Advantages and Limitations of Current Microgravity Platforms for Space Biology Research
Abstract
:1. Introduction
2. Ground Microgravity Simulators
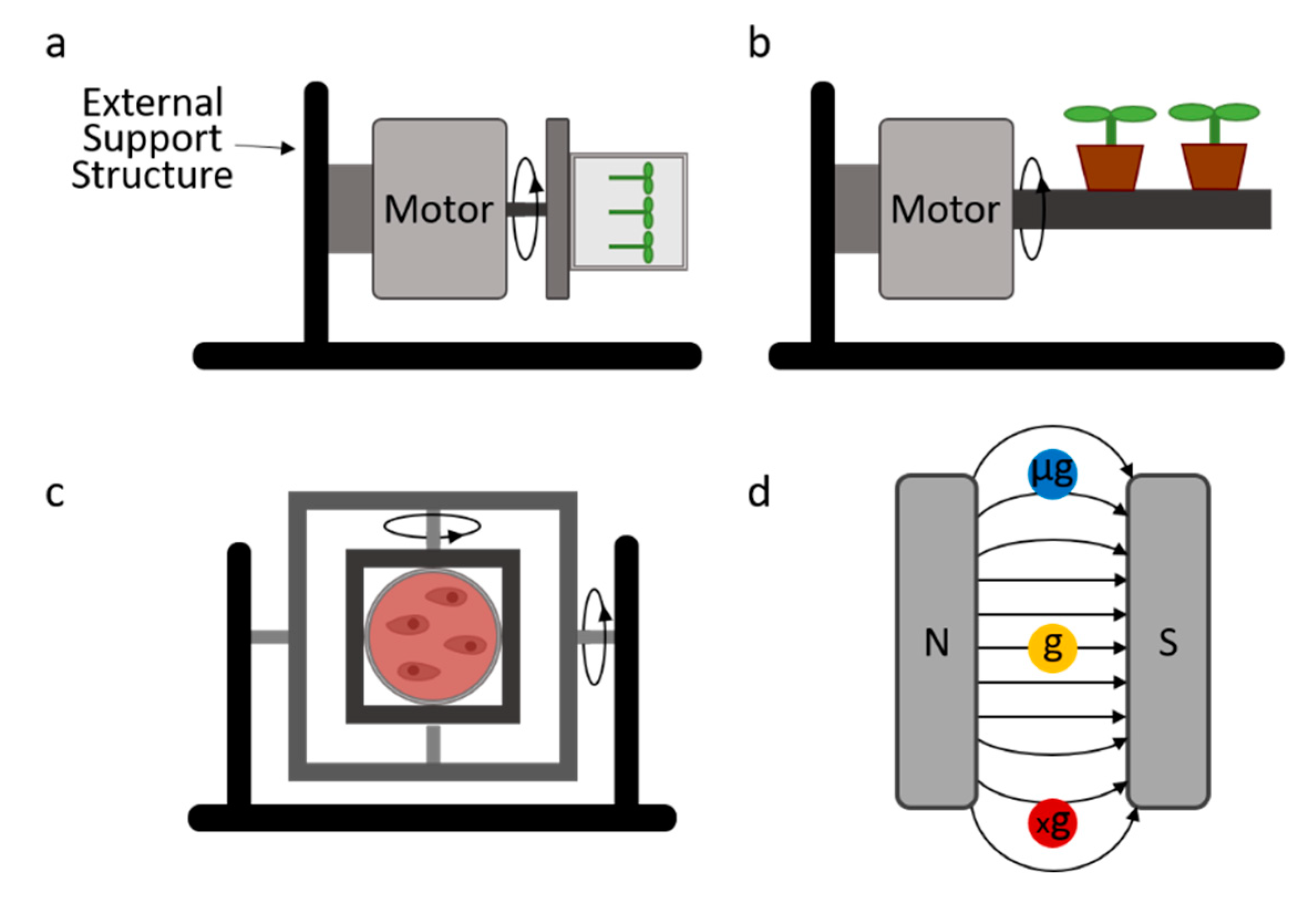
2.1. Clinostat 1D/2D/RWV
2.2. RPM
2.3. Diamagnetic Levitation
3. Ground Microgravity Analogues
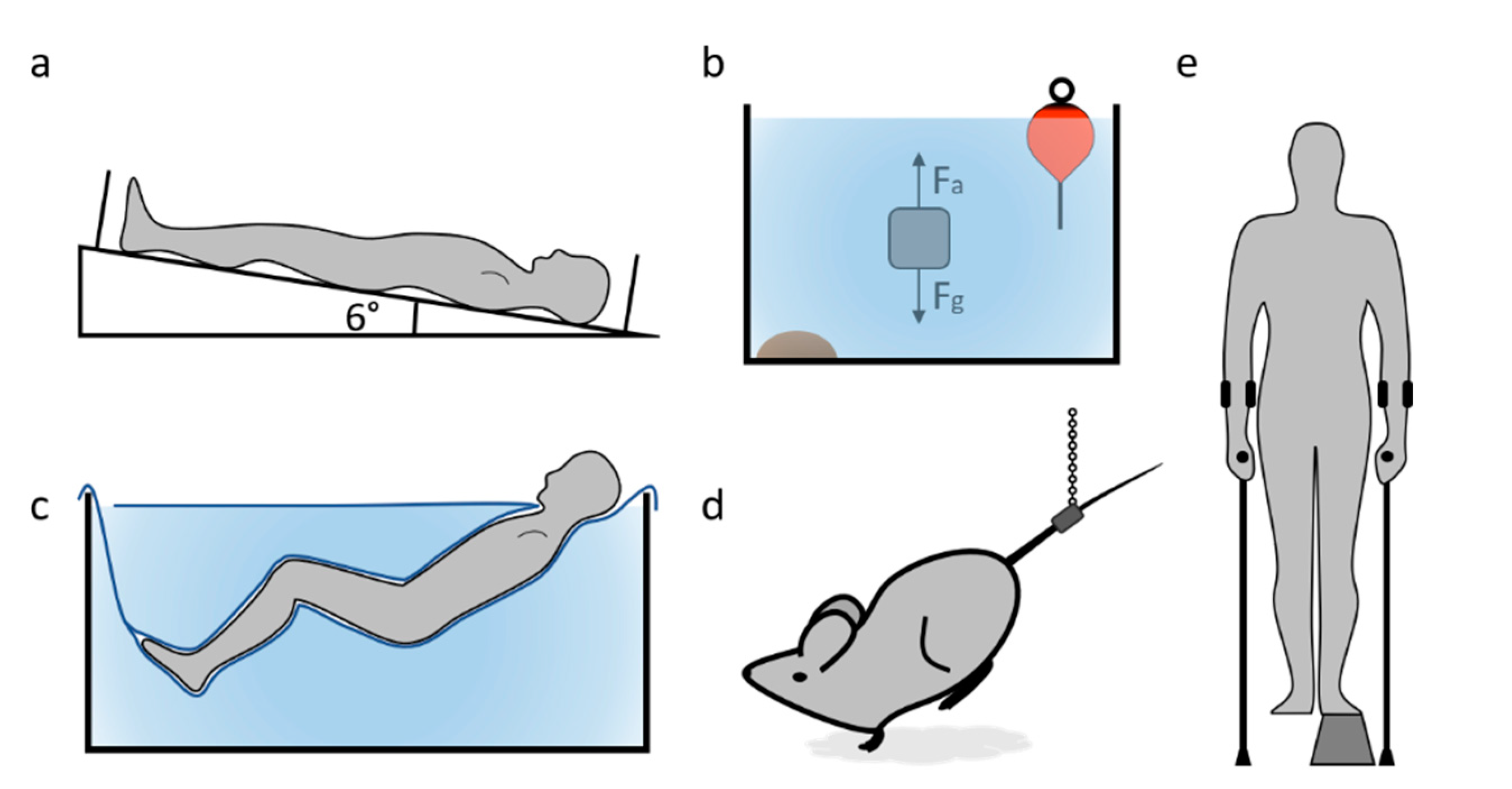
3.1. Bed Rest
3.2. Neutral Buoyancy
3.3. Dry Immersion
3.4. The Unilateral Lower Limb Suspension (ULLS)
3.5. The Rodent Hindlimb Unloading (HU)
4. Non-Orbiting Microgravity Facilities
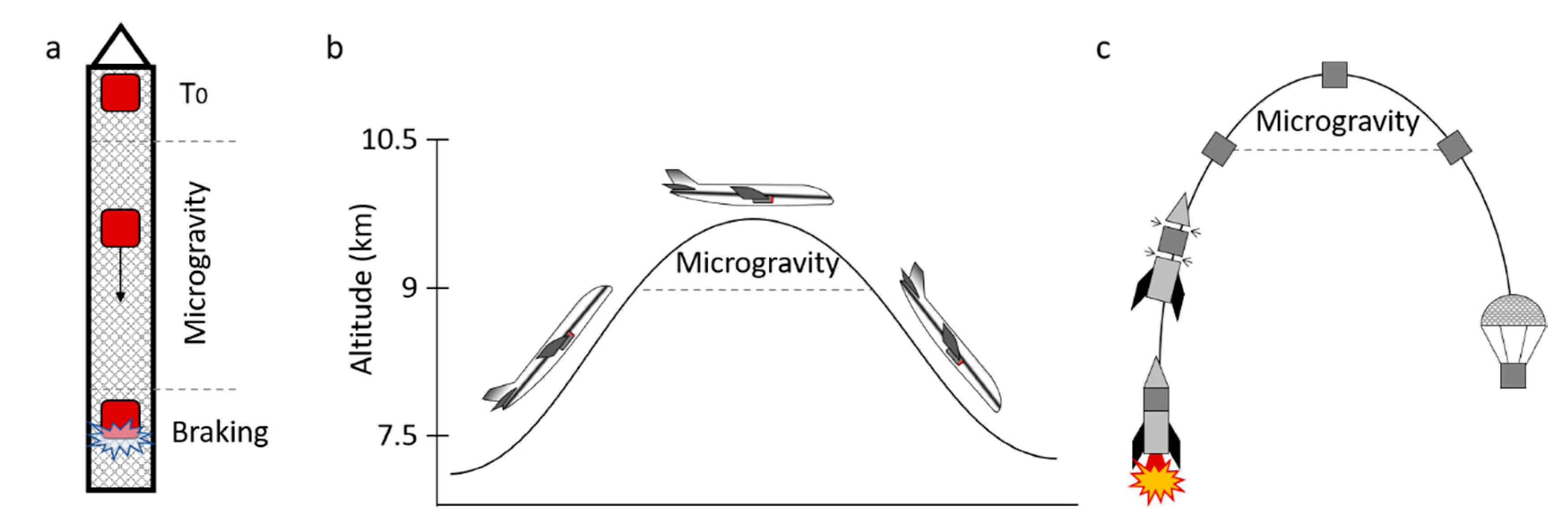
4.1. Drop Towers
4.2. Parabolic Flight
4.3. Sounding Rockets
5. Orbiting Microgravity Facilities
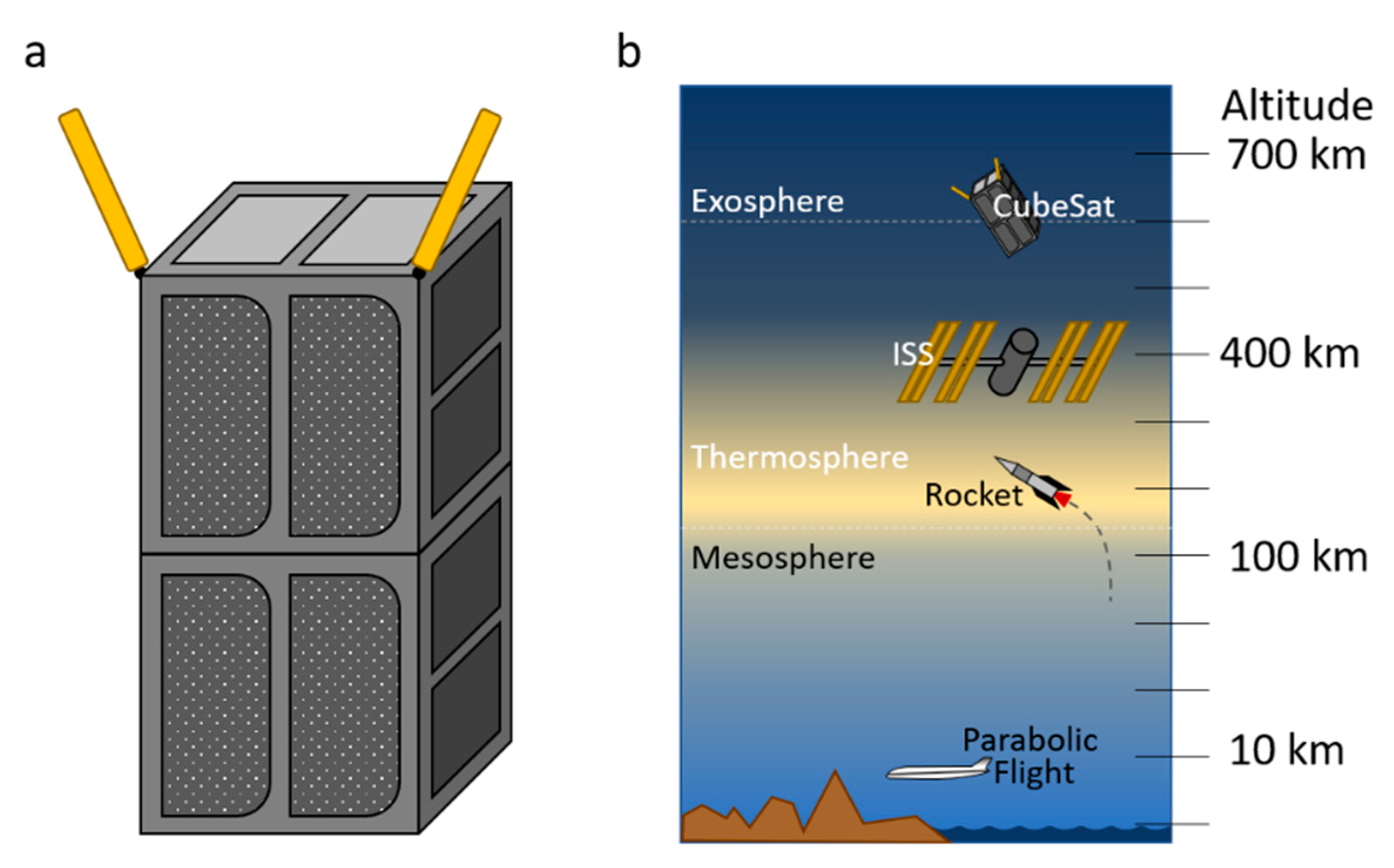
5.1. CubeSats
5.2. The International Space Station
5.2.1. Mass and Volume
5.2.2. Environmental Requirements
5.2.3. Time Constraints
5.2.4. Controls
5.2.5. Experimental Design
6. Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De la Torre, R.; Sancho, L.G.; Horneck, G.; de los Ríos, A.; Wierzchos, J.; Olsson-Francis, K.; Cockell, C.S.; Rettberg, P.; Berger, T.; de Vera, J.P.P.; et al. Survival of lichens and bacteria exposed to outer space conditions—Results of the Lithopanspermia experiments. Icarus 2010, 208, 735–748. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Rabbow, E.; Schill, R.O.; Harms-Ringdahl, M.; Rettberg, P. Tardigrades survive exposure to space in low Earth orbit. Curr. Biol. 2008, 18, 729–731. [Google Scholar] [CrossRef] [Green Version]
- De Vera, J.P.; Alawi, M.; Backhaus, T.; Baqué, M.; Billi, D.; Böttger, U.; Berger, T.; Bohmeier, M.; Cockell, C.; Demets, R.; et al. Limits of Life and the Habitability of Mars: The ESA Space Experiment BIOMEX on the ISS. Astrobiology 2019, 19, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Horneck, G. Responses of Bacillus subtilis spores to space environment: Results from experiments in space. Orig. Life Evol. Biosph. 1993, 23, 37–52. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef] [Green Version]
