Use of a Heating System to Control the Probiotic Beverage Production in Batch Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Equipment and Measurements
2.1.1. Batch Bioreactor
2.1.2. Heating System
2.1.3. CO2 Measurement System
2.1.4. Substances for the Fermentation Process
2.2. Mathematical Model of the Fermentation Process
2.2.1. Constant Temperature Model
- X(t)—the concentration of the microorganisms (g/L),
- S(t)—the concentration of the substrate (g/L),
- P(t)—the concentration of the product (mol/L),
- μm—maximum microorganisms’ growth rate (h−1),
- Km—substrate saturation constant (g/L),
- α—parameter that describes the relation between product yield and microorganism growth,
- β—parameter that describes the product yield independent of the microorganism growth (h−1).
2.2.2. Temperature-Dependent Model
- PΔ(s)—output of the temperature-dependent model (i.e., fermentation product deviation due to change in bioreactor’s temperature),
- TΔ(s)—input of the temperature-dependent model (i.e., bioreactor’s temperature deviation)
- G1(s)—transfer function that denotes the long-term influence of the temperature deviation TΔ(s) on fermentation product deviation PΔ(s), and is especially important after the transient response caused by temperature change,
- G2(s)—transfer function that denotes the short-term influence of the temperature deviation TΔ(s) on fermentation product deviation PΔ(s), it has an impact during the transient response caused by temperature change,
- k1, T1—gain and time constant of the first-order transfer function G1(s),
- k2, T2, z—gain, time constant and damping of the second-order term in transfer function G2(s),
- Td—time constant of the differentiator which is in series with the second-order term in transfer function G2(s), and
- s—complex variable.
2.2.3. Complete Model
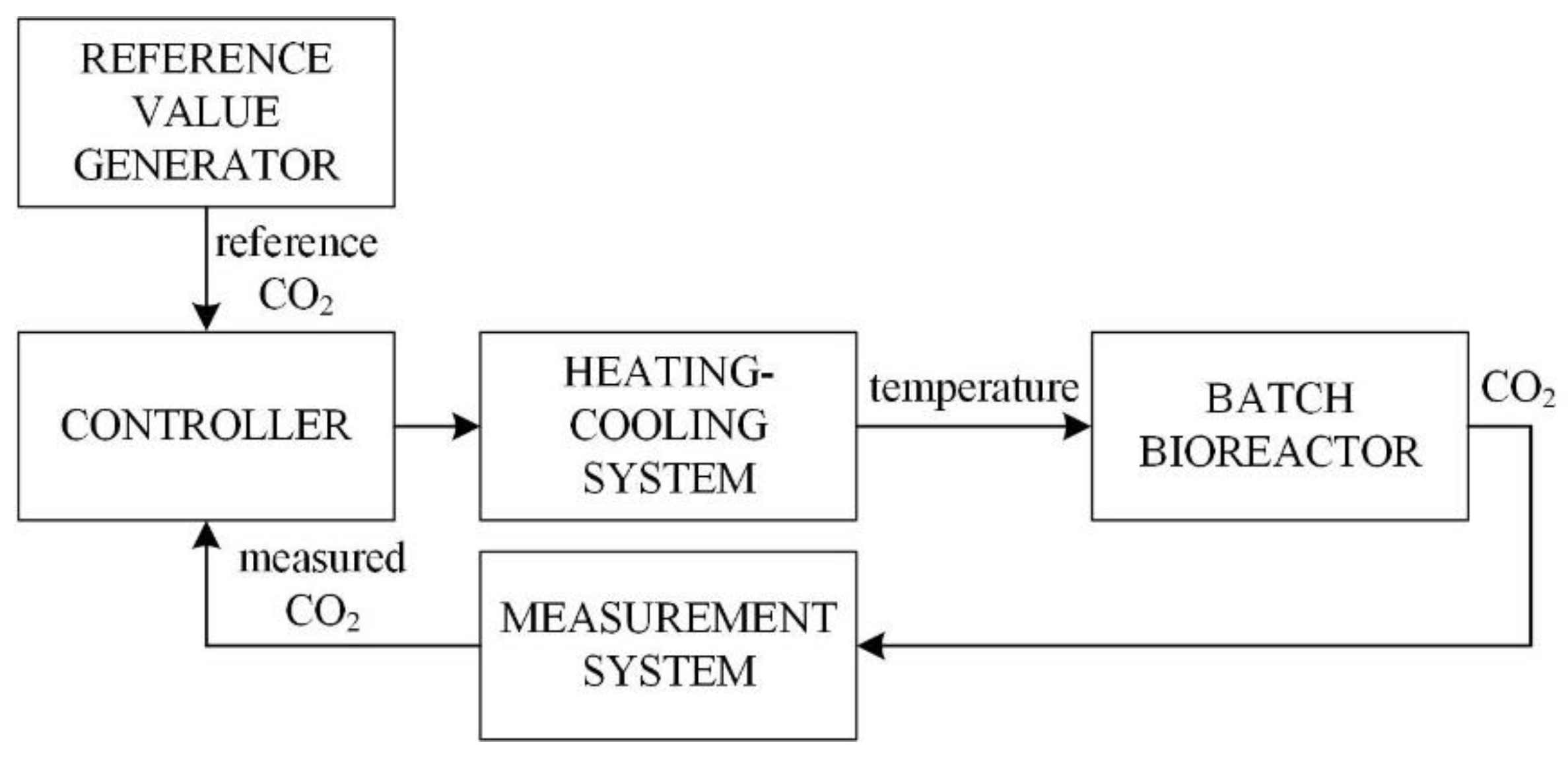
2.3. Control System for the Fermentation Process
- -
- The reference value generator that determines the reference value of the CO2, which provides a sufficient amount of high-quality fermentation product in short fermentation time;
- -
- The measurement system which measures the CO2 concentration;
- -
- The controller, its input is the error between the reference CO2 value and measured CO2 concentration. Based on this deviation, the controller calculates the required temperature change to ensure that the measured CO2 value will be as close as possible to the reference CO2 value;
- -
- The heating/cooling system that provides that the temperature in the reactor is as required by the controller.
