Behavior of Astrocytes Derived from Human Neural Stem Cells Flown onto Space and Their Progenies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
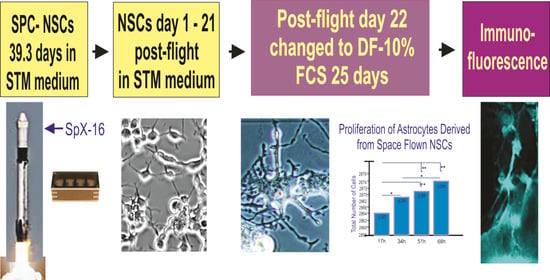
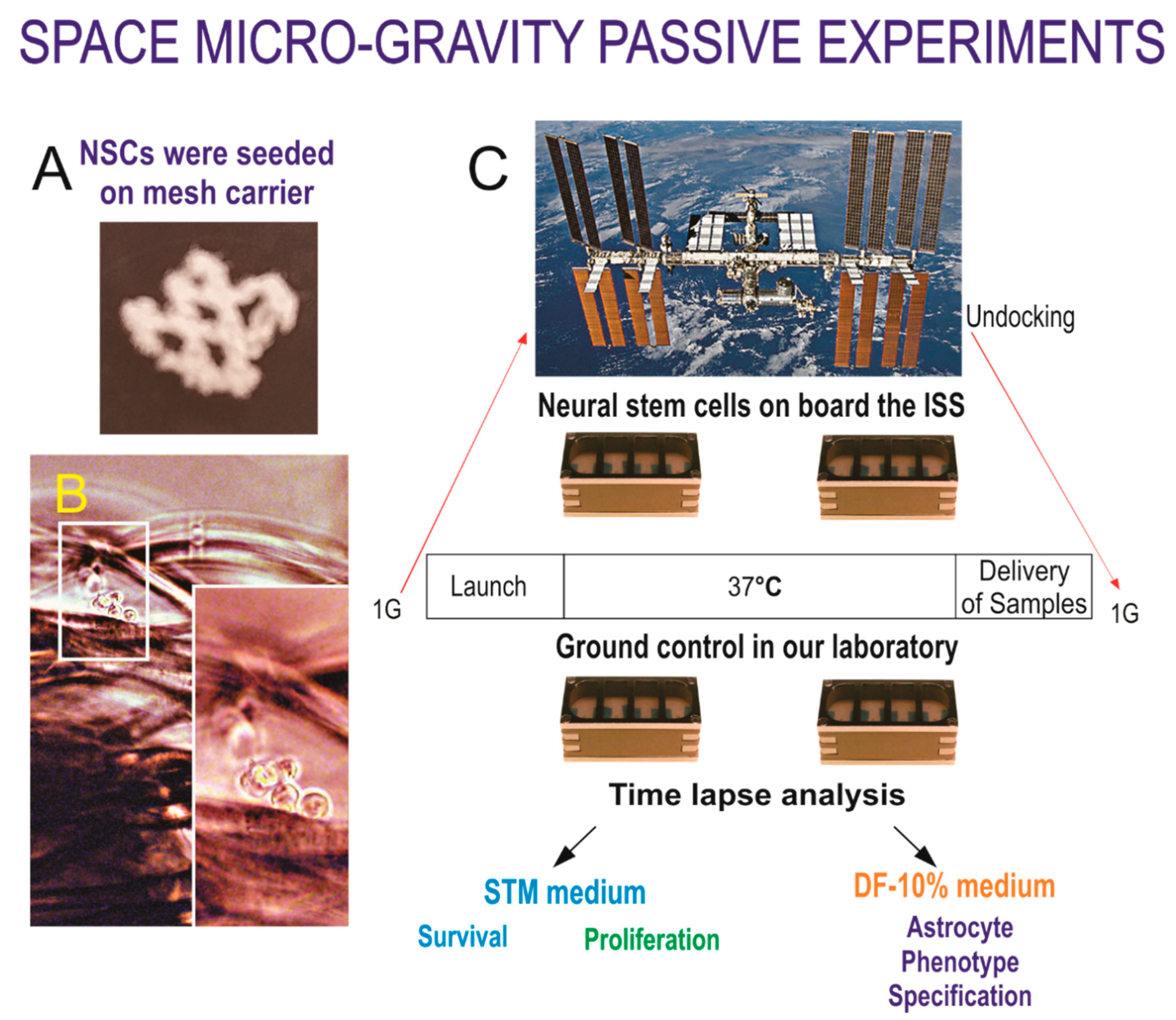
2.2. Space Flight
2.3. Recovery of the Hardware and Harvesting of Samples
2.4. Time-Lapse Microscopy
2.5. Immunofluorescence
3. Results
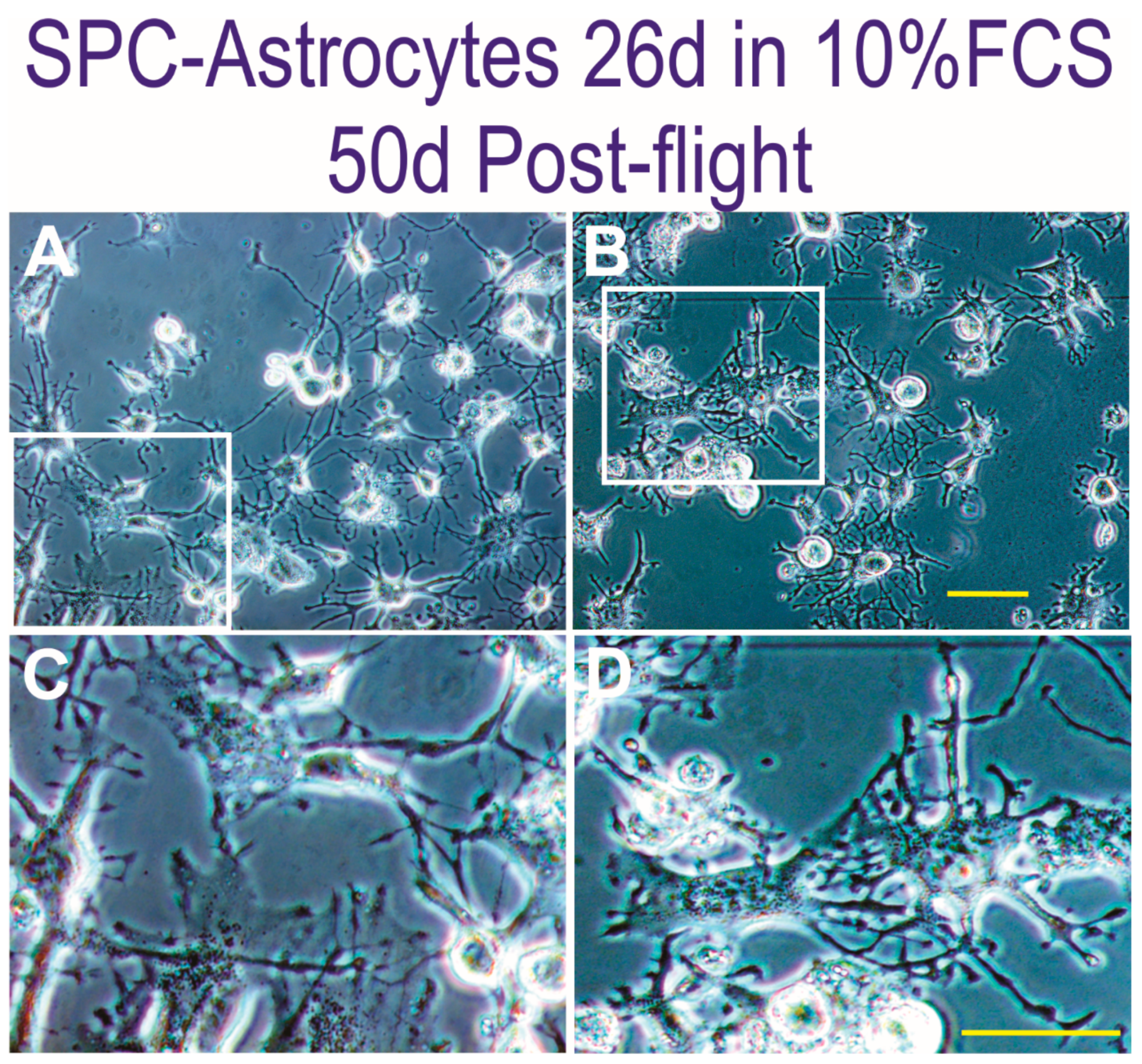
3.1. Space Flown NSCs Give Rise to Astrocytes
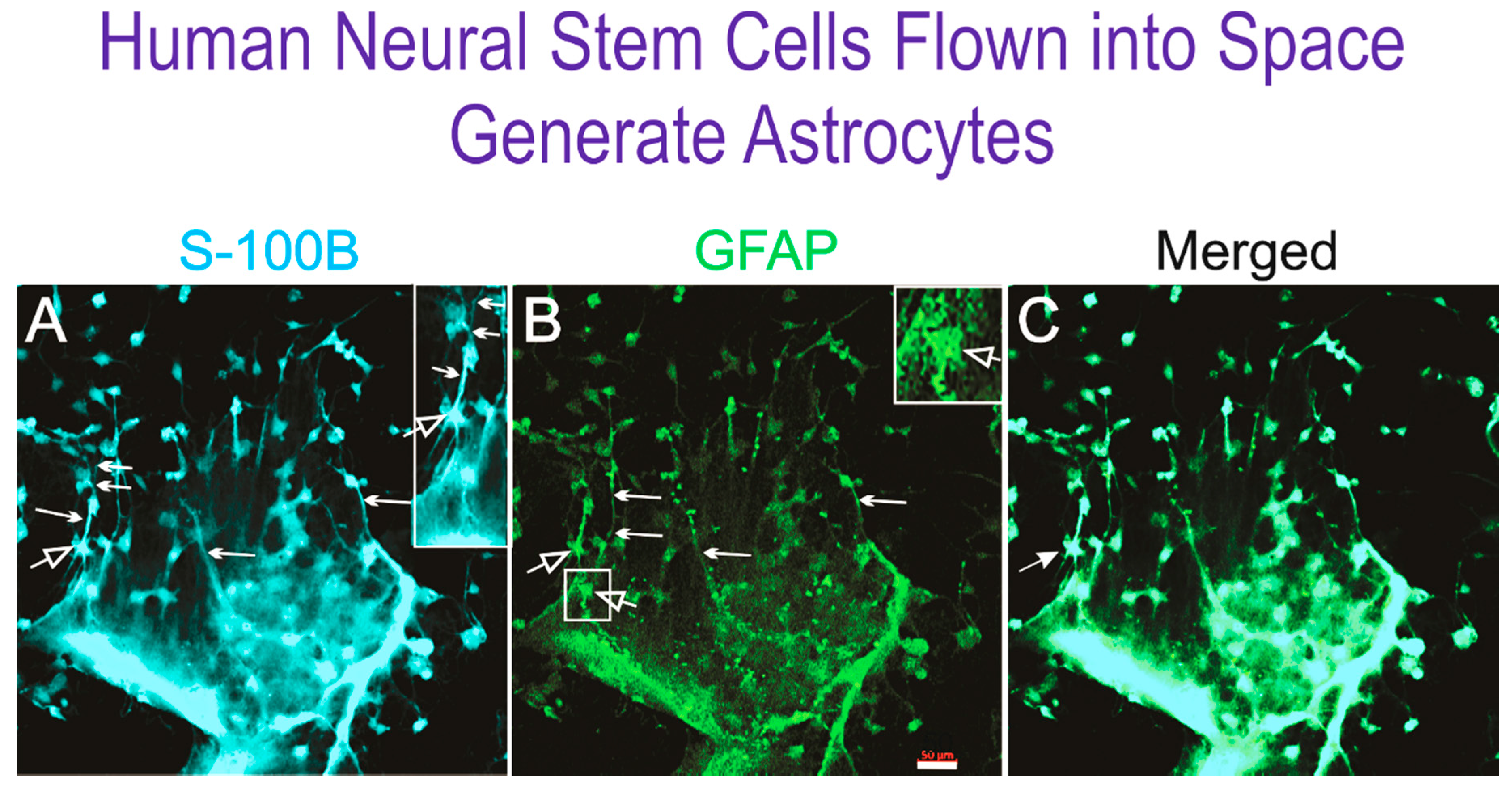
3.2. Immunofluorescent Detection of Astrocyte Markers
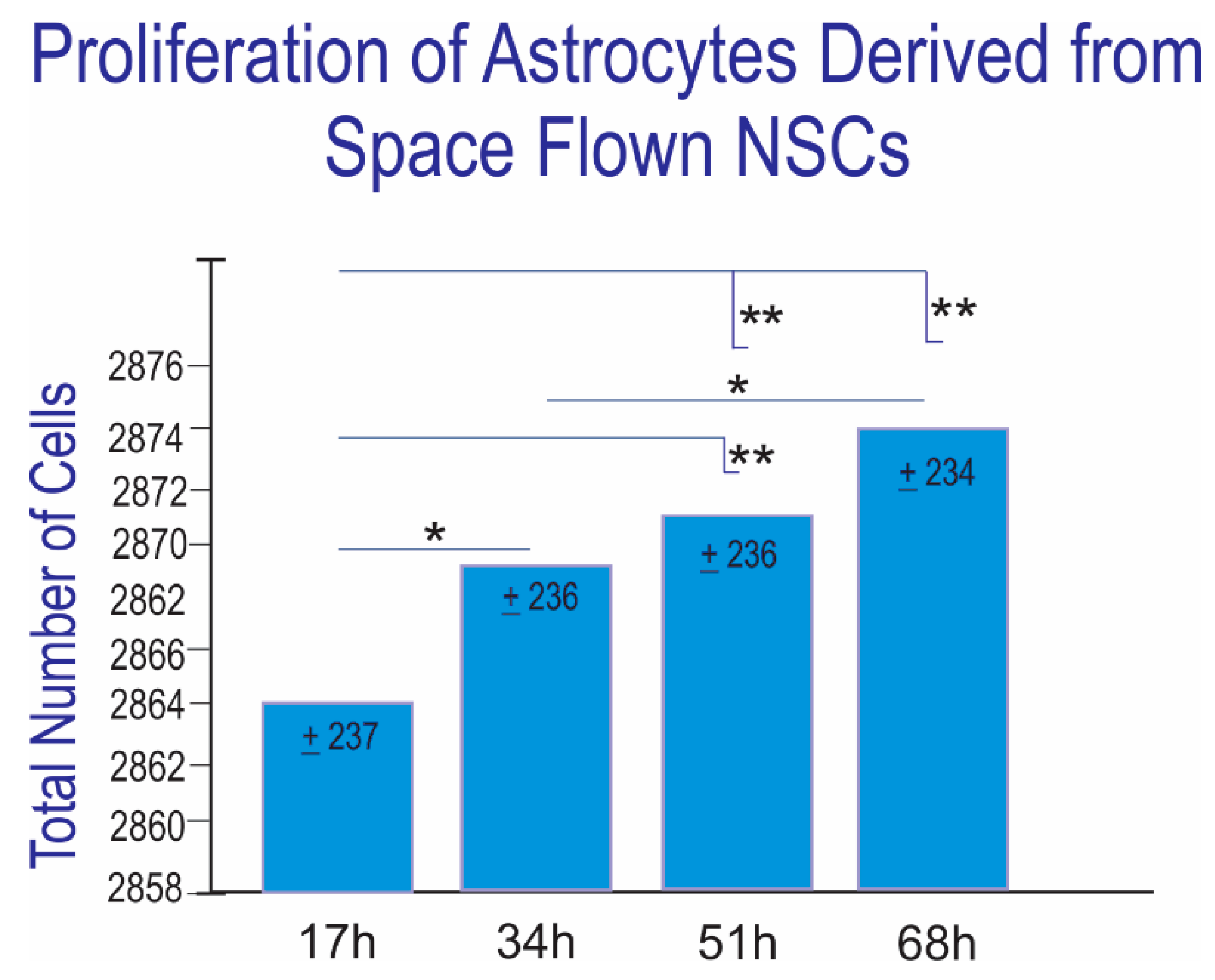
3.3. Proliferation of Newly Formed Astrocytes Derived from NSCs Flown to Space
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kononikhin, A.S.; Starodubtseva, N.L.; Pastushkova, L.K.; Kashirina, D.N.; Fedorchenko, K.Y.; Brhozovsky, A.G.; Popov, I.A.; Larina, I.M.; Nikolaev, E.N. Spaceflight induced changes in the human proteome. Expert Rev. Proteom. 2017, 14, 15–29. [Google Scholar] [CrossRef]
- Espinosa-Jeffrey, A.; Paez, P.M.; Cheli, V.T.; Spreuer, V.; Wanner, I.; de Vellis, J. Impact of simulated microgravity on oligodendrocyte development: Implications for central nervous system repair. PLoS ONE 2013, 8, e76963. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, C.; Vergnes, L.; Carpo, N.; Schibler, M.J.; Bentolila, L.A.; Karouia, F.; Espinosa-Jeffrey, A. Human Neural Stem Cells Flown into Space Proliferate and Generate Young Neurons. Appl. Sci. 2019, 9, 4042. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, C.; Barres, B.A. Regulation of synaptic connectivity by glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef]
- Freeman, M.R.; Rowitch, D.H. Evolving concepts of gliogenesis: A look way back and ahead to the next 25 years. Neuron 2013, 80, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Sung, K.; Jimenez-Sanchez, M. Autophagy in Astrocytes and its Implications in Neurodegeneration. J. Mol. Biol. 2020, 432, 2605–2621. [Google Scholar] [CrossRef]
- Seifert, G.; Schilling, K.; Steinhäuser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef] [Green Version]
- Shaka, S.; Carpo, N.; Tran, V.; Karouia, F.; Espinosa-Jeffrey, A. Human Neural Stem Cells Proliferate More While in Space, Have a Shorter Cell Cycle and are Larger than Ground Control Cells After Space Flight: Implications for Long-term Space Travel. Special Issue “Microgravity and Space Medicine. Int. J. Mol. Sci. 2020. submitted. [Google Scholar]
- Espinosa-Jeffrey, A.; Becker-Catania, S.; Zhao, P.M.; Cole, R.; de Vellis, J. Phenotype Specification and Development of Oligodendrocytes and Neurons from Rat Stem Cell Cultures Using two Chemically Defined Media. Special Issue on Stem cells. J. Neurosci. Res. 2002, 69, 810–825. [Google Scholar] [CrossRef]
- Espinosa de los Monteros, A.; Zhao, A.P.; de Vellis, J. Transplantation of oligodendrocyte progenitors and CG4 cells into the developing rat brain: Differences and similarities. In Cellular and Molecular Mechanisms of Regeneration and Functional Repair in the CNS; Jeserich, G., Althaus, H., Richter-Landsberg, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Guizzetti, M.; Kavanagh, T.J.; Costa, L.G. Measurements of Astrocyte Proliferation. Methods Mol Biol. 2011, 758, 349–359. [Google Scholar] [CrossRef]
- Boghani, Z.; Steele, W.J.; Cykowski, M.D.; Ballester, L.Y.; Britz, G. Creutzfeldt Cell Rich Glioblastoma: A Diagnostic Dilemma. Cureus 2017, 9, e1749. [Google Scholar] [CrossRef] [Green Version]
- Kettenmann, H.; Ransom, B.R. Neuroglia. xix; Oxford University Press: New York, NY, USA, 2005; p. 601. [Google Scholar]