- Onofri, S.; Selbmann, L.; Pacelli, C.; Zucconi, L.; Rabbow, E.; De Vera, J.P. Survival, DNA, and Ultrastructural Integrity of a Cryptoendolithic Antarctic Fungus in Mars and Lunar Rock Analogs Exposed Outside the International Space Station. Astrobiology 2019, 19, 170–182. [Google Scholar] [CrossRef]
- Morita, M.T. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 2010, 61, 705–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, T.; Van Loon, J.J.W.A.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef]
- Aubert, A.E.; Larina, I.; Momken, I.; Blanc, S.; White, O.; Prisk, G.K.; Linnarsson, D. Towards human exploration of space: The THESEUS review series on cardiovascular, respiratory, and renal research priorities. NPJ Microgravity 2016, 2, 16031. [Google Scholar] [CrossRef] [Green Version]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human pathophysiological adaptations to the space environment. Front. Physiol. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, J.P.; Crucian, B.E.; de Quervain, D.J.F.; Grimm, D.; Montano, N.; Praun, S.; Roozendaal, B.; Schelling, G.; Thiel, M.; Ullrich, O.; et al. Towards human exploration of space: The THESEUS review series on immunology research priorities. NPJ Microgravity 2016, 2, 16040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune system dysregulation during spaceflight: Potential countermeasures for deep space exploration missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Blaber, A.P.; Zuj, K.A.; Goswami, N. Cerebrovascular autoregulation: Lessons learned from spaceflight research. Eur. J. Appl. Physiol. 2013, 113, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- White, O.; Clément, G.; Fortrat, J.O.; Pavy-Letraon, A.; Thonnard, J.L.; Blanc, S.; Wuyts, F.L.; Paloski, W.H. Towards human exploration of space: The THESEUS review series on neurophysiology research priorities. NPJ Microgravity 2016, 2, 16023. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.R.; Asemani, D.; Nietert, P.J.; Eckert, M.A.; Inglesby, D.C.; Bloomberg, J.J.; George, M.S.; Brown, T.R. Prolonged microgravity affects human brain structure and function. Am. J. Neuroradiol. 2019, 40, 1878–1885. [Google Scholar] [CrossRef] [Green Version]
