Abstract
A monkey phantom is of significant value for electromagnetic radiation (EMR) dosimetry simulations. Furthermore, phantoms in various postures are needed because living beings are exposed to EMR in various postures during their daily routine. In this study, we attempted to produce monkey phantoms based on three daily postures of a rhesus monkey. From our Visible Monkey project, we selected surface models with 177 monkey structures. In the surface models, 52 virtual joints were created to allow for changes from the anatomical position to quadrupedal and sitting positions using commercial software. The surface models of the three positions were converted into monkey voxel phantoms. These phantoms were arranged in three positions, and the number of voxels and mass of each structure were analyzed. The phantoms in anatomical, quadrupedal, and sitting positions have a total of 5,054,022, 5,174,453, and 4,803,886 voxels, respectively. The mass of 177 structures in three positions were also calculated based on the number of voxels. By comparing the monkey phantom with the phantom of a female human, we confirmed thicker skin, less fat, heavier muscle, and a lighter skeleton in monkeys than those in humans. Through posture-transformed monkey phantoms, more precise EMR simulations could be possible. The ultimate purpose of this study is to determine the effects of EMR on humans. For this purpose, we will create posture-transformed human phantoms in a following study using the techniques employed herein and the human phantoms from our previous study.
1. Introduction
Researchers in institutes around the world are developing various human phantoms [1,2,3,4,5,6,7,8,9]. These human phantoms are used to examine the effects of daily electromagnetic radiation dosimetry (EMR) on humans, such as the specific absorption rate (SAR). They also deploy electronic mobile devices and medical devices such as computed tomography (CT) and magnetic resonance imaging (MRI) [7,10,11,12].
Although human phantoms are useful in various fields, they have two limitations. The first has to do with reliability. To verify the reliability of a human phantom in any simulation, it is ideal to compare the effects of a human phantom with those of an actual human being. However, EMR tests on living humans are impossible because the effects are unknown and thus tests on living subjects have been banned for ethical reasons. Therefore, as a substitute, live monkeys are used in various biological and physical experiments deemed too difficult or dangerous for humans [13,14,15,16,17,18]. In EMR fields, a living monkey can also replace a living human, and a monkey phantom can also replace a living monkey. Consequently, verification of the reliability of a human phantom is possible using monkey phantoms because we can logically deduce the relation between the human phantom and a living human through a comparison of a monkey phantom with a living monkey. The second hindrance is the availability of only one posture in existing phantoms. Most phantoms are produced in a supine position from CT or MRI data [1,2,3,4,5,6,7,8]. To accurately simulate the EMR effects on a phantom, the phantoms must be exposed to electromagnetic waves during specific postures because the tissues and organs of living creatures may be influenced differently depending on posture [9,19,20]. In a recent study, a posture-transformed human phantom was produced for EMR simulation. However, the techniques required to create the phantom were so professional that researchers unfamiliar with mathematical algorithms cannot easily repeat the technique [9].
For verifying MRI stability, EMR simulations based on a monkey phantom of only one anatomical position has been recently produced [21]. The monkey phantom was made from data of our Visible Monkey project [22]. However, if an EMR simulation is conducted not only for medical devices but also for electronic devices on a monkey phantom, then the original posture of the phantom will be unsuitable because living monkeys are exposed to electromagnetic radiation in different postures during their daily routines. To address this limitation, a phantom was needed that can assume common postures in the everyday life of a monkey [23,24,25,26].
The primary aim of this study was to present posture-transformed monkey phantoms that are able to assume anatomical, quadrupedal, and sitting positions among the most common everyday postures used for EMR simulations. The secondary aim was to present easy methods for making posture-transformed phantoms using commercial software. To achieve these aims, in this study, surface models of Visible Monkeys in anatomical positions [27] were transformed into models in quadrupedal and sitting positions and then converted into voxel phantoms.
2. Materials and Methods
In our Visible Monkey project, real-color sectioned images of the whole body of a female rhesus monkey (intervals of 0.05 and 0.5 mm, pixel size of 0.024 mm, and color depth of 48 bits) were produced [22]. Based on the sectioned images, surface models of 177 structures in an anatomical position were created [27]. Thus, 177 surface models in a stereolithography (STL) format were adopted for this study.