3. Results and Discussion
3.1. Identification of the Mathematical Model
3.1.1. Identification of the Constant Temperature Model
3.1.2. Identification of the Temperature-Dependent Model
3.1.3. Simulation of the Complete Model
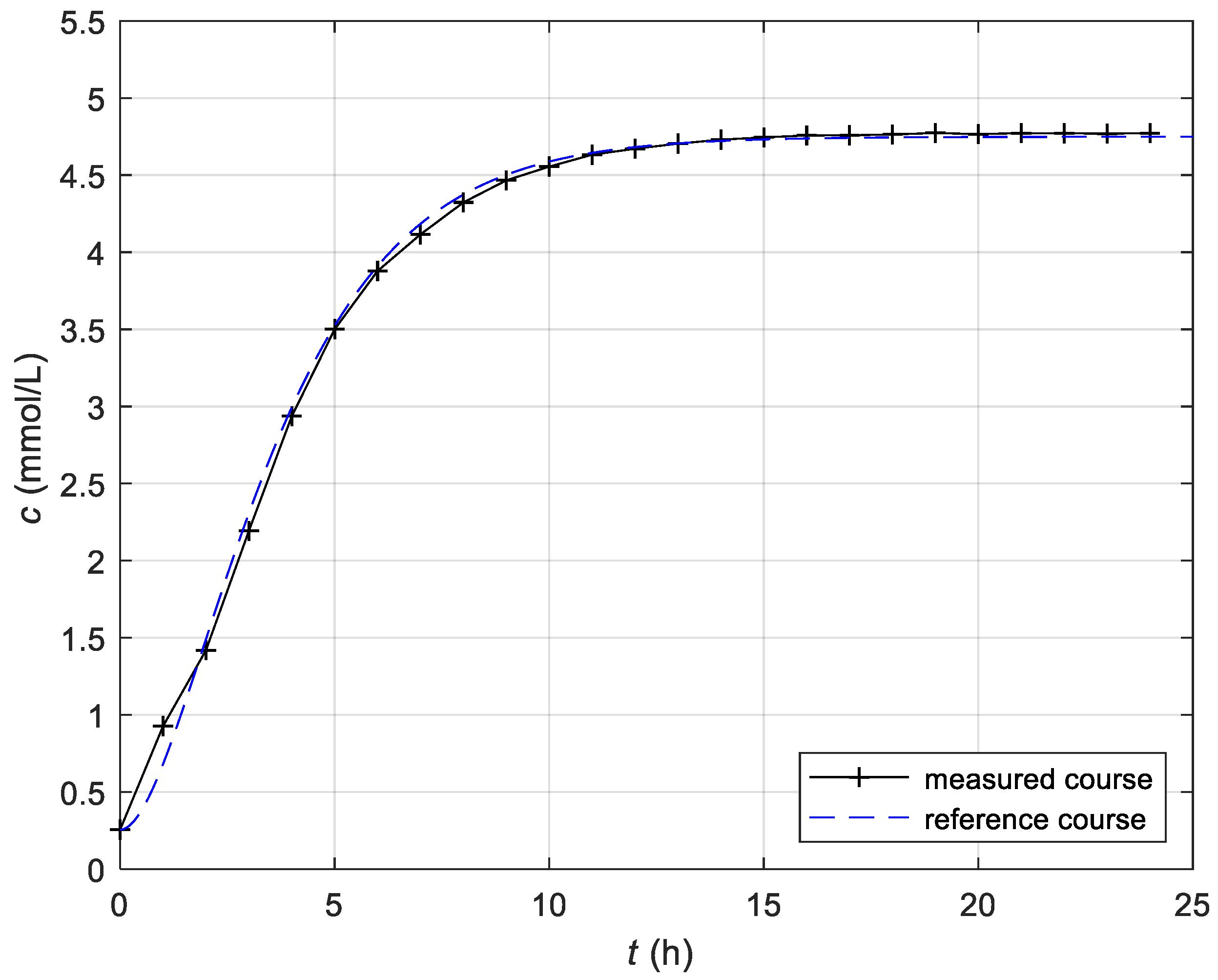
3.2. Determination of the Reference Course of CO2 Concentration
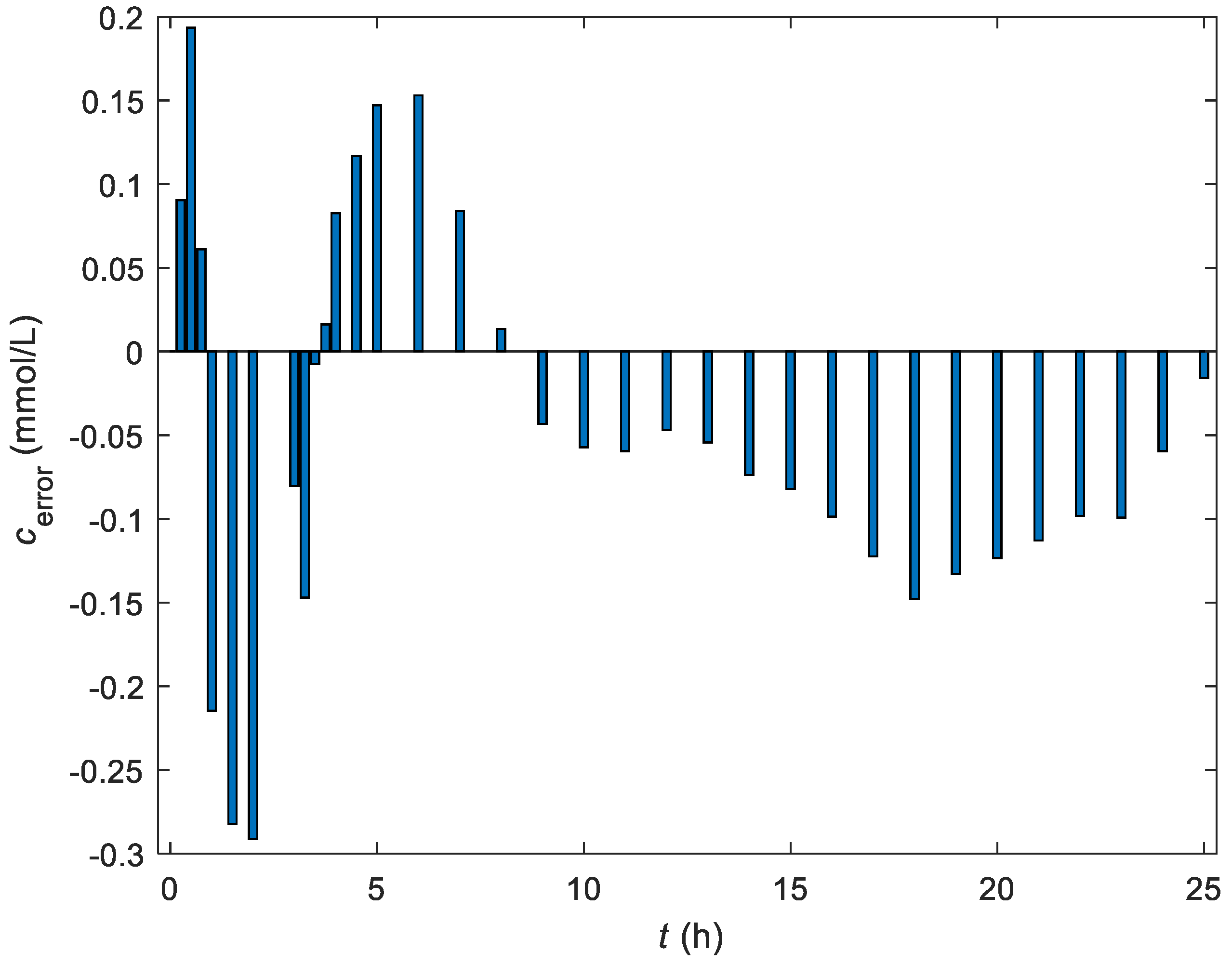
