Fibrinogen and Fibrin Differentially Regulate the Local Hydrodynamic Environment in Neutrophil–Tumor Cell–Endothelial Cell Adhesion System
Abstract
:1. Introduction
2. Methods
2.1. Preparation of Cells and Molecules
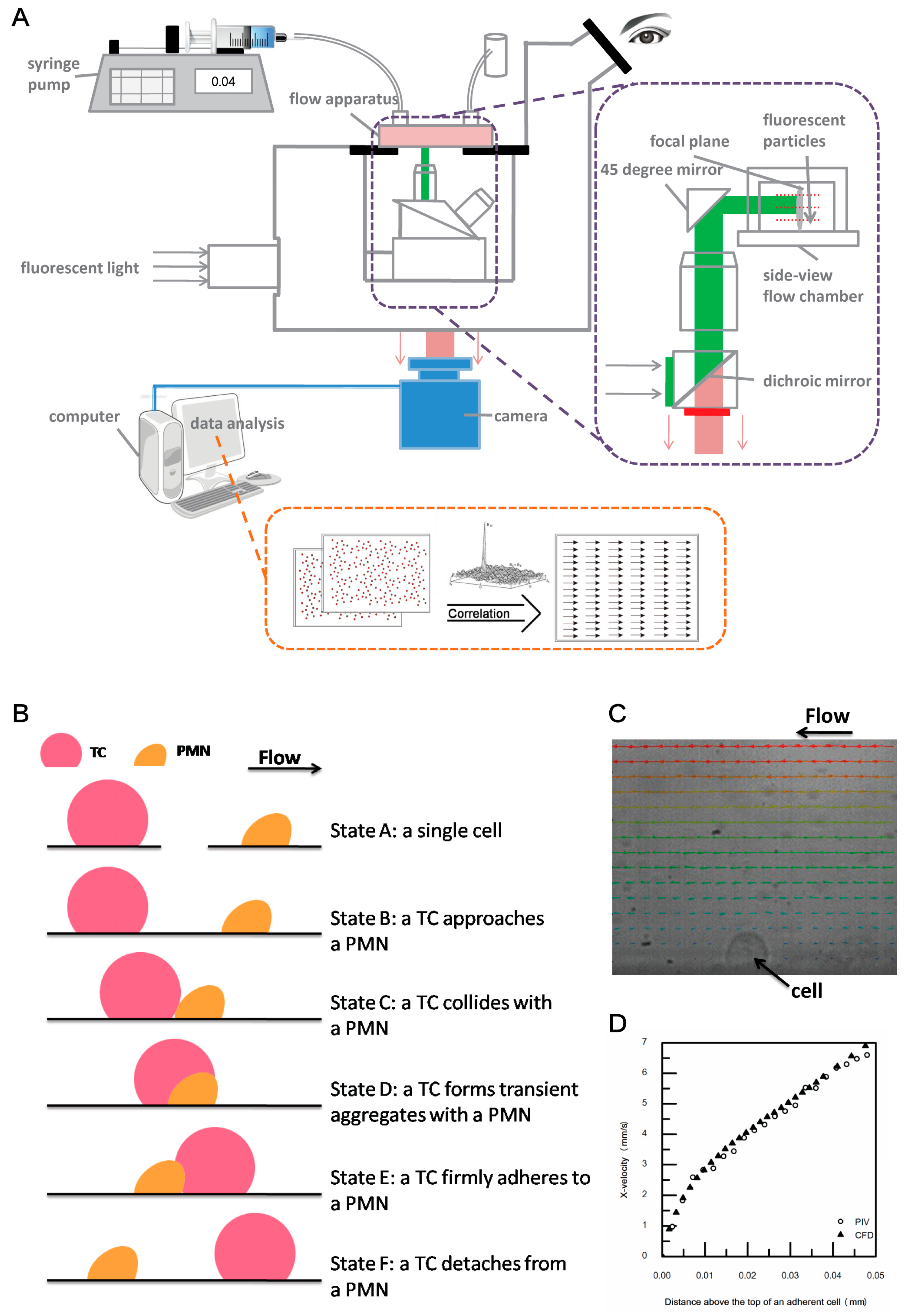
2.2. Coupled Side-View µPIV System Modification and Experimental Procedure
2.3. Data Analysis
2.4. CFD Simulations
3. Results
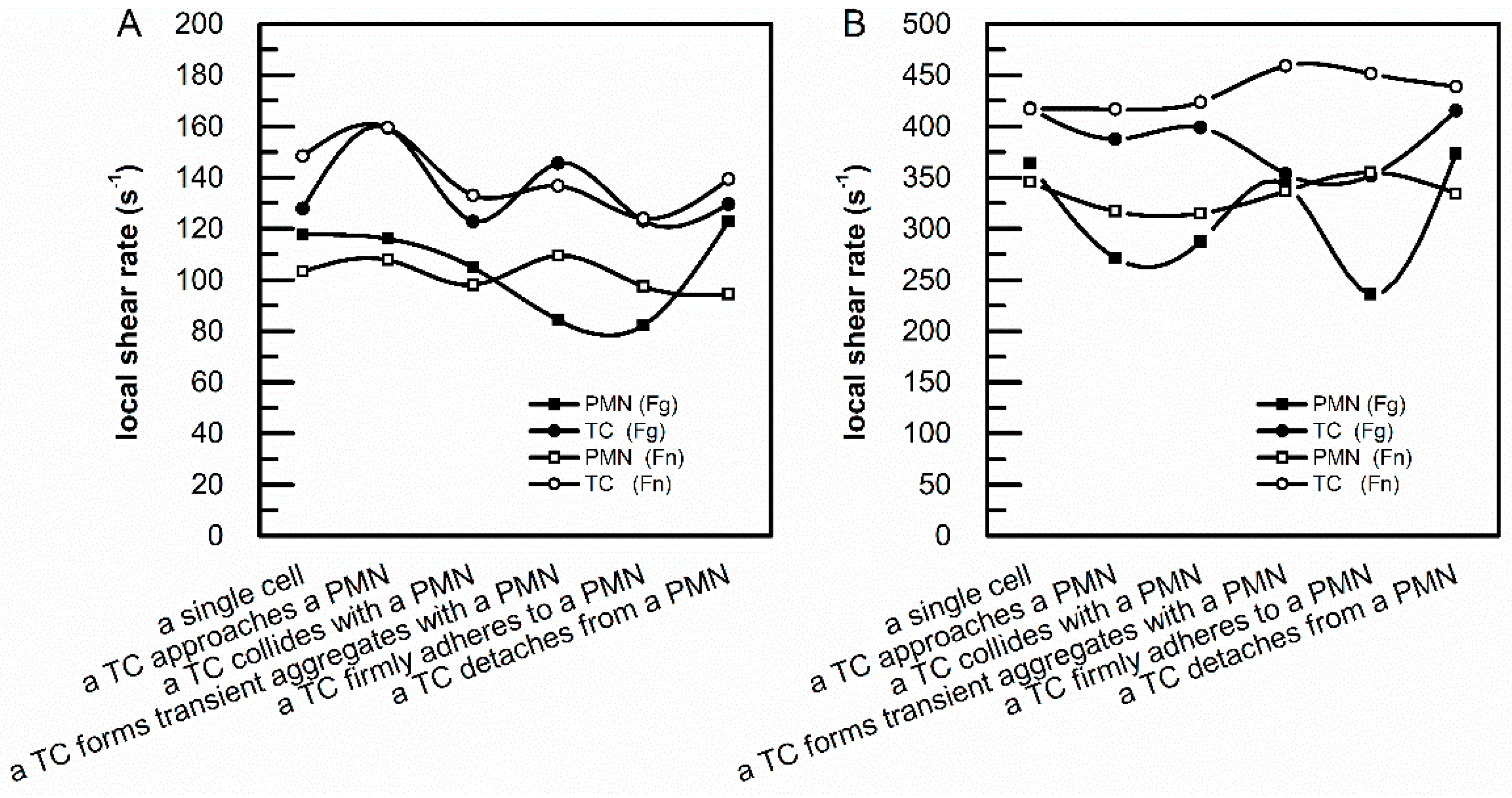
3.1. sFg and sFn Regulated the Local Shear Rates above an Adherent PMN and an Adherent TC on an Endothelial Monolayer
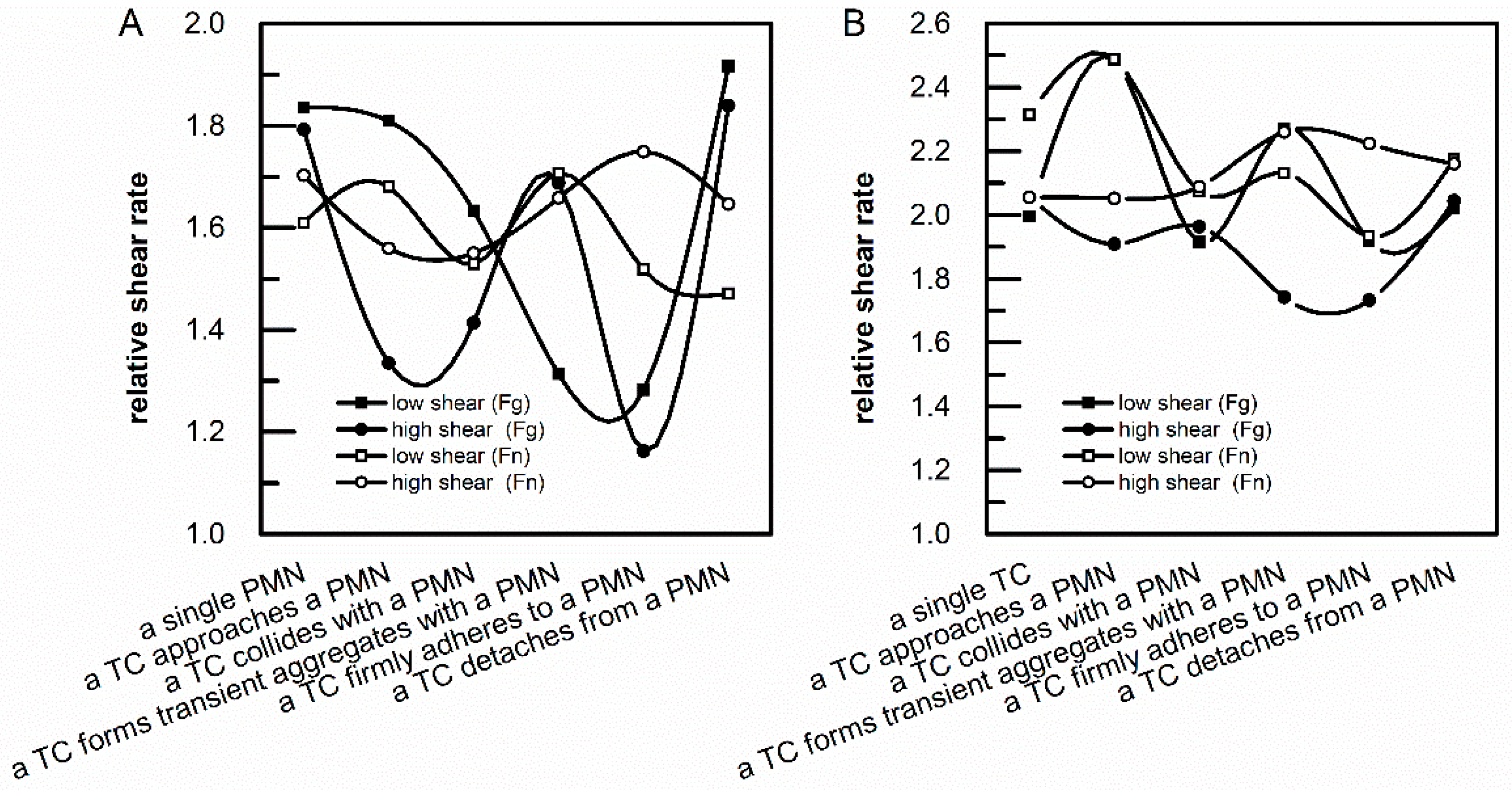
3.2. Analysis of Relative Shear Rates above an Adherent PMN and an Adherent TC
3.3. Calculation of Cell Reynolds Numbers
3.4. Drag Forces Acted on Adherent PMNs and TCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dong, Y.; Feijen, J.; Zhong, Z. Peptide-decorated polymeric nanomedicines for precision cancer therapy. J. Control. Release 2018, 290, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Micalizzi, D.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Slattery, M.; Wagner, D.; Simon, S.; Dong, C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann. Biomed. Eng. 2008, 36, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Slattery, M.J.; Dong, C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp. Cell Res. 2005, 310, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Slattery, M.J.; Liang, S.; Peng, H.H. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol. Cell. Biomech. 2005, 2, 145–159. [Google Scholar]
- Hoskins, M.H.; Kunz, R.F.; Bistline, J.E.; Dong, C. Coupled flow-structure-biochemistry simulations of dynamic systems of blood cells using an adaptive surface tracking method. J. Fluids Struct. 2009, 25, 936–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C. Adhesion and signaling of tumor cells to leukocytes and endothelium in cancer metastasis. In Cellular and Biomolecular Mechanics and Mechanobiology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 477–521. [Google Scholar]