- Bergouignan, A.; Stein, T.P.; Habold, C.; Coxam, V.; O’gorman, D.; Blanc, S. Towards human exploration of space: The THESEUS review series on nutrition and metabolism research priorities. NPJ Microgravity 2016, 2, 16029. [Google Scholar] [CrossRef]
- Mulavara, A.P.; Feiveson, A.H.; Fiedler, J.; Cohen, H.; Peters, B.T.; Miller, C.; Brady, R.; Bloomberg, J.J. Locomotor function after long-duration space flight: Effects and motor learning during recovery. Exp. Brain Res. 2010, 202, 649–659. [Google Scholar] [CrossRef]
- Sachs, J. Über orthotrope und plagiotrope Pflanzenteile. Arb. Bot. Inst. Wurzbg. 1882, 2, 226–284. [Google Scholar]
- Dedolph, R.R.; Dipert, M.H. The Physical Basis of Gravity Stimulus Nullification by Clinostat Rotation. Plant Physiol. 1971, 47, 756–764. [Google Scholar] [CrossRef]
- Cogoli, M. The fast rotating clinostat: A history of its use in gravitational biology and a comparison of ground-based and flight experiment results. ASGSB Bull. 1992, 5, 59–67. [Google Scholar] [PubMed]
- Shen-Miller, J.; Hinchman, R.; Gordon, S.A. Thresholds for Georesponse to Acceleration in Gravity-Compensated Avena Seedlings. Plant Physiol. 1968, 43, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Galland, P.; Finger, H.; Wallacher, Y. Gravitropism in Phycomyces: Threshold determination on a clinostat centrifuge. J. Plant Physiol. 2004, 161, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Laurinavičius, R.; Švegždiene, D.; Buchen, B.; Sievers, A. Determination of the threshold acceleration for the gravitropic stimulation of cress roots and hypocotyls. Adv. Sp. Res. 1998, 21, 1203–1207. [Google Scholar] [CrossRef]
- Chauvet, H.; Pouliquen, O.; Forterre, Y.; Legué, V.; Moulia, B. Inclination not force is sensed by plants during shoot gravitropism. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kiss, J.Z.; Wolverton, C.; Wyatt, S.E.; Hasenstein, K.H.; van Loon, J.J.W.A. Comparison of Microgravity Analogs to Spaceflight in Studies of Plant Growth and Development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef] [Green Version]