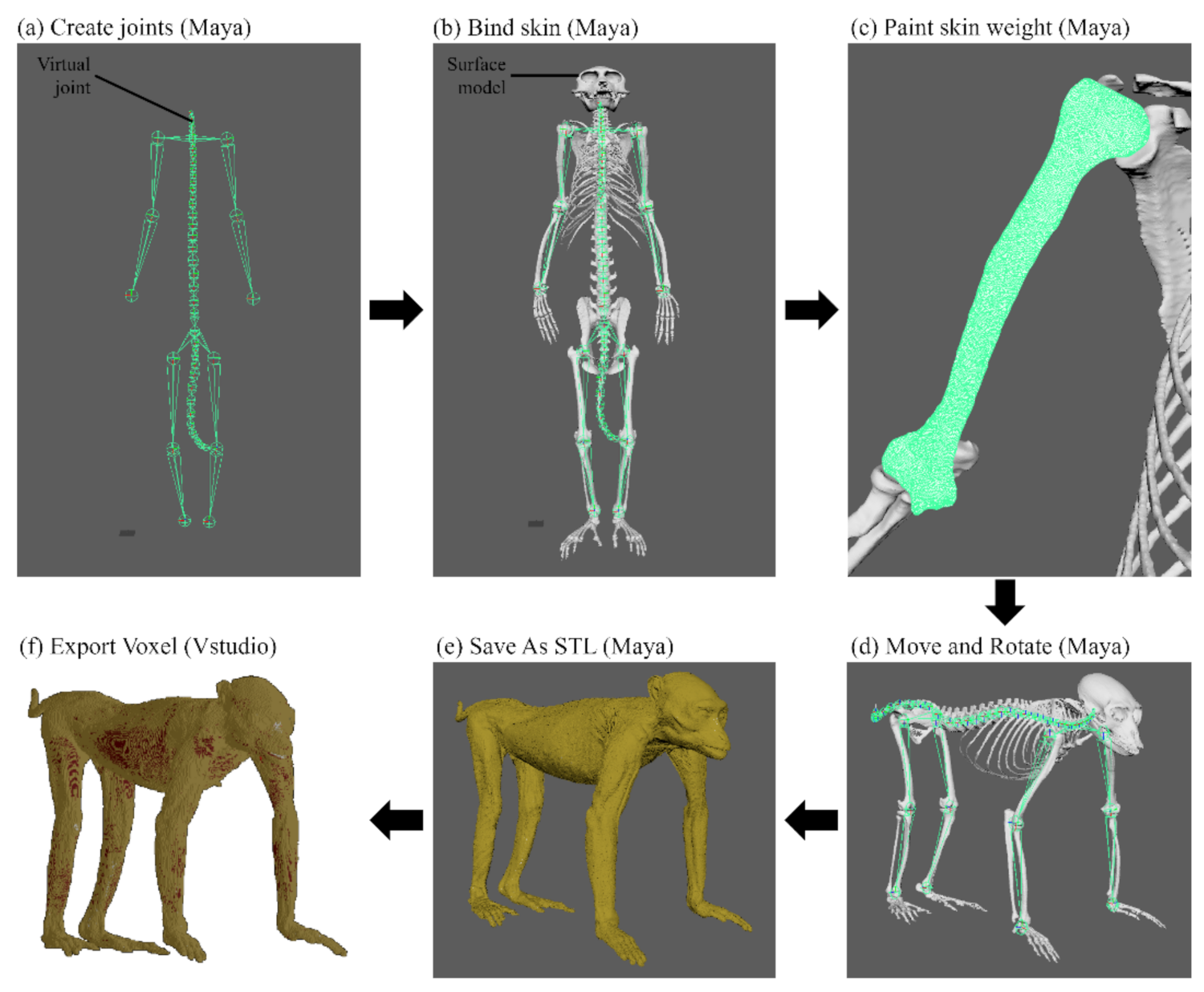
Using Maya version 2018 (Autodesk, Inc., San Rafael, CA, USA), the posture of the surface models in STL format was changed as follows. In total, 52 virtual joints were created—i.e., all intervertebral spaces (7 cervical, 12 thoracic, 7 lumbar, 1 sacral, and 13 caudal vertebral joints), shoulder (2 joints), elbow (2 joints), wrist (2 joints), hip (2 joints), knee (2 joints), and ankle (2 joints)—using the Create Joints tool (Figure 1a). After creating the virtual joints, all surface models were bound to the joints using the Bind Skin tool (Figure 1b). The influence values of the virtual joints in the Paint Skin Weights tool were adjusted to create realistic shapes of the surface models when rotated on an axis at the joint (Figure 1c). All surface models were changed from the anatomical position to the quadrupedal position by controlling the virtual joints using the Move and Rotate tools (Figure 1d). In the same manner, the postures of the surface models were changed from an anatomical position to a sitting position. The shape of quadrupedal and sitting positions were repeatedly revised and examined according to posture descriptions of primate studies targeting multiple subjects [24,25,26].