- Mueller, B.M.; Reisfeld, R.A.; Edgington, T.S.; Ruf, W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc. Natl. Acad. Sci. USA 1992, 89, 11832–11836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickles, F.R.; Patierno, S.; Fernandez, P.M. Tissue factor, thrombin, and cancer. Chest 2003, 124, 58S–68S. [Google Scholar] [CrossRef] [PubMed]
- Gobel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders-A Systematic Review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arter. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef] [Green Version]
- Sugama, Y.; Tiruppathi, C.; Andersen, T.; Fenton, J.; Malik, A. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: A mechanism for stabilizing neutrophil adhesion. J. Cell Biol. 1992, 119, 935–944. [Google Scholar] [CrossRef]
- Zimmerman, G.A.; Mcintyre, T.M.; Prescott, S.M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J. Clin. Investig. 1985, 76, 2235–2246. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Shi, C.; Erhardt, P.W.; Pavlovsky, A.; Soloviev, D.A.; Bledzka, K.; Ustinov, V.; Zhu, L.; Qin, J.; et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbalpha. Nat. Commun. 2017, 8, 15559. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, T.; Zhang, P.; Fu, C.; Dong, C. Fibrin serves as a divalent ligand that regulates neutrophil-mediated melanoma cells adhesion to endothelium under shear conditions. Am. J. Physiol. Cell Physiol. 