- van Loon, J.J.W.A. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Sp. Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Brungs, S.; Egli, M.; Wuest, S.L.; Peter, P.C.; Jack, J.J.; Ngo Anh, T.J.; Hemmersbach, R. Facilities for Simulation of Microgravity in the ESA Ground-Based Facility Programme. Microgravity Sci. Technol. 2016, 28, 191–203. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated Microgravity: Critical Review on the Use of Random Positioning Machines for Mammalian Cell Culture. Biomed. Res. Int. 2015, 2015, 97147. [Google Scholar] [CrossRef] [Green Version]
- Wuest, S.L.; Stern, P.; Casartelli, E.; Egli, M. Fluid dynamics appearing during simulated microgravity using Random Positioning Machines. PLoS ONE 2017, 12, e0170826. [Google Scholar] [CrossRef] [PubMed]
- Leguy, C.A.D.; Delfos, R.; Pourquie, M.J.B.M.; Poelma, C.; Krooneman, J.; Westerweel, J.; Van Loon, J.J.W.A. Fluid motion for microgravity simulations in a random positioning machine. Gravit. Sp. Biol. 2011, 25, 36–39. [Google Scholar]
- Unsworth, B.R.; Lelkes, P.I. Growing tissues in microgravity. Nat. Med. 1998, 4, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; Van Loon, J.; Ulbrich, C.; Magnusson, N.E.; Infanger, M.; Bauer, J. Growing tissues in real and simulated microgravity: New methods for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 555–566. [Google Scholar] [CrossRef] [Green Version]
- Borst, A.G.; Van Loon, J.J.W.A. Technology and developments for the random positioning machine, RPM. Microgravity Sci. Technol. 2009, 21, 287–292. [Google Scholar] [CrossRef]
- Herranz, R.; Larkin, O.J.; Dijkstra, C.E.; Hill, R.J.A.; Anthony, P.; Davey, M.R.; Eaves, L.; van Loon, J.J.W.A.; Medina, F.J.; Marco, R. Microgravity simulation by diamagnetic levitation: Effects of a strong gradient magnetic field on the transcriptional profile of Drosophila melanogaster. BMC Genom. 2012, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Valles, J.M.; Maris, H.J.; Seidel, G.M.; Tang, J.; Yao, W. Magnetic levitation-based Martian and Lunar gravity simulator. Adv. Sp. Res. 2005, 36, 114–118. [Google Scholar] [CrossRef]
