Contact Melting of Metals Explained via the Theory of Quasi-Liquid Layer
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Funding
Conflicts of Interest
References
- Savvin, V.S.; Mikhaleva, O.V.; Povzner, A.A. Contact Melting Studies of the Phase Composition of the Pb-Bi Diffusion Zone. Inorg. Mater. 2002, 38, 683–687. [Google Scholar] [CrossRef]
- Savvin, V.S.; Aitukaev, A.D. Sintering of Bi–Tl, Bi–In, Bi–Pb, and Hg–In Contacts. Inorg. Mater. 2004, 40, 147–151. [Google Scholar] [CrossRef]
- Savvin, V.S.; Azavi, A.K.; Kadochnikova, A.S.; Povzner, A.A. Investigation of the Phase Composition of the Diffusion Zone of the Bismuth Indium System upon Contact Melting. Phys. Met. Metallogr. 2005, 99, 520–526. [Google Scholar]
- Savvin, V.S.; Mikhaleva, O.V.; Zubova, Y.A. Atomic Diffusion from Liquid to Solid Phase during Contact Melting. Tech. Phys. Lett. 2007, 33, 417–419. [Google Scholar] [CrossRef]
- Savvin, V.S.; Kazachkova, Y.A.; Povzner, A.A. Phase formation in contact of dissimilar metals. J. Phys. Conf. Ser. 2008, 98, 052002. [Google Scholar] [CrossRef] [Green Version]
- Savvin, V.S.; Pomytkina, Y.Y.; Anokhina, N.N. Contact Melting in Simple Eutectic System. EPJ Web Conf. 2011, 15, 01020. [Google Scholar] [CrossRef] [Green Version]
- Savvin, V.S.; Anokhina, N.N.; Povzner, A.A. Processes at the Liquid/Crystal Boundary upon Contact Melting in the System with Intermediate Solid Phases. Phys. Met. Metallogr. 2012, 113, 406–410. [Google Scholar] [CrossRef]
- Savitskii, A.P.; Martsunova, L.S.; Zhdanov, V.V. Contact Melting in Intermetallic Systems. Adgez. Rasplavov Paika Mater. 1977, 2, 55–57. [Google Scholar]
- Zalkin, V.M. Nature of Eutectic Alloys and Contact Melting Effect; Metallurgiya: Moscow, Russia, 1987. [Google Scholar]
- Gufan, A.Y.; Akhkubekov, A.A.; Zubkhadzhiev, M.A.V.; Kumykov, Z.M. Adhesion theory of contact melting. Bull. RAS Phys. 2005, 69, 632–638. [Google Scholar]
