Preparation and Electron-Beam Surface Modification of Novel TiNi Material for Medical Applications
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
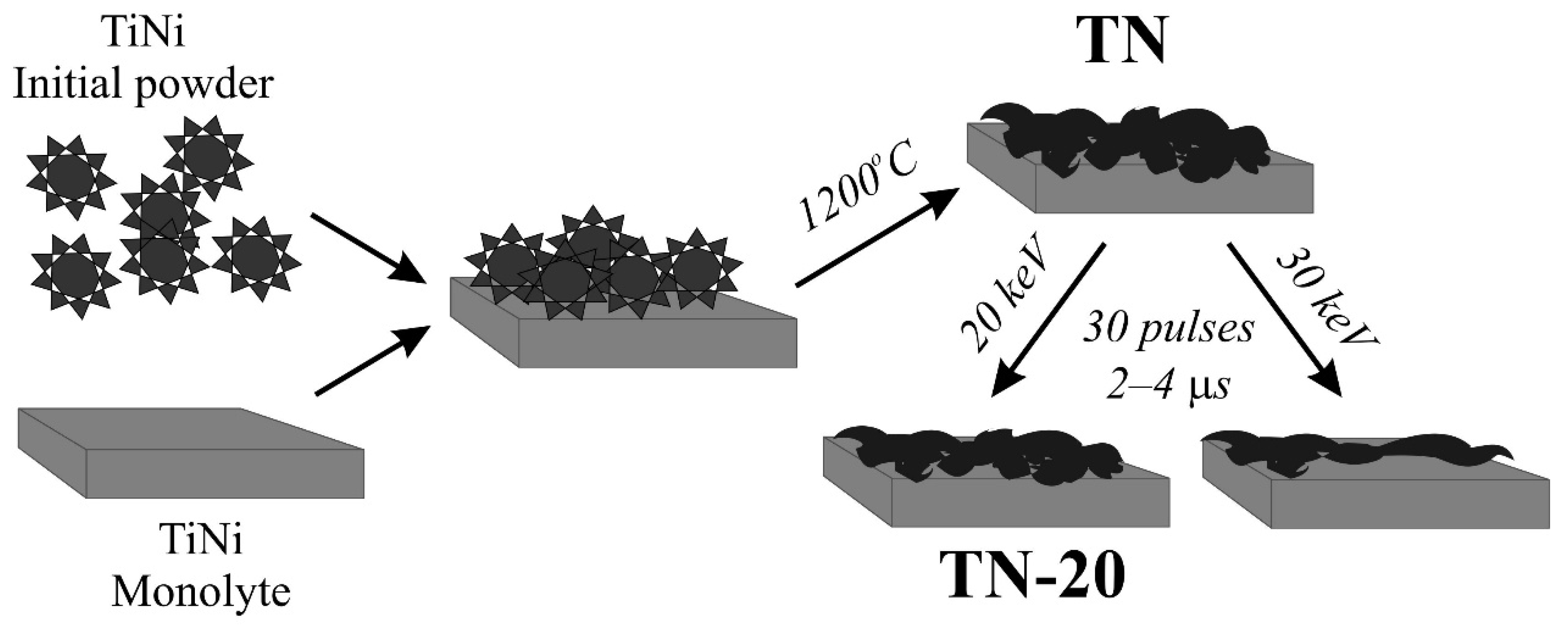
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Electrochemical Measurements
2.5. Cell Growth Experiment
3. Results and Discussion
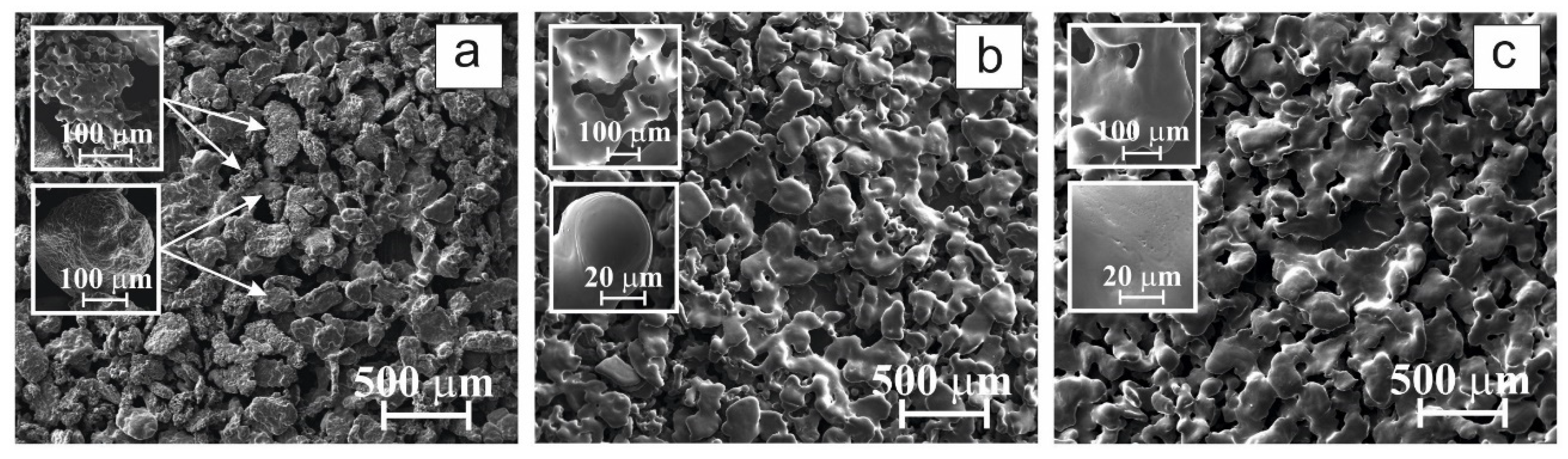
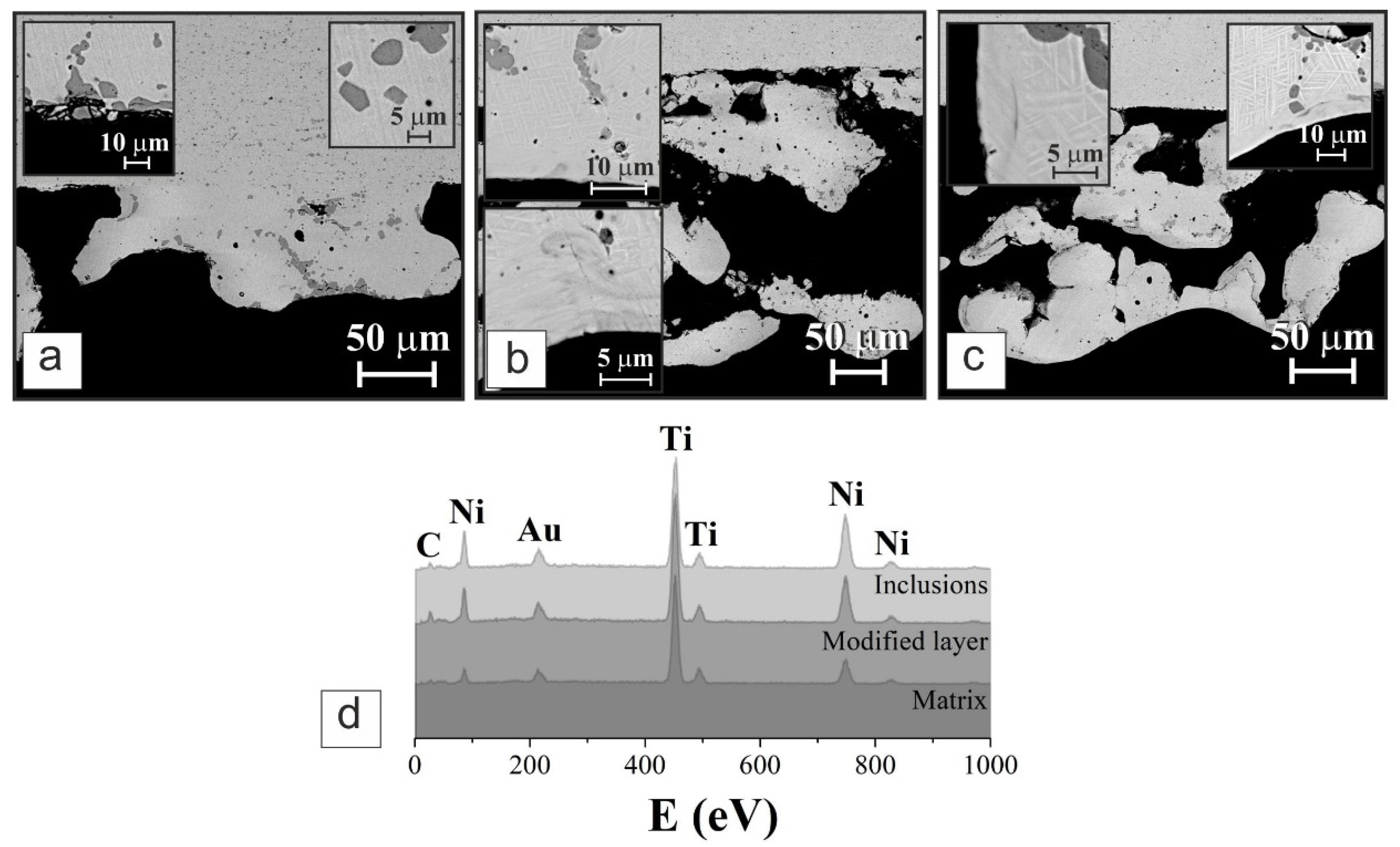
3.1. Structure and Composition
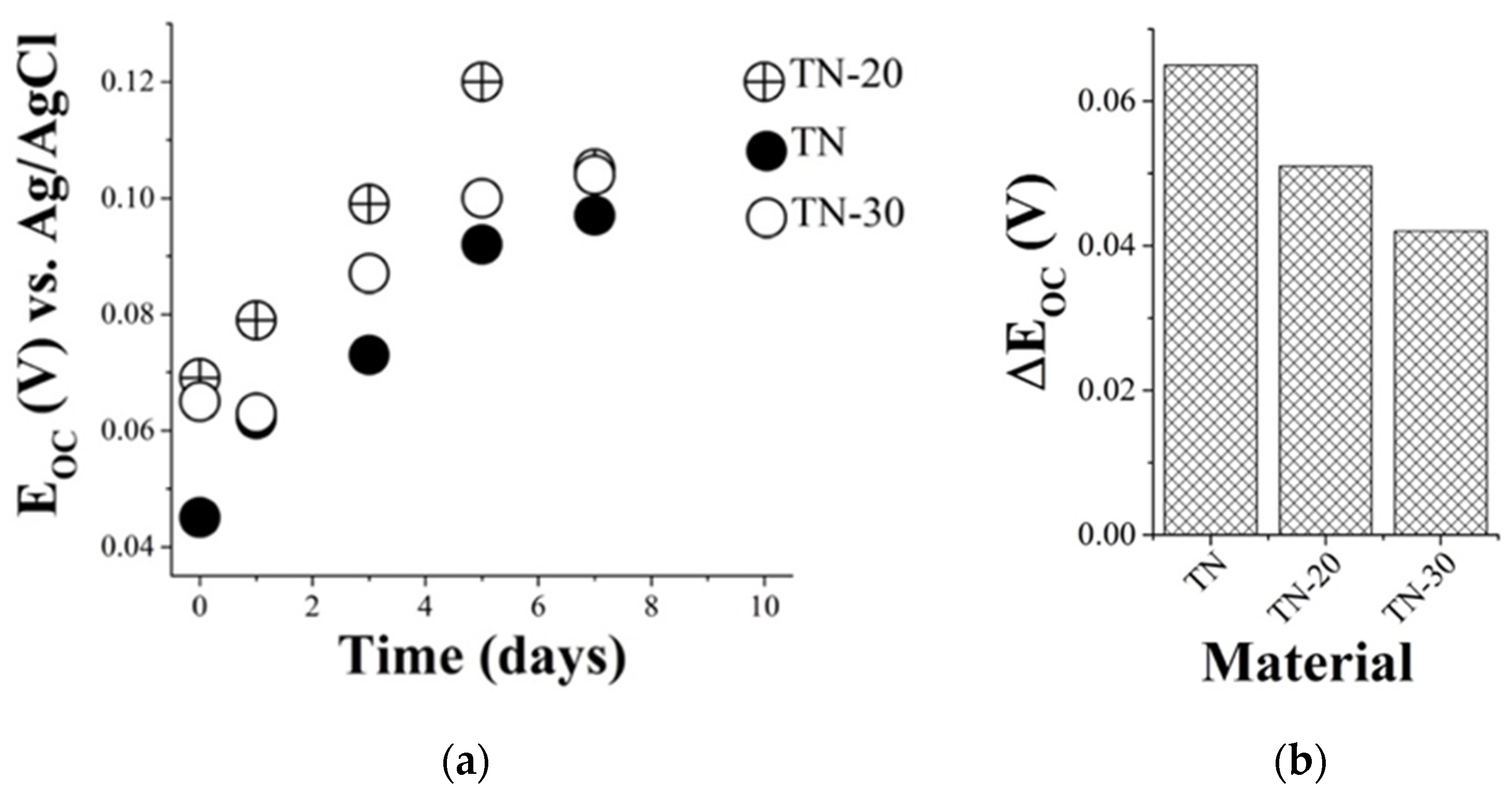
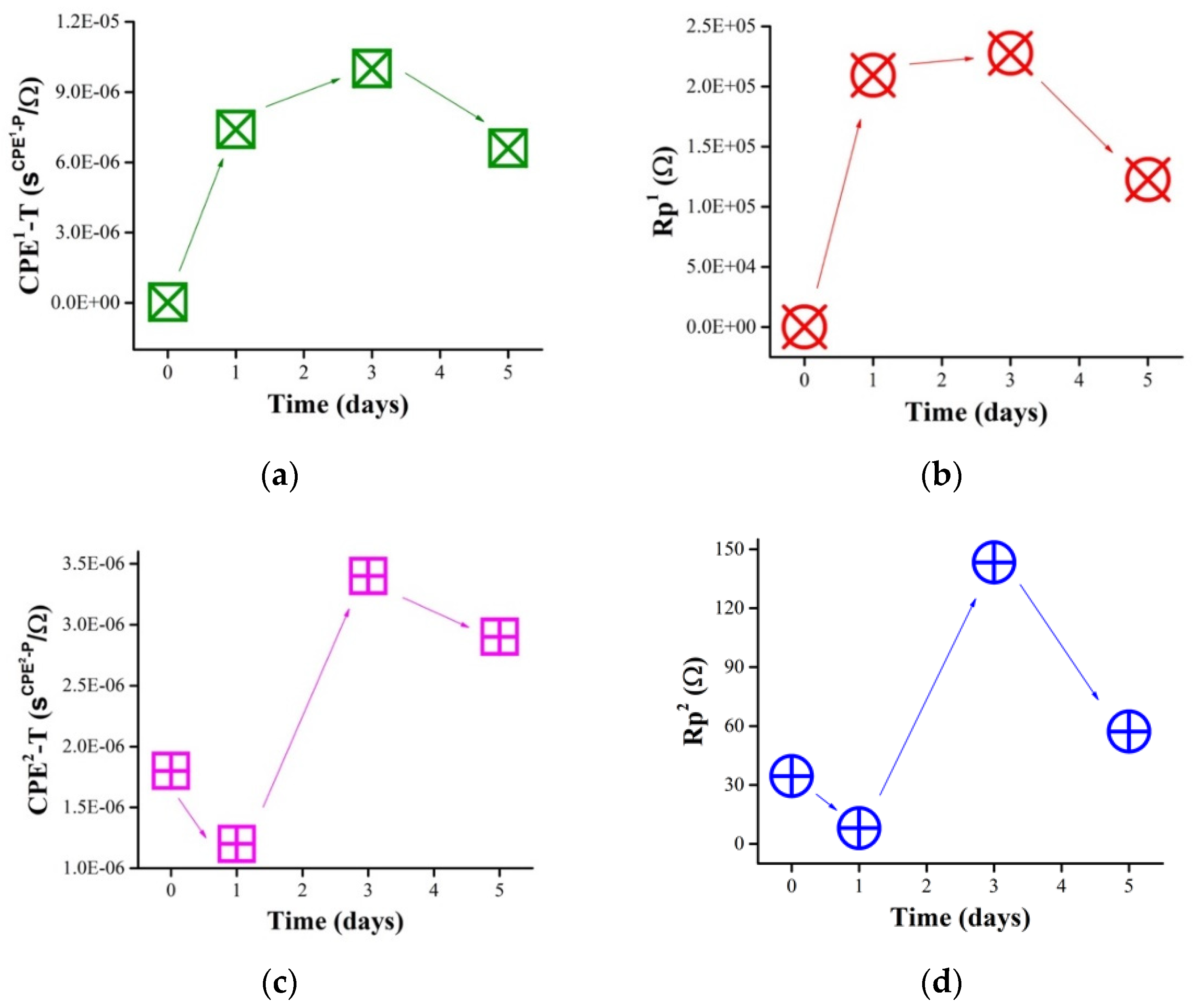
3.2. Electrochemical Tests and Cell Growth
3.2.1. Effect of Saline Medium on Samples
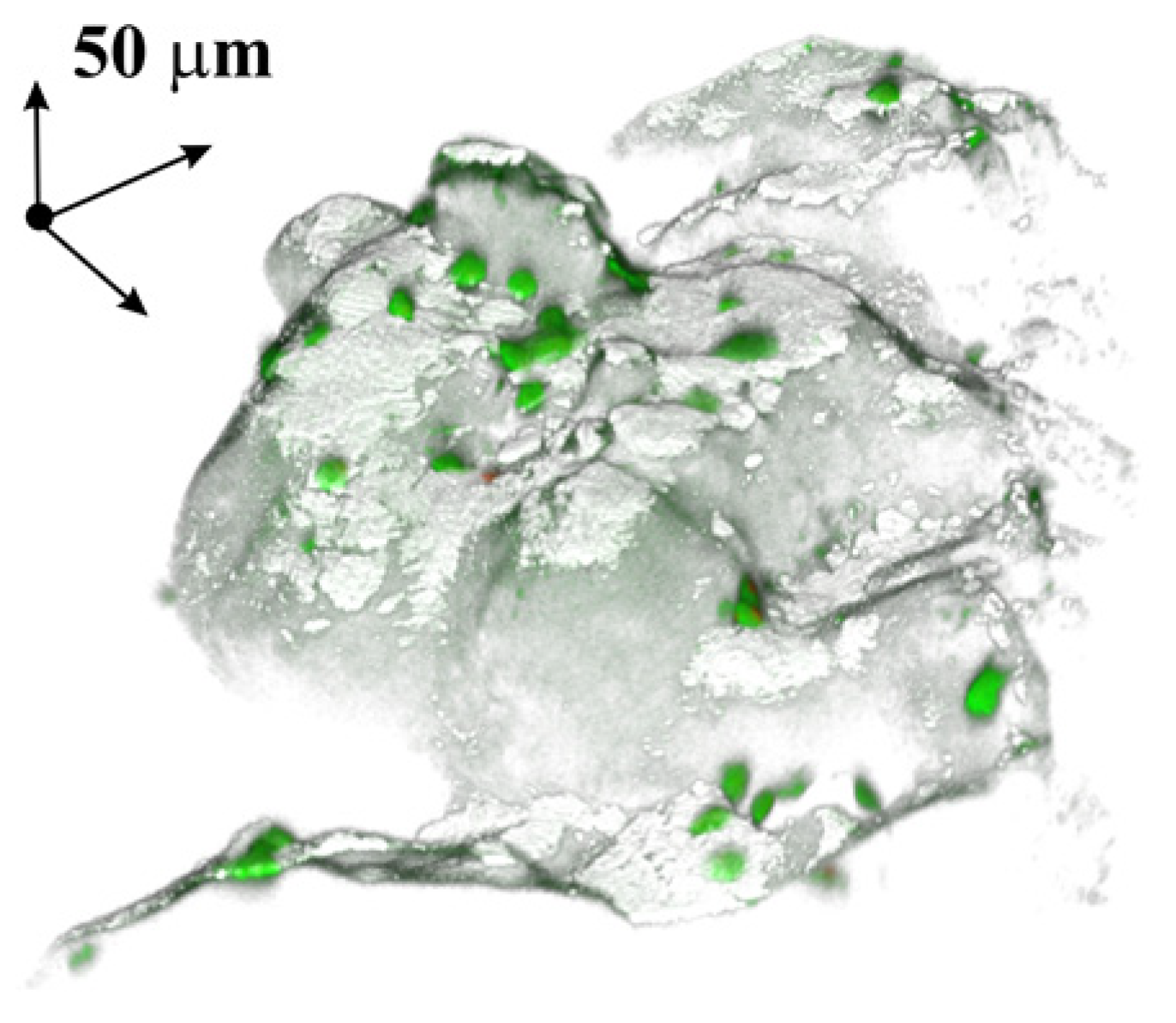
3.2.2. Cell Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Buehler, W.J.; Gilfrich, J.W.; Wiley, R.C. Effect of low-temperature phase changes on the mechanical properties of alloys near composition TiNi. J. Appl. Phys. 1963, 34, 1475–1477. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti-Ni based shape memory alloys. Prog. Mater Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Saburi, T. Ti-Ni shape memory alloys. In Shape Memory Materials; Otsuka, K., Waymann, C.M., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 49–96. [Google Scholar]
- Parvizi, S.; Hashemi, S.M.; Asgarinia, F.; Nematollahi, M.; Elahinia, M. Effective parameters on the final properties of NiTi-based alloys manufactured by powder metallurgy methods: A review. Prog. Mater. Sci. 2021, 117, 100739. [Google Scholar] [CrossRef]