- Glover, P.M.; Cavin, I.; Qian, W.; Bowtell, R.; Gowland, P.A. Magnetic-field-induced vertigo: A theoretical and experimental investigation. Bioelectromagnetics 2007, 28, 349–361. [Google Scholar] [CrossRef]
- Maret, G.; Dransfeld, K. Biomolecules and Polymers in High Steady Magnetic Fields. In Strong and Ultrastrong Magnetic Fields and Their Applications; Springer: Berlin/Heidelberg, Germany, 1985; pp. 143–204. [Google Scholar]
- Nechitailo, G.S.; Mashinsky, A.L.; Kuznetsov, A.A.; Chikov, V.M.; Kuznetsov, O.A. Influence of nonuniform magnetic fields on orientation of plant seedlings in microgravity conditions. Adv. Sp. Res. 2001, 28, 639–643. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Ploutz-Snyder, L. Evaluating countermeasures in spaceflight analogs. J. Appl. Physiol. 2016, 120, 915–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavy-Le Traon, A.; Sigaudo, D.; Vasseur, P.; Maillet, A.; Fortrat, J.O.; Hughson, R.L.; Gauquelin-Koch, G.; Gharib, C. Cardiovascular responses to orthostatic tests after a 42-day head-down bed-rest. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 77, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Nicogossian, A.E.; Huntoon, C.; Pool, S. Space Physiology and Medicine; Lea and Febiger: Philadelphia, PA, USA, 1994; ISBN 9780812115956. [Google Scholar]
- Hoson, T. Apoplast as the site of response to environmental signals. J. Plant Res. 1998, 111, 167–177. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K. New aspects of gravity responses in plant cells. Int. Rev. Cytol. 2003, 229, 209–244. [Google Scholar] [CrossRef] [PubMed]
- Navasiolava, N.M.; Custaud, M.A.; Tomilovskaya, E.S.; Larina, I.M.; Mano, T.; Gauquelin-Koch, G.; Gharib, C.; Kozlovskaya, I.B. Long-term dry immersion: Review and prospects. Eur. J. Appl. Physiol. 2011, 111, 1235–1260. [Google Scholar] [CrossRef] [Green Version]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry immersion as a ground-based model of microgravity physiological effects. Front. Physiol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach-Huntoon, C.; Grigoriev, A.; Natochin, Y. Fluid and Electrolyte Regulation in Spaceflight; American Astronautical Society: San Diego, CA, USA, 1998; ISBN 978-0877034438. [Google Scholar]