2012, 302, C1189–C1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggerstaff, J.P.; Weidow, B.; Vidosh, J.; Dexheimer, J.; Patel, S.; Patel, P. Soluble fibrin inhibits monocyte adherence and cytotoxicity against tumor cells: Implications for cancer metastasis. Thromb. J. 2006, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refaai, M.A.; Riley, P.; Mardovina, T.; Bell, P.D. The Clinical Significance of Fibrin Monomers. Thromb. Haemost. 2018, 118, 1856–1866. [Google Scholar] [CrossRef] [Green Version]
- Campello, E.; Henderson, M.W.; Noubouossie, D.F.; Simioni, P.; Key, N.S. Contact System Activation and Cancer: New Insights in the Pathophysiology of Cancer-Associated Thrombosis. Thromb. Haemost. 2018, 118, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, T.; Gaddes, E.; Wang, Y.; Dong, C. Perspectives: Interplay Between Melanoma Regulated Fibrin and Receptor Mediated Adhesion Under Shear Flow. Cell. Mol. Bioeng. 2014, 1–10. [Google Scholar] [CrossRef]
- Fu, Y.; Kunz, R.; Wu, J.; Dong, C. Study of local hydrodynamic environment in cell-substrate adhesion using side-view μPIV technology. PLoS ONE 2012, 7, e30721. [Google Scholar] [CrossRef]
- Gopalan, P.K.; Smith, C.W.; Lu, H.; Berg, E.L.; McIntire, L.V.; Simon, S.I. Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin. J. Immunol. 1997, 158, 367–375. [Google Scholar]
- Cao, J.; Usami, S.; Dong, C. Development of a side-view chamber for studying cell-surface adhesion under flow conditions. Ann. Biomed. Eng. 1997, 25, 573–580. [Google Scholar] [CrossRef]
- Cao, J.; Donell, B.; Deaver, D.R.; Lawrence, M.B.; Dong, C. In vitro side-view imaging technique and analysis of human T-leukemic cell adhesion to ICAM-1 in shear flow. Microvasc. Res. 1998, 55, 124–137. [Google Scholar] [CrossRef]