- Saber, O.; Ansari, S.A.; Alshoaibi, A. Development of Ti/Ni nanolayered structures to be a new candidate for energy storage applications. Appl. Sci. 2020, 10, 6935. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Carpegna, G.; Alovisi, M.; Salvatore Paolino, D.; Marchetti, A.; Gibello, U.; Scotti, N.; Pasqualini, D.; Scattina, A.; Chiandussi, G.; Berutti, E. Evaluation of pressure distribution against root canal walls of NiTi rotary instruments by finite element analysis. Appl. Sci. 2020, 10, 2981. [Google Scholar] [CrossRef]
- Bajt, A.; Krajnovic, A.; Lakota, K.; Zigon, P.; Sodin-Semrl, S.; Kralj-Iglic, V.; Iglic, A. The importance of antibacterial surfaces in biomedical applications. In Advances in Biomembranes and Lipid Self-Assembly; Iglic, A., Rappolt, M., Garcia-Saez, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 28, pp. 115–165. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef]
- Yan, X.J.; Gugel, H.; Huth, S.; Theisen, W. Microstructures and properties of laser cladding NiTi alloy with W for biomedical applications. Mater. Lett. 2011, 65, 2934–2936. [Google Scholar] [CrossRef]
- Zhu, S.L.; Yang, X.J.; Hu, F.; Deng, S.H.; Cui, Z.D. Processing of porous TiNi shape memory alloy from elemental powders by Ar-sintering. Mater. Lett. 2004, 58, 2369–2373. [Google Scholar] [CrossRef]