- Tesch, P.A.; Lundberg, T.R.; Fernandez-Gonzalo, R. Unilateral lower limb suspension: From subject selection to “omic” responses. J. Appl. Physiol. 2016, 120, 1207–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Palus, S.; Springer, J.I.; Doehner, W.; von Haehling, S.; Anker, M.; Anker, S.D.; Springer, J. Models of sarcopenia: Short review. Int. J. Cardiol. 2017, 238, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Grindeland, R.E.; Ballard, R.W.; Connolly, J.P.; Vasques, M.F. COSMOS 2044 mission. Overview. J. Appl. Physiol. 1992, 73, 1S–3S. [Google Scholar] [CrossRef]
- Grindeland, R. Comparison of hypergravity and microgravity effects on rat physiology: An overview. Aviat Sp. Environ. Med. 1998, 69, 59. [Google Scholar]
- Riley, D.A.; Ellis, S.; Giometti, C.S.; Hoh, J.F.Y.; Ilyina-Kakueva, E.I.; Oganov, V.S.; Slocum, G.R.; Bain, J.L.W.; Sedlak, F.R. Muscle sarcomere lesions and thrombosis after spaceflight and suspension unloading. J. Appl. Physiol. 1992, 73, 33S–43S. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Karim, A.; Elmoselhi, A.B. Muscle unloading: A comparison between spaceflight and ground-based models. Acta Physiol. 2020, 228, e13431. [Google Scholar] [CrossRef]
- 4 Drop Tower—European Space Agency. Available online: http://wsn.spaceflight.esa.int/docs/EUG2LGPr3/EUG2LGPr3-4-DropTower.pdf (accessed on 23 October 2020).
- Ruyters, G.; Friedrich, U. From the Bremen Drop Tower to the international space station ISS—Ways to weightlessness in the German Space Life Sciences Program. Signal Transduct. 2006, 6, 397–405. [Google Scholar] [CrossRef]
- 5 Parabolic Flights—European Space Agency. Available online: http://wsn.spaceflight.esa.int/docs/EUG2LGPr3/EUG2LGPr3-5-ParabolicFlights.pdf (accessed on 23 October 2020).