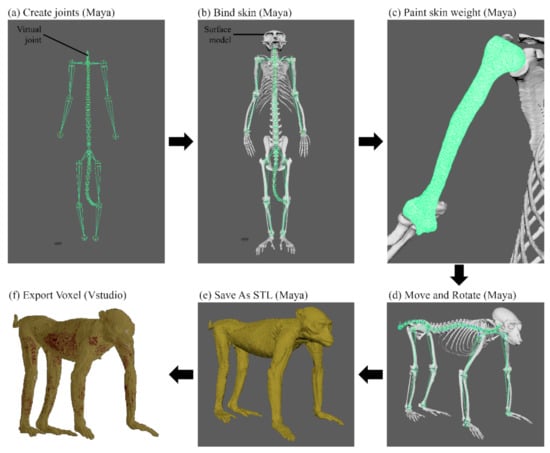
Figure 1.
Overall process of changing positions from the monkey surface models to voxel models. (a) 52 virtual joints were created using the Create Joints tool in Maya. (b) Surface models of the monkey were bound to the virtual joints using the Bind Skin tool in Maya. (c) The shape of the surface models was set to anatomically correct using the Paint Skin Weight tool in Maya. (d) The anatomical position of the monkey surface models were changed to a quadrupedal position using the Move and Rotate tools on virtual joints in Maya. (e) The surface models were saved in STL format in Maya. (f) The surface models of the quadrupedal position were converted into voxel models using the Export Voxel tool in Vstudio.
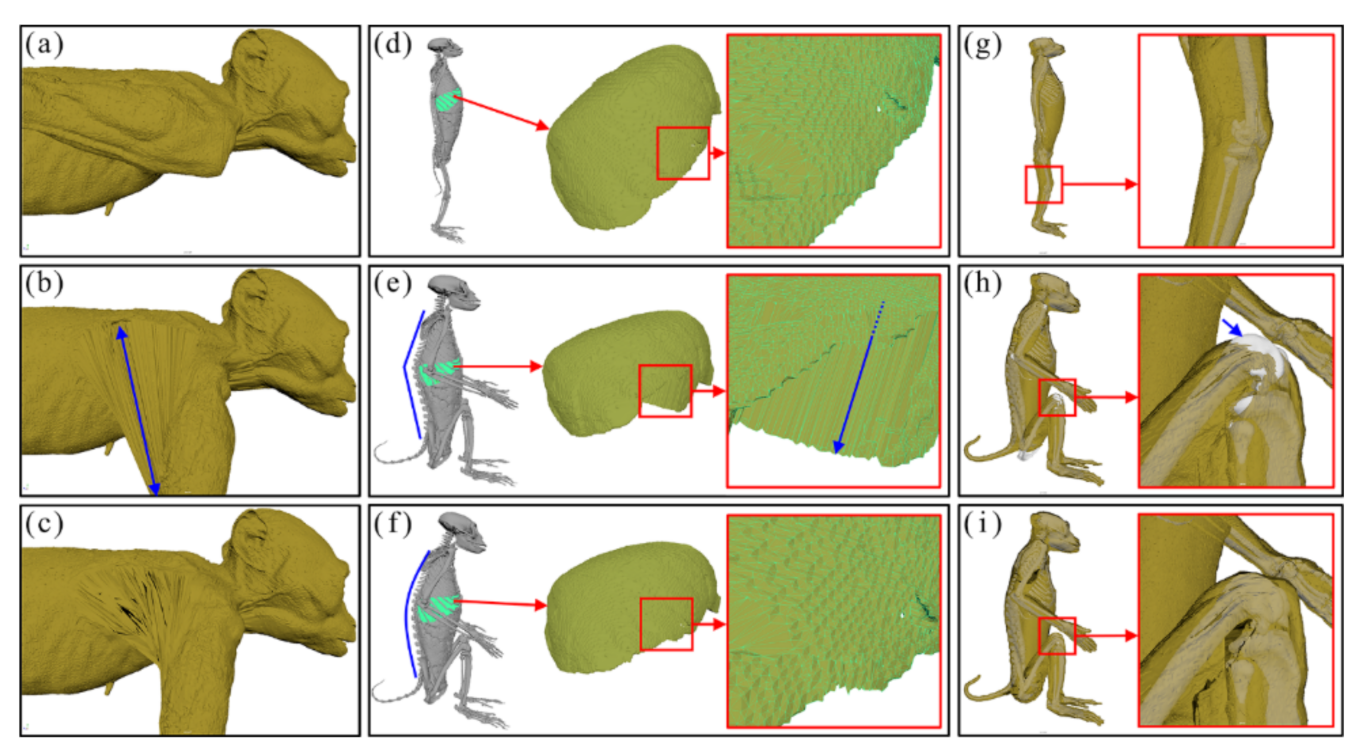
In the surface models of three positions, abnormal shapes were found and repaired using the Move, Smooth, Relax, Grab, and Flatten tools in Maya as follows. First, in the surface models of the anatomical position (Figure 2a), the skin surfaces (meshes) of an arm around the axilla overlapped with those of the thorax despite no such overlap in reality. For this reason, when the upper limbs surface models were flexed, the skin meshes expanded abnormally and unrealistically (Figure 2b). To resolve this, we manually modified the meshes near the area (Figure 2c). Second, in the surface models of the sitting position, some meshes of the liver became intruded or merged on themselves when only a vertebral joint was flexed (Figure 2e). To minimize these invasions, we flexed all joints in every vertebra (Figure 2f). Third, in the surface models of the sitting position, meshes of the femur invaded those of the skin on the knee joint during the flexion of the lower limbs (Figure 2h). To prevent such an invasion, the influence value in the Paint Skin Weights tool in Maya was manually repaired again (Figure 2i). The surface models were saved in STL format to create the 177 surface models in both the quadrupedal and sitting positions (Figure 1e).