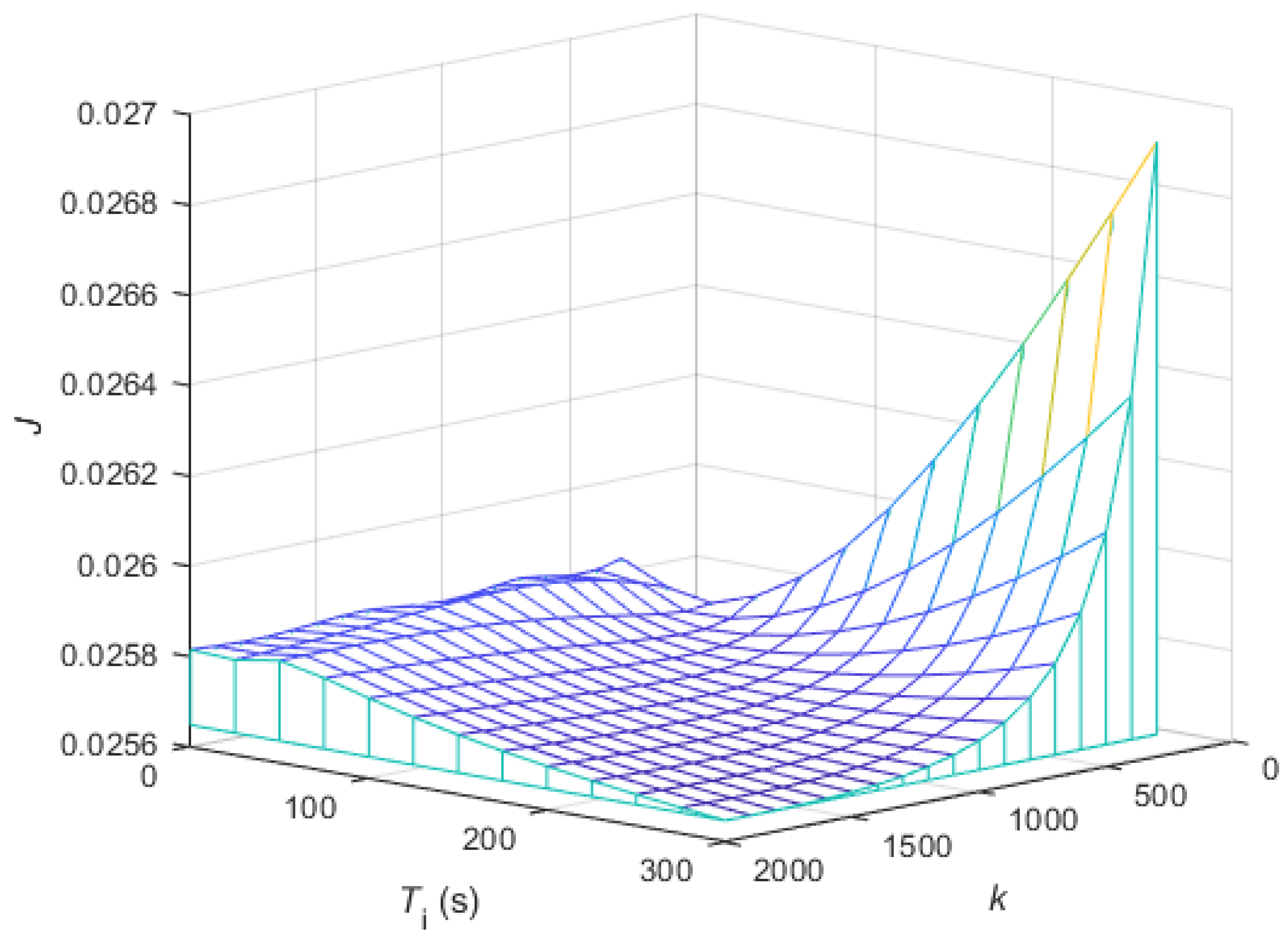
3.3. Tuning of the Controller Parameters
- J—objective function (i.e., cost function),
- e(t)—deviation between the reference and the actual value of CO2 concentration (i.e., control error),
- u(t)—calculated required change of temperature (i.e., controller output) and
- q, r—parameters that enable different weighting of e(t) and u(t) in the objective function.