- Biggerstaff, J.; Seth, N.; Amirkhosravi, A.; Amaya, M.; Fogarty, S.; Meyer, T.; Siddiqui, F.; Francis, J. Soluble fibrin augments platelet/tumor cell adherence in vitro and in vivo, and enhances experimental metastasis. Clin. Exp. Metastasis 1999, 17, 723–730. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Thrombin-unique coagulation system protein with multifaceted impacts on cancer and metastasis. Cancer Metastasis Rev. 2016, 35, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Languino, L.; Plescia, J.; Duperray, A.; Brian, A.; Plow, E.; Geltosky, J.; Altieri, D. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell 1993, 73, 1423–1434. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000, 96, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016, 157, 112–119. [Google Scholar] [CrossRef]
- Felding-Habermann, B.; Ruggeri, Z.; Cheresh, D. Distinct biological consequences of integrin alpha v beta 3-mediated melanoma cell adhesion to fibrinogen and its plasmic fragments. J. Biol. Chem. 1992, 267, 5070–5077. [Google Scholar]
- Huang, R.; Rofstad, E.K. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 92. [Google Scholar] [CrossRef]
- Zhang, P.; Ozdemir, T.; Chung, C.Y.; Robertson, G.P.; Dong, C. Sequential Binding of αvβ3 and ICAM-1 Determines Fibrin-Mediated Melanoma Capture and Stable Adhesion to CD11b/CD18 on Neutrophils. J. Immunol. 2011, 186, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.F.; Moake, J.L.; Nolasco, L.; Bernardo, A.; Arceneaux, W.; Shrimpton, C.N.; Schade, A.J.; McIntire, L.V.; Fujikawa, K.; Lopez, J.A. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 2002, 100, 4033–4039. [Google Scholar] [CrossRef] [Green Version]
- Grassle, S.; Huck, V.; Pappelbaum, K.I.; Gorzelanny, C.; Aponte-Santamaria, C.; Baldauf, C.; Grater, F.; Schneppenheim, R.; Obser, T.; Schneider, S.W. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arter. Thromb. Vasc. Biol. 2014, 34, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Ribes, J.A.; Francis, C.W.; Wagner, D.D. Fibrin induces release of von Willebrand factor from endothelial cells. J. Clin. Investig. 