- Shelhamer, M. Parabolic flight as a spaceflight analog. J. Appl. Physiol. 2016, 120, 1442–1448. [Google Scholar] [CrossRef] [Green Version]
- 6 Sounding Rockets—European Space Agency. Available online: http://wsn.spaceflight.esa.int/docs/EUG2LGPr3/EUG2LGPr3-6-SoundingRockets.pdf (accessed on 23 October 2020).
- NASA Sounding Rocket Program Overview. Available online: https://rscience.gsfc.nasa.gov/srrov.html (accessed on 29 October 2020).
- Santoni, F.; Gugliermetti, L.; Piras, G.; De Pascale, S.; Pannico, A.; Piergentili, F.; Marzioli, P.; Frezza, L.; Amadio, D.; Gianfermo, A.; et al. GreenCube: Microgreens cultivation and growth monitoring on-board a 3U CubeSat. In Proceedings of the Conference: 2020 IEEE 7th International Workshop on Metrology for AeroSpace, Pisa, Italy, 22–24 June 2020; pp. 130–135. [Google Scholar] [CrossRef]
- Meneghin, A.; Brucato, J.R.; Poggiali, G.; Nascetti, A.; Anfossi, L.; Mirasoli, M. Astrobio Cubesat: A Mini Laboratory Payload for Space Environment Astrobiology Experiments. In Proceedings of the EANA 2019—19th EANA Astrobiology Conference, Orléans, France, 3–6 September 2019; pp. 2–3. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Ricco, A.J.; Agasid, E.; Beasley, C.; Diaz-Aguado, M.; Ehrenfreund, P.; Friedericks, C.; Ghassemieh, S.; Henschke, M.; Hines, J.W.; et al. The O/OREOS mission: First science data from the Space Environment Survivability of Living Organisms (SESLO) payload. Astrobiology 2011, 11, 951–958. [Google Scholar] [CrossRef]
- Ricco, A.J.; Hanel, R.; Bhattacharya, S.; Boone, T.; Tan, M.; Mousavi, A.; Padgen, M.; Gentry, D.; Rademacher, A.; Schooley, A.; et al. The biosentinel bioanalytical microsystem: Characterizing DNA radiation damage in living organisms beyond earth orbit. In Proceedings of the Solid-State Sensors, Actuators and Microsystems Workshop Hilton Head Island, Hilton Head Island, SC, USA, 5–9 June 2016; pp. 352–355. [Google Scholar] [CrossRef]
- Polat, H.C.; Virgili-Llop, J.; Romano, M. Survey, Statistical Analysis and Classification of Launched CubeSat Missions with Emphasis on the Attitude Control Method. JoSS 2016, 5, 513–530. [Google Scholar]
- Lemmer, K. Propulsion for CubeSats. Acta Astronaut. 2017, 134, 231–243. [Google Scholar] [CrossRef]
- Marinan, A.; Nicholas, A.; Cahoy, K. Ad hoc CubeSat constellations: Secondary launch coverage and distribution. In Proceedings of the IEEE Aerospace Conference Proceedings, Big Sky, MT, USA, 2–9 March 2013. [Google Scholar] [CrossRef]
- Schoolcraft, J.; Klesh, A.; Werne, T. MarCO: Interplanetary mission development on a cubesat scale. AIAA 2016, 1–8. [Google Scholar] [CrossRef]
- The Global Exploration Roadmap 2018. Available online: https://www.globalspaceexploration.org/wordpress/wp-content/isecg/GER_2018_small_mobile.pdf (accessed on 23 October 2020).
- ISS Benefits for Humanity—3rd Edition NASA. Available online: https://www.nasa.gov/mission_pages/station/research/news/b4h-3rd-ed-book/ (accessed on 23 October 2020).
- Bryce, C.C.; Horneck, G.; Rabbow, E.; Edwards, H.G.M.; Cockell, C.S. Impact shocked rocks as protective habitats on an anoxic early Earth. Int. J. Astrobiol. 2015, 14, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Mancinelli, R.L. The affect of the space environment on the survival of Halorubrum chaoviator and Synechococcus (Nägeli): Data from the Space Experiment OSMO on EXPOSE-R. Int. J. Astrobiol. 2015, 14, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Neuberger, K.; Lux-Endrich, A.; Panitz, C.; Horneck, G. Survival of spores of trichoderma longibrachiatum in space: Data from the Space Experiment SPORES on EXPOSE-R. Int. J. Astrobiol. 2015, 14, 129–135. [Google Scholar] [CrossRef]
- Panitz, C.; Horneck, G.; Rabbow, E.; Rettberg, P.; Moeller, R.; Cadet, J.; Douki, T.; Reitz, G. The SPORES experiment of the EXPOSE-R mission: Bacillus subtilis spores in artificial meteorites. Int. J. Astrobiol. 2015, 14, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Annual Highlights of Results from the International Space Station (1 October 2018–1 October 2019). Available online: https://www.nasa.gov/sites/default/files/atoms/files/np-2019-11-010-jsciss_annual_highlights_2019_screen_12-11-19.pdf (accessed on 26 October 2020).