- Bartolo, P.J.; Bidanda, B. Bio-Materials and Prototyping Applications in Medicine; Springer Publishing: New York, NY, USA, 2008; 216p. [Google Scholar]
- Chua, C.K.; Leong, K.F.; Lim, C.S. Rapid Prototyping: Principles and Applications, 2nd ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2010; 540p. [Google Scholar]
- Shishkovsky, I.V.; Scherbakov, V.I.; Morozov, Y.G.; Kuznetsov, M.V.; Parkin, I.P. Surface laser sintering of exothermal powder compositions: The thermal and SEM/EDX study. J. Therm. Anal. Calorim. 2008, 91, 427–436. [Google Scholar] [CrossRef]
- Elsayed, A.; Umeda, J.; Kondoh, K. Effect of quenching media on the properties of TiNi shape memory alloys fabricated by powder metallurgy. J. Alloys Compd. 2020, 842, 155931. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Xie, H.; Qu, J.; Chen, X.; Chen, F.; Song, Q.; Yin, H. Engineering the porosity and superelastic behaviors of NiTi alloys prepared by an electro-assisted powder metallurgical route in molten salts. J. Alloys Compd. 2019, 794, 455–464. [Google Scholar] [CrossRef]
- Yi, X.; Wang, H.; Sun, K.; Gao, W.; Sun, B.; Shen, G.; Meng, X.; Gao, Z.; Cai, W. Characterization of high-strength Ti-Ni shape memory alloys prepared by hot pressed sintering. J. Alloys Compd. 2021, 854, 157159. [Google Scholar] [CrossRef]
- Nivala, P.T.; James, S.P. Master sintering curves of nickel-titanium and nickel-titanium open-cell foams fabricated by spark plasma sintering. J. Mater. Sci. 2019, 55, 3668–3683. [Google Scholar] [CrossRef]