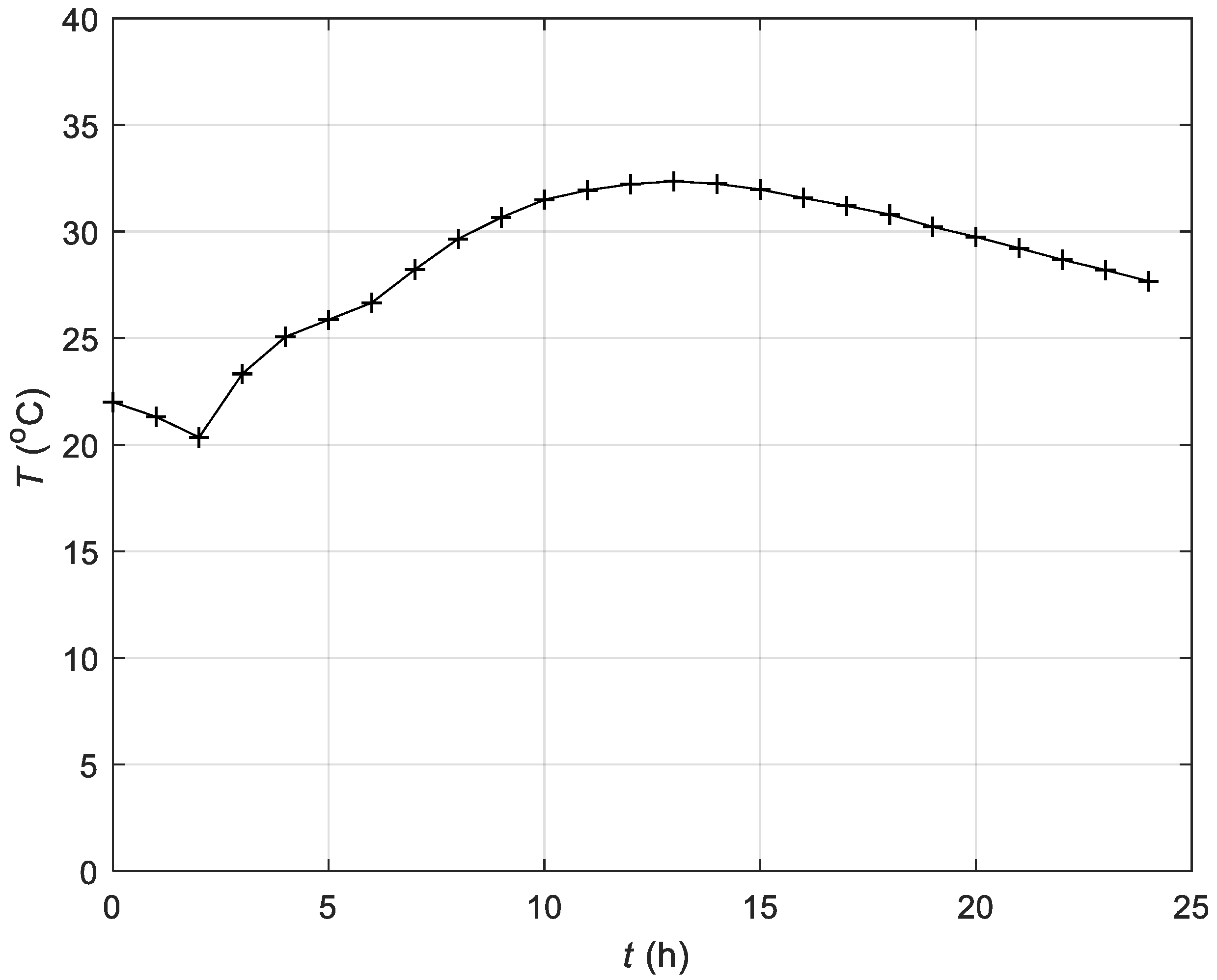
3.4. Experimental Results of the Control of the Fermentation Process in the Batch Bioreactor
4. Conclusions
- -
- An experimental analysis of the batch bioreactor, and finding that it is possible to develop a control system based on temperature changes in the bioreactor;
- -
- Derivation of a new, enhanced model of the fermentation process in the batch bioreactor;
- -
- Identification of the parameters of the mathematical model utilizing the differential evolution optimization method;
- -
- Design of a closed-loop control system for a batch bioreactor;
- -
- Tuning of the controller parameters by means of selected objective functions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguirre-Ezkauriatza, E.J.; Aguilar-Yáñez, J.M.; Ramírez-Medrano, A.; Alvarez, M.M. Production of probiotic biomass (Lactobacillus casei) in goat milk whey: Comparison of batch, continuous and fed-batch cultures. Bioresour. Technol. 2010, 101, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Z.; Chen, T.; Zhao, X. Development of Novel Bioreactor Control Systems Based on Smart Sensors and Actuators. Front. Bioeng. Biotechnol. 2020, 8, 7. [Google Scholar] [CrossRef]
- Yang, J.-D.; Lu, C.; Stasny, B.; Henley, J.; Guinto, W.; Gonzalez, C.; Gleason, J.; Fung, M.; Collopy, B.; Benjamino, M.; et al. Fed-batch bioreactor process scale-up from 3-L to 2,500-L scale for monoclonal antibody production from cell culture. Biotechnol. Bioeng. 2007, 98, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-T.; Erh, M.-H.; Lin, S.-P.; Lo, K.-Y.; Chen, K.-I.; Cheng, K.-C. Enrichment of two isoflavone aglycones in black soymilk by Rhizopus oligosporus NTU 5 in a plastic composite support bioreactor. J. Sci. Food Agric. 2016, 96, 3779–3786. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, Y.; Liu, X.; Zhou, X.; Wang, Z.; Wang, G.; Xiong, Z.; Ai, L. Enhancement of antroquinonol production during batch fermentation using pH control coupled with an oxygen vector. J. Sci. Food Agric. 