- Ndubaku, U.; de Bellard, M.E. Glial cells: Old cells with new twists. Acta Histochem. 2008, 110, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Cassé, F.; Richetin, K.; Toni, N. Astrocytes’ Contribution to Adult Neurogenesis in Physiology and Alzheimer’s Disease. Front. Cell Neurosci. 2018, 12, 432. [Google Scholar] [CrossRef] [Green Version]
- Filous, A.R.; Silver, J. Targeting astrocytes in CNS injury and disease: A translational research approach. Prog. Neurobiol. 2016, 144, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Colodner, K.J.; Montana, R.A.; Anthony, D.C.; Folkerth, R.D.; De Girolami, U.; Feany, M.B. Proliferative potential of human astrocytes. J. Neuropathol. Exp. Neurol. 2005, 64, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Liu, X.; Sampson, J.H.; Bigner, D.D.; Li, C.Y. Rapid Reprogramming of Primary Human Astrocytes into Potent Tumor-Initiating Cells with Defined Genetic Factors. Cancer Res. 2016, 76, 5143–5150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izrael, M.; Slutsky, S.G.; Admoni, T.; Cohen, L.; Granit, A.; Hasson, A.; Itskovitz-Eldor, J.; Krush Paker, L.; Kuperstein, G.; Lavon, N.; et al. Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1G93A and NSG animal models. Stem Cell Res. Ther. 2018, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sato, K.; Hishikawa, N.; Kawahara, Y.; Takemoto, M.; Ohta, Y.; Yamashita, T.; Fujii, K.; Kurozumi, K.; Date, I.; et al. A unique case with positive anti-myelin oligodendrocyte glycoprotein antibody presenting multiple brain lesions. Neurol. Clin. Neurosci. 2020, 8, 92–95. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaka, S.; Carpo, N.; Tran, V.; Espinosa-Jeffrey, A. Behavior of Astrocytes Derived from Human Neural Stem Cells Flown onto Space and Their Progenies. Appl. Sci. 2021, 11, 41. https://doi.org/10.3390/app11010041
Shaka S, Carpo N, Tran V, Espinosa-Jeffrey A. Behavior of Astrocytes Derived from Human Neural Stem Cells Flown onto Space and Their Progenies. Applied Sciences. 2021; 11(1):41. https://doi.org/10.3390/app11010041
Chicago/Turabian StyleShaka, Sophia, Nicholas Carpo, Victoria Tran, and Araceli Espinosa-Jeffrey. 2021. "Behavior of Astrocytes Derived from Human Neural Stem Cells Flown onto Space and Their Progenies" Applied Sciences 11, no. 1: 41. https://doi.org/10.3390/app11010041
APA StyleShaka, S., Carpo, N., Tran, V., & Espinosa-Jeffrey, A. (2021). Behavior of Astrocytes Derived from Human Neural Stem Cells Flown onto Space and Their Progenies. Applied Sciences, 11(1), 41. https://doi.org/10.3390/app11010041