- Paul, A.; Ferl, R. Spaceflight exploration in plant gravitational biology. Methods Mol. Biol. 2015, 1309, 285–305. [Google Scholar] [CrossRef]
- Shiba, D.; Mizuno, H.; Yumoto, A.; Shimomura, M.; Kobayashi, H.; Morita, H.; Shimbo, M.; Hamada, M.; Kudo, T.; Shinohara, M.; et al. Development of new experimental platform ‘MARS’-Multiple Artificial-gravity Research System-to elucidate the impacts of micro/partial gravity on mice. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Brinckmann, E. Centrifuges and their application for biological experiments in space. Microgravity Sci. Technol. 2012, 24, 365–372. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z. Space, the final frontier: A critical review of recent experiments performed in microgravity. Plant Sci. 2016, 243, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Kiss, J.Z.; Edelmann, R.E.; Wood, P.C. Gravitropism of hypocotyls of wild-type and starch-deficient Arabidopsis seedlings in spaceflight studies. Planta 1999, 209, 96–103. [Google Scholar] [CrossRef]
- Global Exploration Roadmap Supplement 2020. Available online: https://www.globalspaceexploration.org/wp-content/uploads/2020/08/GER_2020_supplement.pdf (accessed on 23 October 2020).
| 1D/2D Clinostat/RWV | 3D Clinostat/RPM | Diamagnetic Levitation | |
|---|---|---|---|
| Microgravity Duration | Hours to weeks | Hours to weeks | Minutes to hours |
| Microgravity Quality | ≤10−3 g | 10−4 g | <10−2 g |
| Hypogravity | Y | Y | Y |
| Biological System | Cells, microbes, plants | Cells, microbes, plants | Cells, microbes, plants, animals |
| Cost | Low | Low | Medium |
| Accessibility | Easy | Easy | Easy |
| Bed Rest | ULLS | HU | Neutral Buoyancy | Dry Immersion | |
|---|---|---|---|---|---|
| Microgravity Duration | Hours to weeks | Hours to weeks | Hours to weeks | Hours | Days to months |
| Hypogravity | Yes | No | No | No | No |
| Biological System | Humans | Humans | Animals | Animals, humans | Humans |
| Cost | Medium | Low | Low | Medium | Medium |
| Accessibility | Easy | Medium | Easy | Medium | Medium |
| Drop Tower | Parabolic Flight | Sounding Rocket | CubeSat | ISS | |
|---|---|---|---|---|---|
| Microgravity Duration | 2.2 s up to 9.5 s | 20 s repetitive | 5–20 min | Weeks to months | Months to years |
| Microgravity Quality | 10−6 g | 10−2 g | ≤10−4 g | 10−6 g | 10−6 g |
| Hypogravity | Yes | Yes | No | No | Yes |
| Biological System | Cells, microbes, plants | Cells, microbes, plants, animals, humans | Cells, plants, microbes, animals | Microbes, plants | Cells, microbes, plants, animals, humans |
| Cost | Medium | Medium | Medium | Medium | High |
| Accessibility | Medium | Medium | Medium | Medium | Hard |
| Late access before experiment | 2 h | 10 min | <3 h | 24 h | 24 h |
| Early retrieval after experiment | 45 min | 1 min | 1–2 h | Not Applicable | ≥48 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferranti, F.; Del Bianco, M.; Pacelli, C. Advantages and Limitations of Current Microgravity Platforms for Space Biology Research. Appl. Sci. 2021, 11, 68. https://doi.org/10.3390/app11010068
Ferranti F, Del Bianco M, Pacelli C. Advantages and Limitations of Current Microgravity Platforms for Space Biology Research. Applied Sciences. 2021; 11(1):68. https://doi.org/10.3390/app11010068
Chicago/Turabian StyleFerranti, Francesca, Marta Del Bianco, and Claudia Pacelli. 2021. "Advantages and Limitations of Current Microgravity Platforms for Space Biology Research" Applied Sciences 11, no. 1: 68. https://doi.org/10.3390/app11010068
APA StyleFerranti, F., Del Bianco, M., & Pacelli, C. (2021). Advantages and Limitations of Current Microgravity Platforms for Space Biology Research. Applied Sciences, 11(1), 68. https://doi.org/10.3390/app11010068