- Samal, S.; Molnarova, O.; Prusa, F.; Kopecek, J.; Heller, L.; Sittner, P.; Skodova, M.; Abate, L.; Blanco, I. Net-shape NiTi shape memory alloy by spark plasma sintering method. Appl. Sci. 2021, 11, 1802. [Google Scholar] [CrossRef]
- Velmurugan, C.; Senthilkumar, V.; Biswas, K.; Yadav, S. Densification and microstructural evolution of spark plasma sintered NiTi shape memory alloy. Adv. Powder Technol. 2018, 29, 2456–2462. [Google Scholar] [CrossRef]
- Ju, X.; Dong, H. Plasma surface modification of NiTi shape memory alloy. Surf. Coat. Technol. 2006, 201, 1542–1547. [Google Scholar] [CrossRef]
- Witkowska, J.; Kaminski, J.; Płocinski, T.; Tarnowski, M.; Wierzchon, T. Corrosion resistance of NiTi shape memory alloy after hybrid surface treatment using low-temperature plasma. Vacuum 2017, 137, 92–96. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.-H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef] [Green Version]
- Levintant-Zayonts, N.; Kucharski, S. Surface characterization and wear behavior of ion implanted NiTi shape memory alloy. Vacuum 2009, 83, S220–S223. [Google Scholar] [CrossRef]
- Pequegnat, A.; Michael, A.; Wang, J.; Lian, K.; Zhou, Y.; Khan, M.I. Surface characterizations of laser modified biomedical grade NiTi shape memory alloys. Mater. Sci. Eng. C 2015, 50, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, Z.; Yang, Y.; Wang, X.; Xiong, Z.; Li, Z.; Wang, C.; Cui, L.; Huang, S.; Li, M.; et al. Study on the junction zone of NiTi shape memory alloy produced by selective laser melting via a stripe scanning strategy. Intermetallics 2020, 126, 106947. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, H.; Luo, S.; Yang, J.; Wang, Z. The effect of energy input on reaction, phase transition and shape memory effect of NiTi alloy by selective laser melting. J. Alloys Compd. 2020, 817, 153288. [Google Scholar] [CrossRef]
- Neiman, A.A.; Semin, V.O.; Meisner, L.L.; Ostapenko, M.G. Structural decomposition and phase changes in TiNi surface layer modified by low-energy high-current pulsed electron beam. J. Alloys Compd. 2019, 803, 721–729. [Google Scholar] [CrossRef]
- Liu, C.; Chu, P.K. Corrosion behavior of NiTi alloys used in orthopedic implants and prosthetic devices. In Recent Advances in Biomaterials Research; Hu, J., Ed.; Transworld Research Network: Trivandrum, India, 2008; pp. 59–97. [Google Scholar]
- Anikeev, S.G.; Garin, A.S.; Artyukhova, N.V.; Khodorenko, V.N.; Gunther, V.E. Structural and morphological features of TiNi-based powder manufactured by the method of hybrid-calcium reduction. Russ. Phys. J. 2018, 61, 749–756. [Google Scholar] [CrossRef]
- Markov, A.B.; Mikov, A.V.; Ozur, G.E.; Padei, A.G. A PИTM-CП facility for the surface alloying. Instrum. Exp. Tech. 2011, 54, 862–866. [Google Scholar] [CrossRef]
