacteria and tumor cell lines on the surface of materials. Appl. Surf. Sci. 2020, 530, 147197. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Pires, R.A.; Reis, R.L.; Alves, N.M. Layer-by-layer films based on catechol-modified polysaccharides produced by dip- and spin-coating onto different substrates. J. Biomed. Mater. Res. B 2020, 108, 1412–1427. [Google Scholar] [CrossRef]
- Song, I.T.; Lee, M.; Lee, H.; Han, J.; Jang, J.H.; Lee, M.S.; Koh, G.Y.; Lee, H. PEGylation and HAylation via catechol: Alpha-Amine-specific reaction at N-terminus of peptides and proteins. Acta Biomater. 2016, 43, 50–60. [Google Scholar] [CrossRef]
- Lu, B.; Luo, D.; Zhao, A.; Wang, H.; Zhao, Y.; Maitz, M.F.; Yang, P.; Huang, N. pH responsive chitosan and hyaluronic acid layer by layer film for drug delivery applications. Prog. Org. Coat. 2019, 135, 240–247. [Google Scholar] [CrossRef]
- Kang, E.B.; Lee, G.B.; In, I.; Park, S.Y. pH-sensitive fluorescent hyaluronic acid nanogels for tumor-targeting and controlled delivery of doxorubicin and nitric oxide. Eur. Polym. J. 2018, 101, 96–104. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.; Kim, J.H.; Cho, J.H.; Jin, Y.; Kim, S.; Min, S.; Kim, S.K.; Choi, D.; Cho, S.W. Tissue tapes - Phenolic hyaluronic acid hydrogel patches for off-the-shelf therapy. Adv. Funct. Mater. 2019, 29, 1903863. [Google Scholar] [CrossRef]
- Abdullah Al, N.; Lee, J.E.; In, I.; Lee, H.; Lee, K.D.; Jeong, J.H.; Park, S.Y. Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol. Pharm. 2013, 10, 3736–3744. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.; Kim, Y.B.; Kim, J.; Hyeon, T.; Park, H.; Messersmith, P.B.; Park, T.G. Bioinspired surface immobilization of hyaluronic acid on monodisperse magnetite nanocrystals for targeted cancer imaging. Adv. Mater. 2008, 20, 4154–4157. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic bonds between boronic acid and alginate: Hydrogels with stretchable, self-healing, stimuli-responsive, remoldable, and adhesive properties. Biomacromolecules 2018, 19, 2053–2061. [Google Scholar] [CrossRef]
- Hong, S.H.; Shin, M.; Park, E.; Ryu, J.H.; Burdick, J.A.; Lee, H. Alginate-boronic acid: pH-triggered bioinspired glue for hydrogel assembly. Adv. Funct. Mater. 2019, 30, 1908497. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Ryu, J.H.; Kim, K.; Han, Y.M.; Lee, H. Long-term, feeder-free maintenance of human embryonic stem cells by mussel-inspired adhesive heparin and collagen type I. Acta Biomater. 2016, 32, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.H.; Kim, J.; Lee, I.; Kim, S.; Jeong, Y.K.; Hong, S.; Ryu, J.H.; Kim, T.S.; Park, J.K.; Lee, H.; et al. Mussel-inspired adhesive binders for high-performance silicon nanoparticle anodes in lithium-ion batteries. Adv. Mater. 2013, 25, 1571–1576. [Google Scholar] [CrossRef]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef]
- Jooybar, E.; Abdekhodaie, M.J.; Alvi, M.; Mousavi, A.; Karperien, M.; Dijkstra, P.J. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta. Biomater. 2019, 83, 233–244. [Google Scholar] [CrossRef]
- Yuan, J.; Maturavongsadit, P.; Metavarayuth, K.; Luckanagul, J.A.; Wang, Q. Enhanced bone defect repair by polymeric substitute fillers of multiarm polyethylene glycol-crosslinked hyaluronic acid hydrogels. Macromol. Biosci. 2019, 19, e1900021. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecularmodes of toxic action in cells, from microorganismsto mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Wilker, J.J. The iron-fortified adhesive system of marine mussels. Angew. Chem. Int. Ed. 2010, 49, 8076–8078. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.G.; Fullenkamp, D.E.; He, L.; Holten-Andersen, N.; Lee, K.Y.; Messersmith, P.B. pH-Based regulation of hydrogel mechanical properties through mussel-inspired chemistry and processing. Adv. Funct. Mater. 2013, 23, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 2010, 107, 12850–12853. [Google Scholar] [CrossRef] [PubMed]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angew. Chem. Int. Ed. 2004, 43, 448–450. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, C.; Ryu, J.H. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2021, 11, 21. https://doi.org/10.3390/app11010021
Kim J, Lee C, Ryu JH. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Applied Sciences. 2021; 11(1):21. https://doi.org/10.3390/app11010021
Chicago/Turabian StyleKim, Jongho, Chaemyeong Lee, and Ji Hyun Ryu. 2021. "Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review" Applied Sciences 11, no. 1: 21. https://doi.org/10.3390/app11010021
APA StyleKim, J., Lee, C., & Ryu, J. H. (2021). Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Applied Sciences, 11(1), 21. https://doi.org/10.3390/app11010021