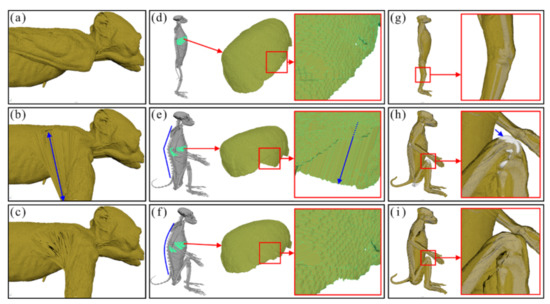
Figure 2.
Three types of surface models with abnormal shapes under changing positions. While changing (a) the anatomical position of the surface models into (b) the quadrupedal position, the skin meshes of the axilla expand abnormally (blue arrow). The meshes of abnormal shape are repaired manually (c). When changing (d) the anatomical position of the surface models to (e) a sitting position by the flexion (blue line) of only a vertebral joint, (e) the meshes of the liver intrude on each other (blue arrow). (f) The invasion is prevented by a flexion (blue curve) of all vertebral joints. When changing (g) the anatomical position of the surface models to a sitting position, (h) the meshes of the femur invade the skin in the knee joint. (i) This invasion is repaired by adjusting the improper influence value of skin in Maya.
In Vstudio version 2.0 (copyright ETRI, Daejeon, Korea), STL files of the surface models in three positions were loaded with a structure list consisting of the assigned identification numbers (ID) for each structure [28]. After converting the loaded surface models into voxel models, the shapes of the voxel models were checked stereoscopically and cross-sectionally. Using the Export Voxel tool, voxel information (pixels, intervals, and ID) of the 177 structures in the voxel models were saved in TXT format to create the monkey phantom for the three positions (Figure 1f) [28,29].
In the TXT files of the three phantom positions, the ID of the 177 structures and the coordinates (x-, y-, and z-axes) of each ID were recorded using the x- and y-axes for the pixel size and the z-axis for the intervals in the voxels. To calculate each ID in the TXT files, a house-developed software was used [29]. Because one ID was written for each voxel in the TXT files, the software calculated the total number of voxels in a body by checking the total for each ID.
The number of voxels for each structure was multiplied by its tissue density using the Gabriel list to determine the mass of the 177 structures [3,30]. To determine the relation between humans and monkeys in the EMR simulation, as a preliminary step, the mass of the structures of the female monkey were compared with those of a VK-phantom of a female human [29].
The study protocol of Visible Monkey was reviewed and approved by the Institutional Review Board (IRB) of the National Primate Research Center of Korea, Research Institute of Bioscience and Biotechnology (IRB No. KRIBB-AEC-18087) [22].