- Radi, A.; Khalil-Allafi, J.; Etminanfar, M.R.; Pourbabak, S.; Schryvers, D.; Amin-Ahmadi, B. Influence of stress aging process on variants of nano-Ni4Ti3 precipitates and martensitic transformation temperatures in NiTi shape memory alloy. Mater. Des. 2018, 142, 93–100. [Google Scholar] [CrossRef]
- Ren, X.; Miura, N.; Zhang, J.; Otsuka, K.; Tanaka, K.; Koiwa, M.; Suzuki, T.; Chumlyakov, Y.I.; Asai, M. A comparative study of elastic constants of Ti-Ni based alloys prior to martensitic transformation. Mater. Sci. Eng. A 2001, A312, 196–206. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Dlouhy, A.; Eggeler, G. Ni4Ti3-precipitation during aging of NiTi shape memory alloys and its influence on martensitic phase transformations. Acta Mater. 2002, 50, 4255–4274. [Google Scholar] [CrossRef]
- Meisner, L.L.; Lotkov, A.I.; Sivokha, V.P.; Rotshtein, V.P.; Barmina, E.G.; Girjakova, Y.L. Structural-phase condition, unelastic and plastic behavior and nanohardness of the TiNi surface layers modified by an ion- and electron irradiation. Mater. Sci. Eng. A 2006, 438–440, 558–562. [Google Scholar] [CrossRef]
- Meisner, L.L.; Sivokha, V.P. Effect of applied stress on the shape memory behavior of TiNi-based alloys with different consequences of martensitic transformations. Phys. B 2004, 344, 93–98. [Google Scholar] [CrossRef]