1987, 79, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Goerge, T.; Kleineruschkamp, F.; Barg, A.; Schnaeker, E.M.; Huck, V.; Schneider, M.F.; Steinhoff, M.; Schneider, S.W. Microfluidic reveals generation of platelet-strings on tumor-activated endothelium. Thromb. Haemost. 2007, 98, 283–286. [Google Scholar] [PubMed]
- Bauer, A.T.; Suckau, J.; Frank, K.; Desch, A.; Goertz, L.; Wagner, A.H.; Hecker, M.; Goerge, T.; Umansky, L.; Beckhove, P.; et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood 2015, 125, 3153–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerk, N.; Strozyk, E.A.; Poppelmann, B.; Schneider, S.W. The mechanism of melanoma-associated thrombin activity and von Willebrand factor release from endothelial cells. J. Investig. Dermatol. 2010, 130, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.J.; Wang, M.; Wang, Y.; Cai, W.; Li, Q.; Zhao, T.T.; Zhang, L.H.; Houck, K.; Chen, X.; Jin, Y.L.; et al. Cancer cell-derived von Willebrand factor enhanced metastasis of gastric adenocarcinoma. Oncogenesis 2018, 7, 12. [Google Scholar] [CrossRef] [Green Version]


| Conditions | PMN | TC | ||||||
|---|---|---|---|---|---|---|---|---|
| Low Shear | High Shear | Low Shear | High Shear | |||||
| L (μm) | H (μm) | L (μm) | H (μm) | L (μm) | H (μm) | L (μm) | H (μm) | |
| isolated | 8.8 | 5.2 | 11.6 | 4.9 | 15.3 | 14.48 | 15.15 | 11.4 |
| approach | 9.3 | 5.55 | 10.3 | 4.9 | 12.3 | 9.3 | 12.2 | 9.9 |
| collision | 8.25 | 4.8 | 6.6 | 6.6 | 14.25 | 13.65 | 14.7 | 12.15 |
| transient aggregate | 8.7 | 8.1 | 7.8 | 6.7 | 13.35 | 13.05 | 14.6 | 13.5 |
| adhesion | 7.65 | 6.3 | 7.05 | 5.85 | 15.3 | 12.0 | 12.3 | 10.05 |
| detach | 9.3 | 4.65 | 10.95 | 5.7 | 14.25 | 11.85 | 15.45 | 11.7 |