3. Results
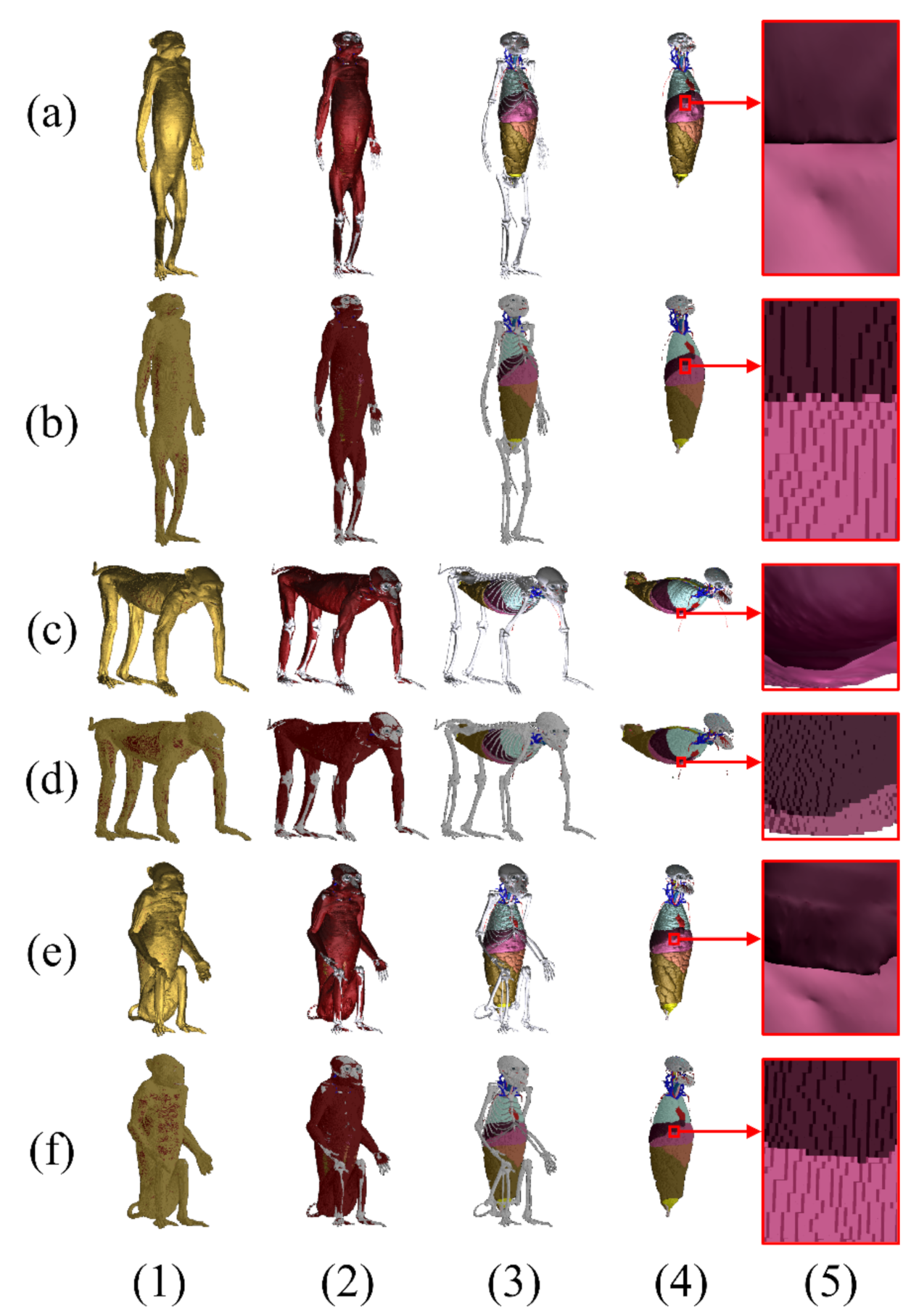
From the surface models of the monkey with 177 structures in the anatomical position (Figure 3a), the surface models of quadrupedal (Figure 3c) and sitting (Figure 3e) positions were produced, each having 177 structures. The surface models of the monkey at the three positions were stored in a developer friendly STL format (Table 1).
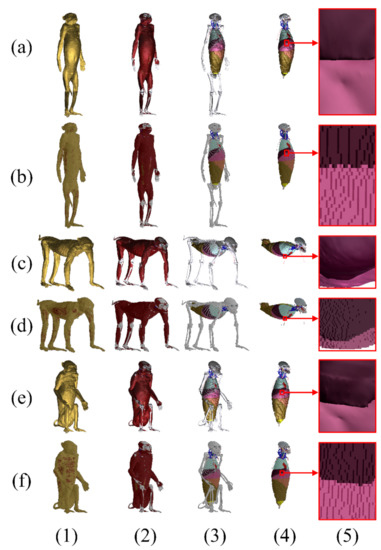
Figure 3.
Surface models and voxel phantoms of a monkey in an (a and b rows) an anatomical position, (c and d rows) a quadrupedal position, and (e and f rows) a sitting position with 177 structures. (Column 1) The surface models and voxel phantoms of the whole body, (column 2) the skin, (column 3) muscle, and (column 4) peeled bones. Differences between (a-5, c-5, e-5) the meshes of the surface models and (b-5, d-5, f-5) the voxels of the phantoms can be seen in the magnified views.

Table 1.
Properties of surface models and phantoms of a rhesus monkey in anatomical, quadrupedal, and sitting positions.