2019, 99, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.M.; Aguirre-Ezkauriatza, E.J.; Ramírez-Medrano, A.; Rodríguez-Sánchez, Á. Kinetic analysis and mathematical modeling of growth and lactic acid production of Lactobacillus casei var. rhamnosus in milk whey. J. Dairy Sci. 2010, 93, 5552–5560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Jiang, S.; Zhang, J.; Zhang, Q.; Ning, Y.; Fang, M.; Liu, S. Parametric optimization and kinetic study of l-lactic acid production by homologous batch fermentation of Lactobacillus pentosus cells. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef]
- Zelle, R.M.; de Hulster, E.; Kloezen, W.; Pronk, J.T.; van Maris, A.J.A. Key Process Conditions for Production of C4 Dicarboxylic Acids in Bioreactor Batch Cultures of an Engineered Saccharomyces cerevisiae Strain. Appl. Environ. Microbiol. 2010, 76, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Dumbrepatil, A.; Adsul, M.; Chaudhari, S.; Khire, J.; Gokhale, D. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl. Environ. Microbiol. 2008, 74, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Schorsch, J.; Castro, C.C.; Couto, L.D.; Nobre, C.; Kinnaert, M. Optimal control for fermentative production of fructo-oligosaccharides in fed-batch bioreactor. J. Process Control 2019, 78, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Cui, Y.; Zhou, Y.; Luo, Z.; Liu, J.; Zhao, M. The industrial applications of cassava: Current status, opportunities and prospects. J. Sci. Food Agric. 2017, 97, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; He, Q.; He, Z.; Hemme, C.L.; Wu, L.; Zhou, J. Continuous Cellulosic Bioethanol Fermentation by Cyclic Fed-Batch Cocultivation. Appl. Environ. Microbiol. 2013, 79, 1580–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.-Y.; Jia, D.-C.; Zhang, K.-D.; Zhu, H.-F.; Zhang, Q.; Jiang, W.-H.; Gu, Y.; Li, F.-L. Ethanol Metabolism Dynamics in Clostridium ljungdahlii Grown on Carbon Monoxide. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Hernández-Cortés, G.; Córdova-López, J.A.; Herrera-López, E.J.; Morán-Marroquín, G.A.; Valle-Rodríguez, J.O.; Díaz-Montaño, D.M. Effect of pH, aeration and feeding non-sterilized agave juice in a continuous agave juice fermentation. J. Sci. Food Agric. 2010, 90, 1423–1428. [Google Scholar] [CrossRef]
- Plessas, S.; Kourkoutas, Y.; Psarianos, C.; Kanellaki, M.; Koutinas, A.A. Continuous baker’s yeast production using orange peel as promoting support in the bioreactor. J. Sci. Food Agric. 2006, 86, 407–414. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Kiel, P. Selection of lactic acid bacteria for acidification of brown juice (grass juice), with the aim of making a durable substrate for L-lysine fermentation. J. Sci. Food Agric. 2008, 88, 976–983. [Google Scholar] [CrossRef]
- de Andrade, G.A.; Pagano, D.J.; Guzmán, J.L.; Berenguel, M.; Fernández, I.; Acién, F.G. Distributed Sliding Mode Control of pH in Tubular Photobioreactors. IEEE Trans. Control Syst. Technol. 2016, 24, 1160–1173. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Cutright, T.J.; Qammar, H.K. Control of a continuous bioreactor using model predictive control. Process Biochem. 2005, 40, 2763–2770. [Google Scholar] [CrossRef]
- Yan, X.; Bergstrom, D.J.; Chen, X.B. Modeling of Cell Cultures in Perfusion Bioreactors. IEEE Trans. Biomed. Eng. 2012, 59, 2568–2575. [Google Scholar] [CrossRef]
- Peroni, C.V.; Kaisare, N.S.; Lee, J.H. Optimal control of a fed-batch bioreactor using simulation-based approximate dynamic programming. IEEE Trans. Control Syst. Technol. 2005, 13, 786–790. [Google Scholar] [CrossRef]
- Romoli, S.; Scaglia, G.J.E.; Serrano, M.E.; Godoy, S.A.; Ortiz, O.A.; Vega, J.R. Control of a Fed-Batch Fermenter Based on a Linear Algebra Strategy. IEEE Lat. Am. Trans. 2014, 12, 1206–1213. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Munoz, J.A.T.; Luna, R.; Correa, J.R.P.; Bocanegra, A.R.D.; Ramirez, H.S.; Castro, R. Robust Control for cultivation of microorganisms in a high density fed-batch bioreactor. IEEE Lat. Am. Trans. 2015, 13, 1927–1933. [Google Scholar] [CrossRef]