| Conditions | PMN | TC | ||||||
|---|---|---|---|---|---|---|---|---|
| Low Shear | High Shear | Low Shear | High Shear | |||||
| L (μm) | H (μm) | L (μm) | H (μm) | L (μm) | H (μm) | L (μm) | H (μm) | |
| isolated | 10.8 | 8.03 | 11.0 | 8.3 | 14.7 | 11.7 | 13.7 | 10.4 |
| approach | 11.7 | 8.55 | 17.7 | 5.85 | 14.55 | 12.6 | 15.0 | 14.1 |
| collision | 6.75 | 4.2 | 8.7 | 5.0 | 14.03 | 12.83 | 13.7 | 12.2 |
| transient aggregate | 11.7 | 9.9 | 7.875 | 5.55 | 12.0 | 10.5 | 13.05 | 10.7 |
| adhesion | 11.25 | 7.05 | 9.2 | 6.5 | 15.3 | 11.85 | 13.4 | 13.0 |
| detach | 9.9 | 5.4 | 10.1 | 5.8 | 13.7 | 12.0 | 14.7 | 10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Li, A.; Wu, J.; Kunz, R.F.; Sun, R.; Ding, Z.; Wu, J.; Dong, C. Fibrinogen and Fibrin Differentially Regulate the Local Hydrodynamic Environment in Neutrophil–Tumor Cell–Endothelial Cell Adhesion System. Appl. Sci. 2021, 11, 79. https://doi.org/10.3390/app11010079
Fu Y, Li A, Wu J, Kunz RF, Sun R, Ding Z, Wu J, Dong C. Fibrinogen and Fibrin Differentially Regulate the Local Hydrodynamic Environment in Neutrophil–Tumor Cell–Endothelial Cell Adhesion System. Applied Sciences. 2021; 11(1):79. https://doi.org/10.3390/app11010079
Chicago/Turabian StyleFu, Yi, Ang Li, Jie Wu, Robert F. Kunz, Ren Sun, Zurong Ding, Jianhua Wu, and Cheng Dong. 2021. "Fibrinogen and Fibrin Differentially Regulate the Local Hydrodynamic Environment in Neutrophil–Tumor Cell–Endothelial Cell Adhesion System" Applied Sciences 11, no. 1: 79. https://doi.org/10.3390/app11010079
APA StyleFu, Y., Li, A., Wu, J., Kunz, R. F., Sun, R., Ding, Z., Wu, J., & Dong, C. (2021). Fibrinogen and Fibrin Differentially Regulate the Local Hydrodynamic Environment in Neutrophil–Tumor Cell–Endothelial Cell Adhesion System. Applied Sciences, 11(1), 79. https://doi.org/10.3390/app11010079