Three monkey phantoms, i.e., in anatomical (Figure 3b), quadrupedal (Figure 3d), and sitting (Figure 3f) positions, were stored in TXT format (Table 1). In the TXT files for the three positions, the coordinates (x-, y-, z-axes) and ID of each structure were recorded. Using these TXT files, the researchers could simulate the EMR research of the monkeys [7,10].
Using monkey phantoms stored as TXT files, the number of voxels of each structure were analyzed. The total number of voxels of the anatomical, quadrupedal, and sitting positions were 5,054,022, 5,174,453, and 4,803,886 voxels, respectively. The discrepancies in the total number of voxels among the three positions were under 5%, which was similar to a previous study [31]. In the phantoms, the muscle tissue had the greatest number of voxels (1,699,435 anatomical position voxels, 1,767,197 quadrupedal position voxels, and 1,645,241 sitting position voxels), and the superficial temporal vein had the lowest number of voxels (5 anatomical position voxels, 3 quadrupedal position voxels, and 2 sitting position voxels). However, the number of voxels of the arteries and veins, including the superficial temporal vein, were inaccurate because their segmentations had not yet been completed. Excluding the arteries and veins, the cornea had the smallest number of voxels (18 anatomical position voxels, 22 quadrupedal position voxels, and 19 sitting position voxels) (see the Appendix A), which was the same for cornea of the VK-phantom of a female human [29].
In general, structures with a large or small number of voxels tend to either be heavy or light, respectively. However, the rank order of numbers of voxels does not always correspond to the rank order of mass because their densities differ depending on the tissue type. For example, in the anatomical position, the gallbladder ranked 66th in number of voxels, whereas it ranked 94th in mass. The differences in the total number of voxels between changed positions and the anatomical position were set at under 5%. Unfortunately, these differences (i.e., under 5%) were unavoidable because the changes in the positions caused spatial changes in the surface models, leading to changes in the volumes of the surface models. Eventually, volume changes in the surface models resulted in differences in the number of voxels (Appendix A).
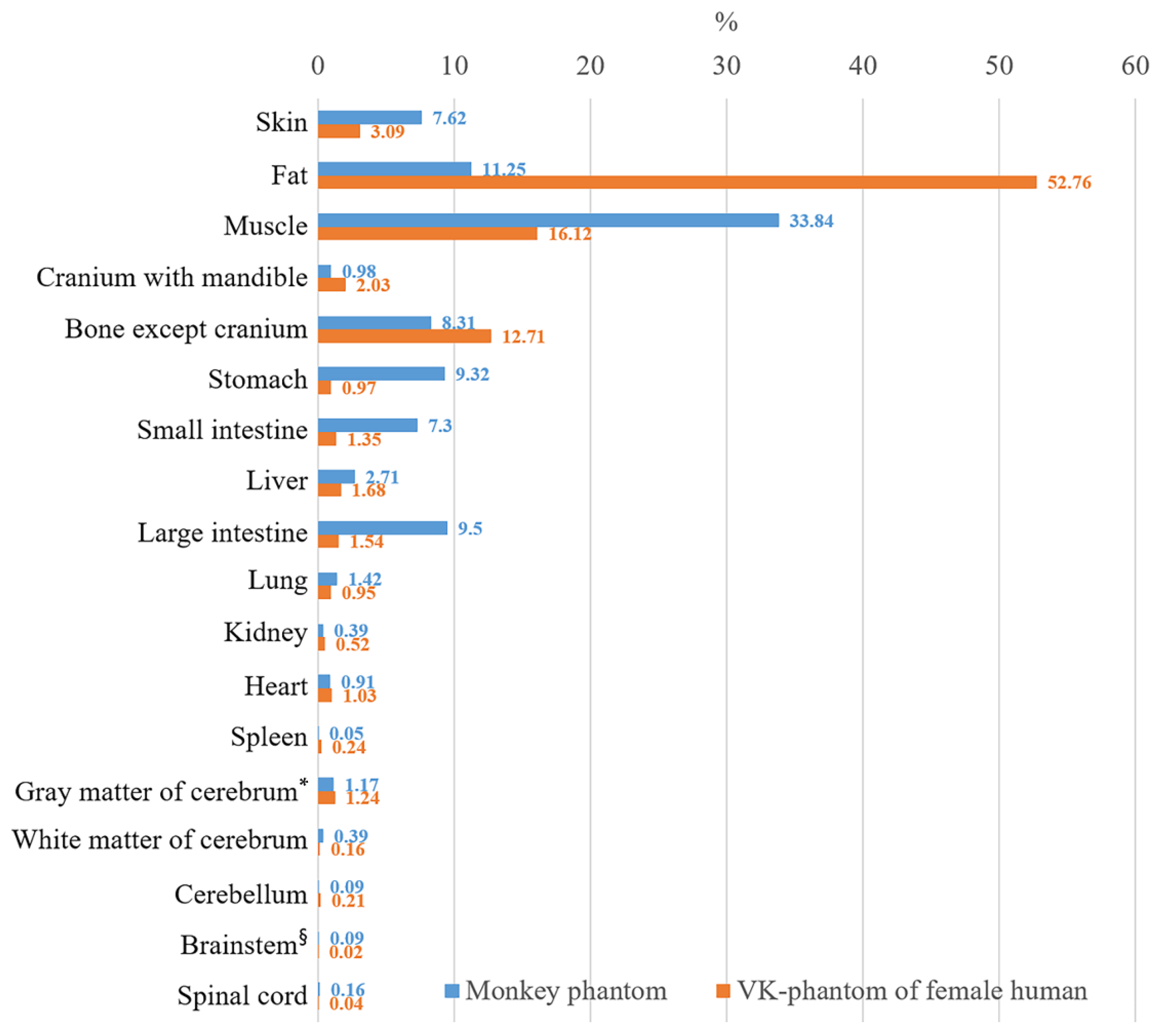