- Williams, D.; Yousefpour, P.; Wellington, E.M. On-line adaptive control of a fed-batch fermentation of Saccharomyces cerevisiae. Biotechnol. Bioeng. 1986, 28, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Estakhrouiyeh, M.R.; Vali, M.; Gharaveisi, A. Application of fractional order iterative learning controller for a type of batch bioreactor. IET Control Theory Appl. 2016, 10, 1374–1383. [Google Scholar] [CrossRef]
- Arévalo, H.; Snáchez, F.; Ruiz, F.; Guerrero, D.; Patino, D.; Alméciga-Díaz, C.; Rodríguez-López, A. Gain-Scheduled Oxygen Concentration Control System for a Bioreactor. IEEE Lat. Am. Trans. 2018, 16, 2689–2697. [Google Scholar] [CrossRef]
- Chitra, M.; Pappa, N.; Abraham, A. Dissolved Oxygen Control of Batch Bioreactor using Model Reference Adaptive Control scheme. IFAC-Pap. 2018, 51, 13–18. [Google Scholar] [CrossRef]
- Ritonja, J.; Gorsek, A.; Pecar, D. Control of Milk Fermentation in Batch Bioreactor. Elektron. Elektrotechnika 2020, 26, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Cinar, A.; Parulekar, S.J.; Undey, C.; Birol, G. Batch Fermentation: Modeling: Monitoring, and Control; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-8247-4849-4. [Google Scholar]
- Henson, M.A. Exploiting cellular biology to manufacture high-value products—Biochemical reactor modelling and control. IEEE Control Syst. Mag. 2006, 54–62. [Google Scholar]
- Goršek, A.; Ritonja, J.; Pečar, D. Mathematical model of CO2 release during milk fermentation using natural kefir grains. J. Sci. Food Agric. 2018, 98, 4680–4684. [Google Scholar] [CrossRef]
- Price, K.; Storn, R.M.; Lampinen, J.A. Differential Evolution: A Practical Approach to Global Optimization; Natural Computing Series; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 978-3-540-20950-8. [Google Scholar]