- Meisner, L.L.; Markov, A.B.; Proskurovsky, D.I.; Rotshtein, V.P.; Ozur, G.E.; Meisner, S.N.; Yakovlev, E.V.; Poletika, T.M.; Girsova, S.L.; Semin, V.O. Effect of inclusions on cratering behavior in TiNi shape memory alloys irradiated with a low-energy, high-current electron beam. Surf. Coat. Technol. 2016, 302, 495–506. [Google Scholar] [CrossRef]
- Meisner, L.L.; Markov, A.B.; Rotshtein, V.P.; Ozur, G.E.; Meisner, S.N.; Yakovlev, E.V.; Semin, V.O.; Mironov, Y.P.; Poletika, T.M.; Girsova, S.L.; et al. Microstructural characterization of Ti-Ta-based surface alloy fabricated on TiNi SMA by additive pulsed electron-beam melting of film/substrate system. J. Alloys Compd. 2018, 730, 376–385. [Google Scholar] [CrossRef]
- Rondelli, G.; Torricelli, P.; Fini, M.; Rimondini, L.; Giardino, R. In vitro corrosion study by EIS of an equiatomic NiTi alloy and an implant quality AISI 316 stainless steel. J. Biomed. Mater. Res. B 2006, 79, 320–324. [Google Scholar] [CrossRef]
- Li, K.; Huang, X.; Zhao, Z.S.; Li, Y.; Fu, Y.Q. Electrochemical and corrosion behaviors of sputtered TiNi shape memory films. Smart Mater. Struct. 2016, 25, 035039. [Google Scholar] [CrossRef] [Green Version]

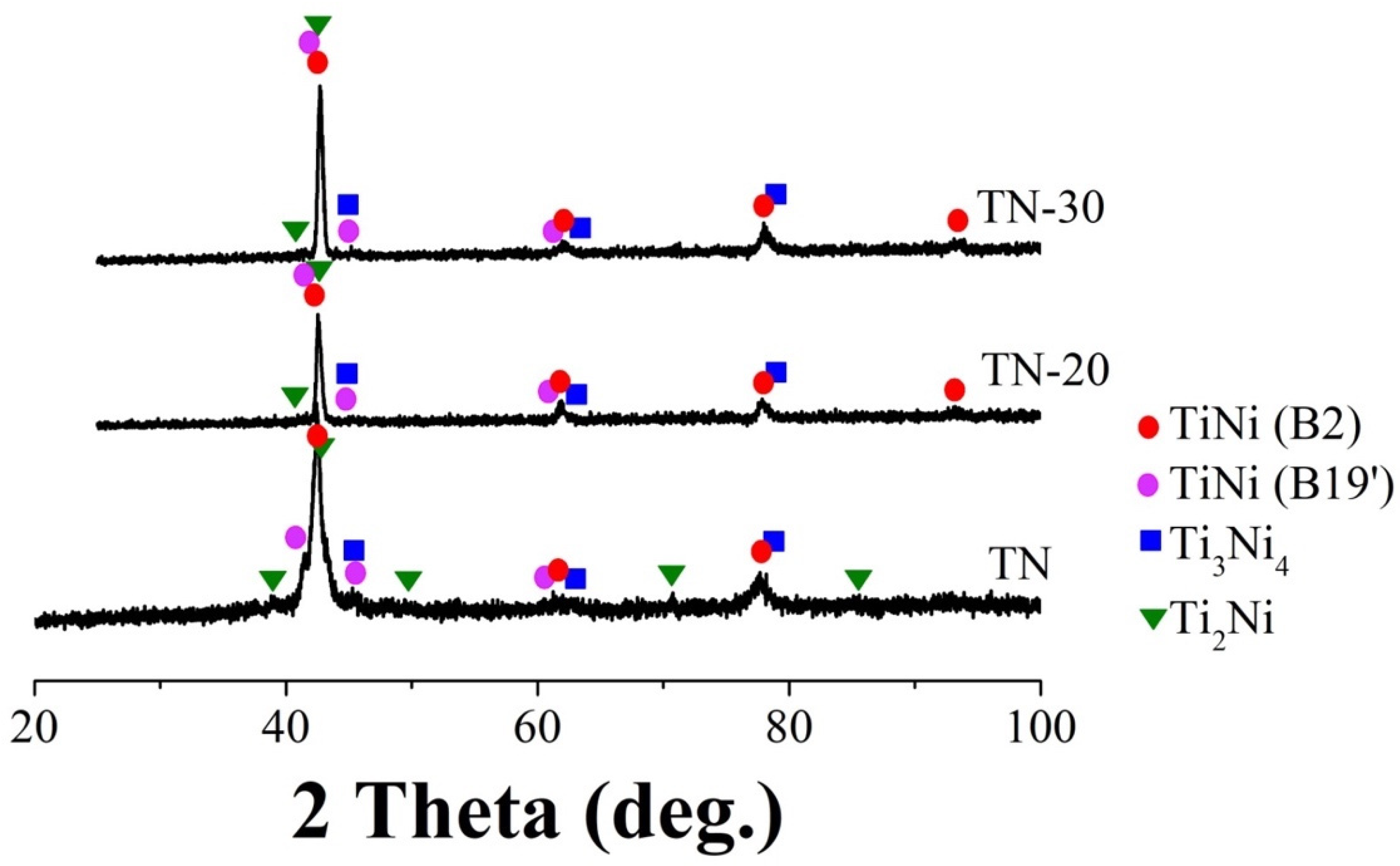


| Phase | Content in the Samples (wt%) | ||
|---|---|---|---|
| TN | TN-20 | TN-30 | |
| TiNi | 73 | 87 | 78 |
| Ti3Ni4 | 13 | 7 | 14 |
| Ti2Ni | 14 | 6 | 8 |
| Sample | Rz (µm) | Ra (µm) |
|---|---|---|