Most experiments using monkeys as subjects are conducted for human health. As a preliminary step to determine the relation between humans and monkeys in EMR simulations, we compared the proportion of mass of each structure to the total mass of the human phantoms from a previous study and the monkey phantoms [29]. The results showed that a monkey phantom has larger proportions of skin (7.62%) and muscle (33.84%) than a VK-phantom (3.09% and 16.12%, respectively), whereas the proportion of bone (9.7%) of a monkey phantom is smaller than that of a VK-phantom (Figure 4, Appendix A). It is reasonable for monkeys to have thicker skin, less fat, heavier muscle, and lighter skeletons than humans because they are arboreal animals living in a wild environment [32].
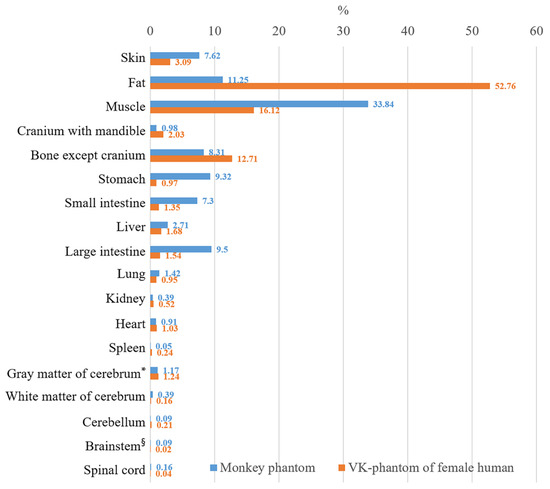
Figure 4.
Comparison of the mass proportion of each structure in the monkey phantom and the VK-phantom of female human [29]. The mass proportions of skin, muscle, and structures of alimentary system of the monkey phantom are larger than those of the VK-phantom. The monkey phantom has larger proportions of cerebral white matter, brainstem, and spinal cord than those of the human. (* Cerebral gray matter including thalamus, hypothalamus, and hippocampus; § Brainstem including midbrain, pons, and medulla oblongata).
The monkey phantoms also have a larger proportion of tissues in the alimentary system, including the teeth (0.2%), tongue (0.14%), esophagus (0.2%), stomach (9.32%), small intestine (7.03%), large intestine (9.5%), and liver (2.71%) compared with a VK-phantom (Figure 4, Appendix A). It is also logical for monkeys to have larger gastrointestinal (GI) organs than humans because they eat foods that are low in digestibility such as plants, which requires a relatively robust GI system with elaborate fermenting chambers [33]. However, the GI system of this phantom was larger than those of actual monkeys because gas was generated in the GI organs during the time between sacrifice and freezing of the monkey.
Not surprisingly, in the central nervous system, the monkey phantom had a smaller proportion of gray matter (1.13%), cingulate gyrus (0.02%), and cerebellum (0.09%) than the VK-phantom, whereas the white matter (0.39%), midbrain (0.03%), pons (0.03%), medulla oblongata (0.03%), and spinal cord (0.16%) of the monkey were larger (Figure 4, Appendix A). It is reasonable for monkeys to have a large paleencephalon (e.g., brainstem) and a relatively small neoencephalon compared to humans given that humans have a more evolved brain (e.g., gray matter in the cerebral cortex). Moreover, it is natural for monkeys to have a smaller proportion of cerebellar tissue than humans because humans use their hands more often and more elaborately by virtue of their bipedalism.