- Dash, J.G.; Fu, H.; Wettlaufe, J.S. The premelting of ice and its environmental consequences. Rep. Prog. Phys. 1995, 58, 115–167. [Google Scholar] [CrossRef]
- Doppenscmidt, A.; Butt, H.-J. Measuring the Thickness of the Liquid-like Layer on Ice Surfaces with Atomic Force Microscopy. Langmuir 2000, 16, 6709–6714. [Google Scholar] [CrossRef]
- Sazaki, G.; Zepeda, S.; Nakatsubo, S.; Yokomine, M.; Furukawa, Y. Quasi-liquid layers on ice crystal surfaces are made up of two different phases. Proc. Natl. Acad. Sci. USA 2012, 109, 1052–1055. [Google Scholar] [CrossRef] [Green Version]
- Benet, J.; Llombart, P.; Sanz, E.; MacDowell, L.G. Premelting-Induced Smoothening of the Ice-Vapor Interface. Phys. Rev. Lett. 2016, 117, 096101. [Google Scholar] [CrossRef] [Green Version]
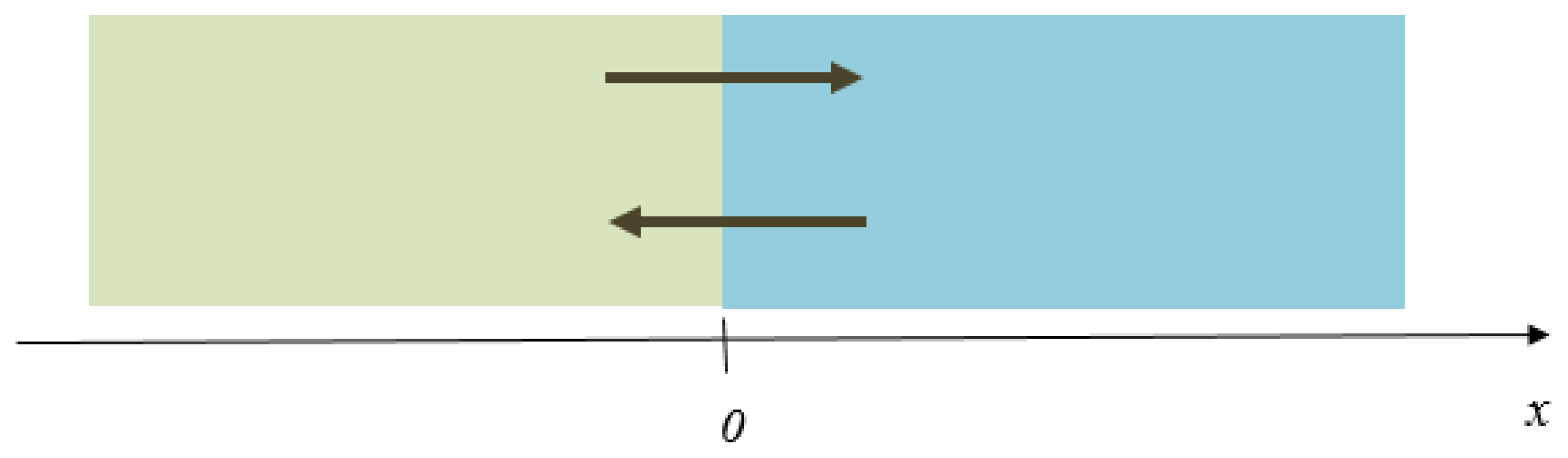
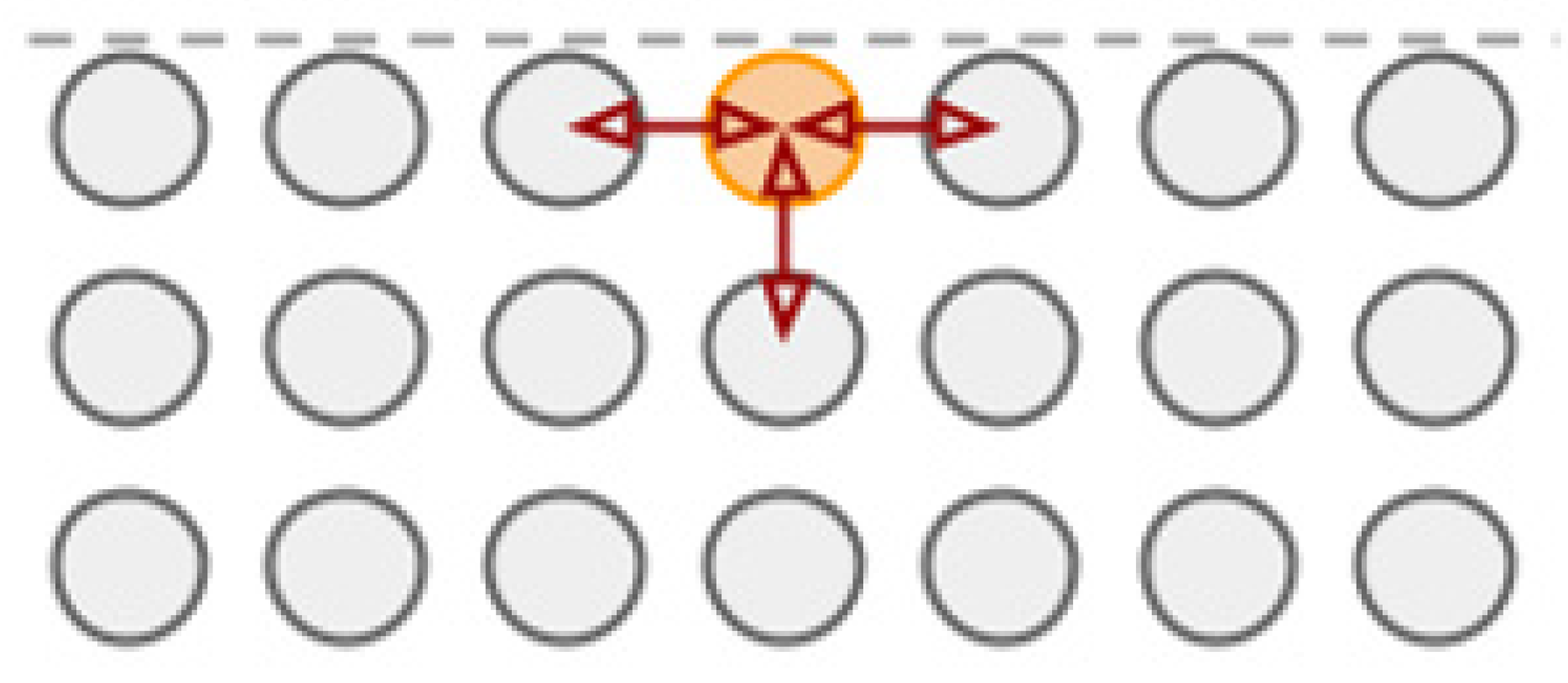
| Sample Materials Used in the Experiment and Their Melting Points, K [2,6,7] | Experimental Contact Melting Temperature, K | Maximum Pre-Melting Temperature (First Metal) | Minimum Pre-Melting Temperature (Second Metal) | Where is the Quasi-Liquid Film Located? |
|---|---|---|---|---|
| Pb (601)–Sn (505) | 463 | 501 | 421 | Sn |
| Sn (505)–In (430) | 400 | 421 | 358 | In |
| Tl (577)–Bi (545) | 483 | 480 | 454 | Tl, Bi |
| Tl (577)–Sn (505) | 455 | 480 | 421 | Sn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melkikh, A.V. Contact Melting of Metals Explained via the Theory of Quasi-Liquid Layer. Appl. Sci. 2021, 11, 51. https://doi.org/10.3390/app11010051
Melkikh AV. Contact Melting of Metals Explained via the Theory of Quasi-Liquid Layer. Applied Sciences. 2021; 11(1):51. https://doi.org/10.3390/app11010051
Chicago/Turabian StyleMelkikh, Alexey V. 2021. "Contact Melting of Metals Explained via the Theory of Quasi-Liquid Layer" Applied Sciences 11, no. 1: 51. https://doi.org/10.3390/app11010051