| TN | 360 | 80 |
| TN-20 | 340 | 70 |
| TN-30 | 280 | 60 |
| Analyzed Point | Element | Content (at.%) |
|---|---|---|
| Matrix | Ti | 45 |
| Ni | 55 | |
| Surface layer | Ti | 53 |
| Ni | 47 | |
| Inclusions | Ti | 64 |
| Ni | 36 |
| Time (Days) | Lifetime (s) | |
|---|---|---|
| τ1 | τ2 | |
| 0 | 10−6 | 10−5 |
| 1 | 100 | 10−6 |
| 3 | 100 | 10−4 |
| 5 | 10−1 | 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anikeev, S.G.; Shabalina, A.V.; Kulinich, S.A.; Artyukhova, N.V.; Korsakova, D.R.; Yakovlev, E.V.; Vlasov, V.A.; Kokorev, O.V.; Hodorenko, V.N. Preparation and Electron-Beam Surface Modification of Novel TiNi Material for Medical Applications. Appl. Sci. 2021, 11, 4372. https://doi.org/10.3390/app11104372
Anikeev SG, Shabalina AV, Kulinich SA, Artyukhova NV, Korsakova DR, Yakovlev EV, Vlasov VA, Kokorev OV, Hodorenko VN. Preparation and Electron-Beam Surface Modification of Novel TiNi Material for Medical Applications. Applied Sciences. 2021; 11(10):4372. https://doi.org/10.3390/app11104372
Chicago/Turabian StyleAnikeev, Sergey G., Anastasiia V. Shabalina, Sergei A. Kulinich, Nadezhda V. Artyukhova, Daria R. Korsakova, Evgeny V. Yakovlev, Vitaly A. Vlasov, Oleg V. Kokorev, and Valentina N. Hodorenko. 2021. "Preparation and Electron-Beam Surface Modification of Novel TiNi Material for Medical Applications" Applied Sciences 11, no. 10: 4372. https://doi.org/10.3390/app11104372