- Åström, K.J.; Hägglund, T. PID Controllers: Theory, Design, and Tuning; The International Society for Measurement and Control: Research Triangle Park, NC, USA, 1988. [Google Scholar]

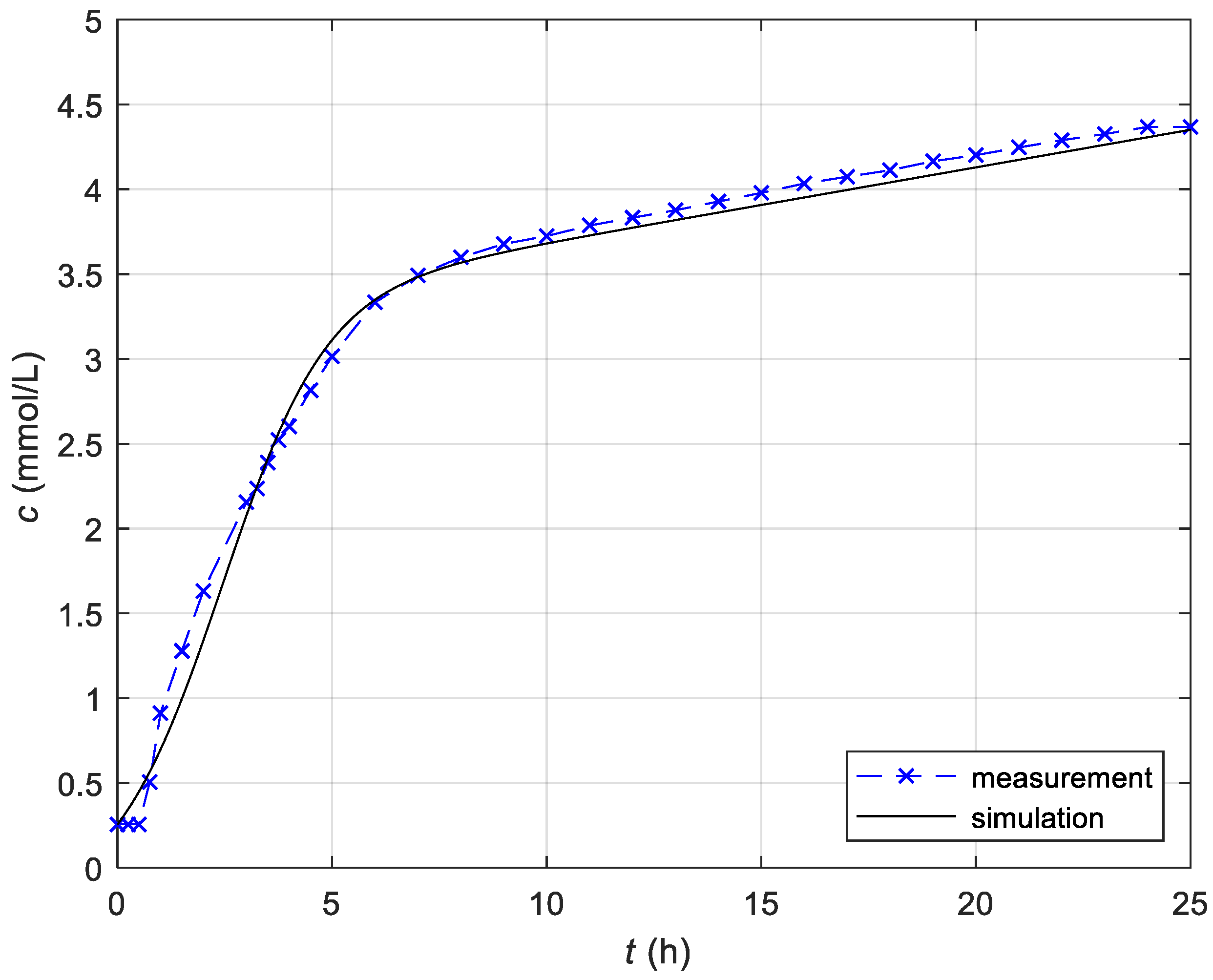
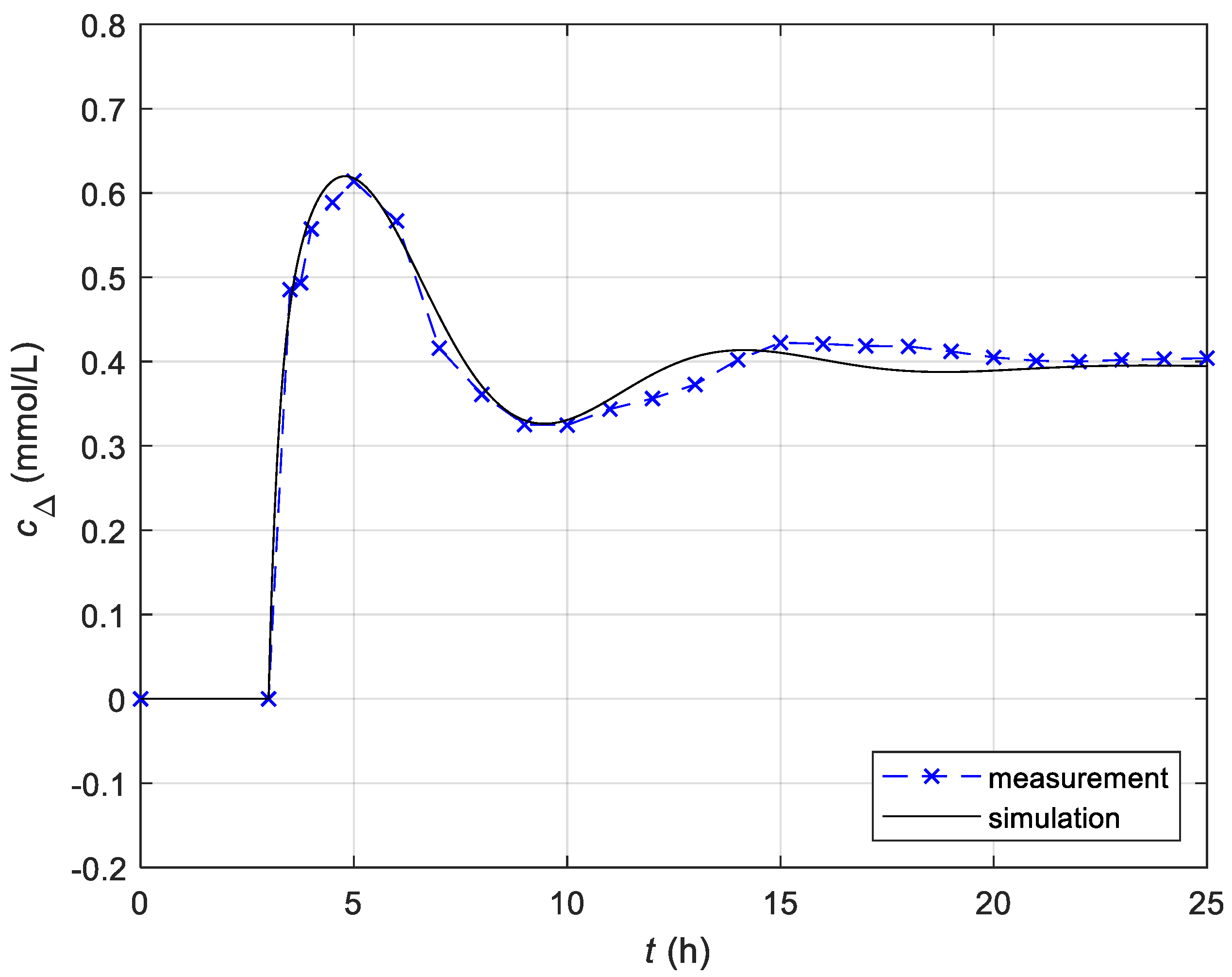
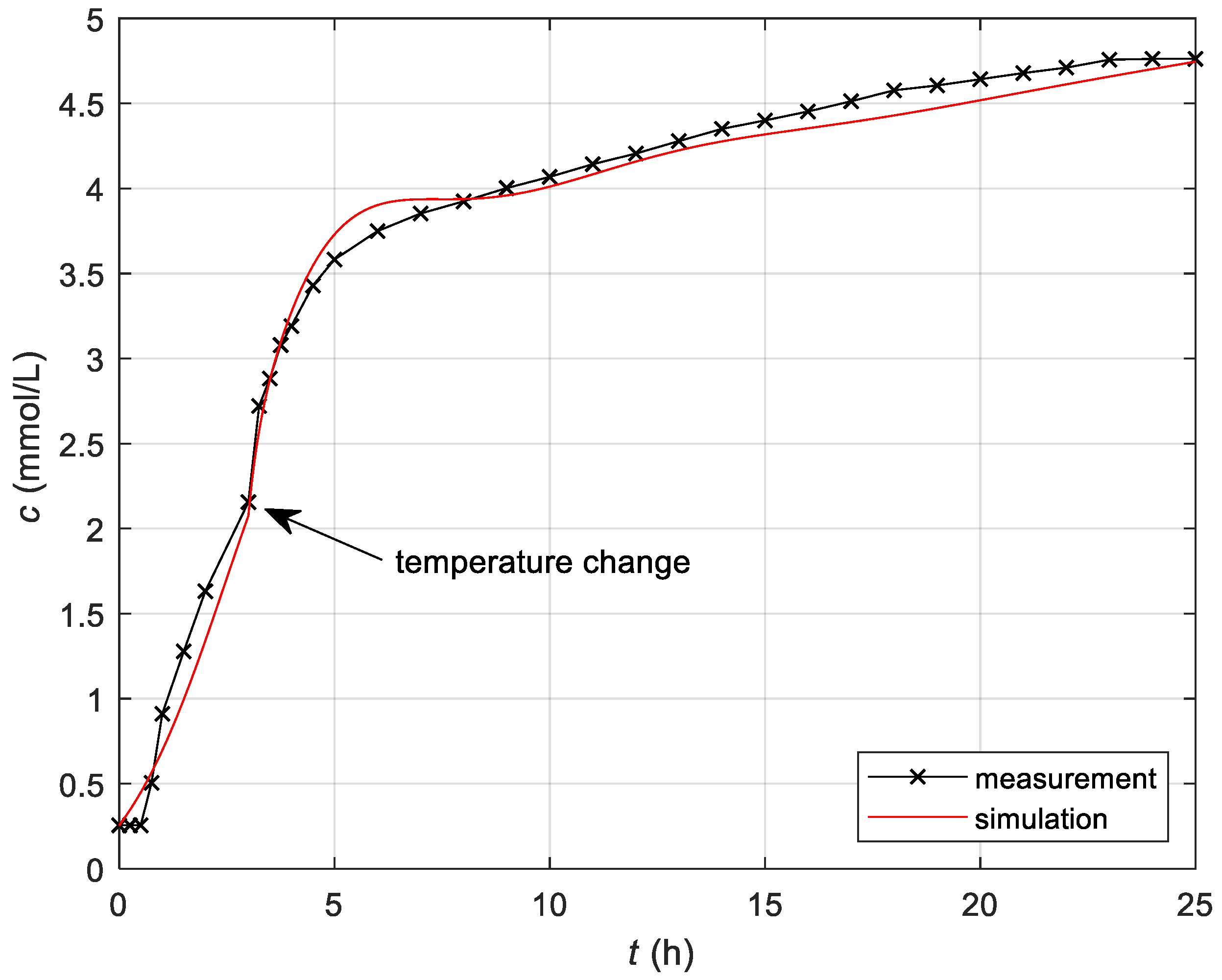
| Measured Initial Values | Identified Parameters |
|---|---|
| X(0) = 6.0 × 10−4 g/L | μm = 6.0 h−1 |
| S(0) = 3.7 × 10−3 g/L | Km = 0.03 g/L |
| P(0) = 2.6 × 10−4 mol/L | α = 0.84 |
| β = 0.01 h−1 |
| Index | Value |
|---|---|
| maximum absolute error | emax = 2.9125 × 10−4 mol/L |
| average absolute error | ea = 8.0249 × 10−5 mol/L |
| integral square error | ISE = 2.5640 × 10−7 |
| integral absolute error | IAE = 1.991 × 10−3 |
| G1(s) | G2(s) |
|---|---|
| k1 = 7.87 × 10−5 | k2 = 1.8 |
| T1 = 800 s | T1 = 5000 s |
| z = 0.36 | |
| Td = 0.2 s |
| Index | Value |
|---|---|
| maximum absolute error | emax = 4.0220 × 10−5 mol/L |
| average absolute error | ea = 1.5853 × 10−5 mol/L |
| integral square error | ISE = 8.0573 × 10−9 |
| integral absolute error | IAE = 3.6025 × 10−4 |
| Index | Value |
|---|---|
| maximum absolute error | emax = 2.9125 × 10−4 mol/L |
| average absolute error | ea = 1.0027 × 10−4 mol/L |
| integral square error | ISE = 3.7115 × 10−7 |
| integral absolute error | IAE = 2.6139 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritonja, J.; Goršek, A.; Pečar, D. Use of a Heating System to Control the Probiotic Beverage Production in Batch Bioreactor. Appl. Sci. 2021, 11, 84. https://doi.org/10.3390/app11010084
Ritonja J, Goršek A, Pečar D. Use of a Heating System to Control the Probiotic Beverage Production in Batch Bioreactor. Applied Sciences. 2021; 11(1):84. https://doi.org/10.3390/app11010084
Chicago/Turabian StyleRitonja, Jožef, Andreja Goršek, and Darja Pečar. 2021. "Use of a Heating System to Control the Probiotic Beverage Production in Batch Bioreactor" Applied Sciences 11, no. 1: 84. https://doi.org/10.3390/app11010084