4. Discussion
Monkey phantoms are beneficial for research on the safety of EMR from ordinary electronic and medical devices because the effect of EMR on humans can be deduced based on the results of monkey phantoms [7,10,11,12]. In addition, the monkey phantoms of daily postures are necessary to accurately examine the EMR effect because the results of the EMR are sensitive to various phantom postures [19,20]. However, strictly speaking, to examine the precise impact of EMR on living humans, living humans should be used in EMR tests; however, doing so is impossible. Therefore, human phantoms have been employed instead of actual humans [3,4,6,34]. However, we still cannot help but consider the reliability of such human phantoms because they have never been compared to living humans in relation to EMR. To verify the reliability of human phantoms, we chose to use monkeys, which are commonly applied as human surrogates. Using a monkey, researchers can directly check the impact of EMR on both living monkeys and monkey phantoms. If the reliability of the monkey phantoms is verified through comparisons with living monkeys, we can deduce logically the relation between human phantoms and living humans without the EMR testing of actual humans.
Because this study was the first step into monkey phantom research, we developed posture-transformed monkey phantoms (Figure 3b,d,f). To develop the posture-transformed monkey phantoms, three points must be considered.
First, monkey phantoms with precise anatomy are required to obtain accurate results with regard to EMR. Although phantoms are easily created using CT and MRI scans, small structures cannot be identified in detail in such phantoms [35,36,37,38]. By contrast, it is difficult to create phantoms from the sectioned images owing to the need for a high resolution and true color [39,40,41,42,43]. Nevertheless, we have successfully created VK-phantoms for 583 male whole-body structures, 459 female whole-body structures, and 300 head structures from the sectioned images of humans [29,44]. Using not only the image data of Visible Monkey but also the experience and software techniques employed for the VK-phantoms, precise phantoms of rhesus monkeys were produced in this study (Figure 3b,d,f) [22,27].
Second, posture-transformed monkey phantoms are required to obtain simulation values in situations identical to real life. EMR simulations usually use phantoms in the anatomical position [3,6,45]. However, in the real world, living things assume different postures, and are thus exposed to EMR in different postures. Consequently, phantoms in various positions are needed for more precise measurements. Although posture-transformed phantoms of whole bodies have been employed in some EMR and SAR studies (the hands of the phantoms were even placed over their ears), these studies did not provide voxel phantoms for other researchers [7,9,10]. Most importantly, not only a monkey phantom but also posture-transformed phantoms with detailed structures were applied. Therefore, in this study, considering the most common postures in the daily routine of a monkey, we chose anatomical, quadrupedal, and sitting positions, and produced phantoms of these three positions (Figure 3 and Table 1 and Appendix A). For reference, monkeys usually walk quadrupedally, and sleep and relax on tree branches in a sitting position.
Third, in the posture-transformed procedure, manual works are required to obtain phantoms with an anatomical correct shape. In the procedures applied in this study, abnormal expansions and intrusions of surface models occurred (Figure 2). These issues led to abnormal shapes of the phantoms, and the abnormal shapes of phantoms led to differences in the number of voxels of structures between positions. Eventually, the differences will affect the reliability and accuracy of the phantoms in EMR and SAR simulations. Therefore, to make reliable and accurate posture-transformed phantoms, researchers who plan to use the methods described in this study must consider these technical issues to minimize the differences in the numbers of voxel of structures between positions, even if manual works for such issues are difficult.
In addition, the total masses of the anatomical, quadrupedal, and sitting positions were approximately 5.5, 5.6, and 5.2 kg, respectively. Because the body mass of the real monkey was 4.3 kg, the discrepancy between the total mass of the anatomical position and the real body mass was approximately 1.2 kg (Appendix A) [22]. This was caused mainly for three reasons. First, the tissue density of Gabriel, which was used for the calculation of the monkey’s body mass (Appendix A), has values of a human not a monkey. Second, in a real monkey, the inner spaces of the organs such as the stomach and intestines are filled with air, whereas the inner space of the organs in the phantom are regarded as the organs not air. Finally, because the segmentations had not yet been completed, the muscle, fat, and blood vessels were not divided in the phantoms. In the near future, we will be able to decrease the discrepancy of the masses because many researchers will find the correct tissue densities and will segment more structures. At that time, we will make new phantoms based on the new densities and new segmented images available.
5. Conclusions
Posture-transformable volume models are necessary for EMR simulations. Consequently, in the study, we presented the first monkey phantom of a small voxel size with 177 structures in the anatomical (Figure 3b), quadrupedal (Figure 3d), and sitting (Figure 3f) positions by changing the postures of the surface models and converting the surface models into volume models. It will be absolutely imperative to compare living monkeys with a virtual monkey (phantom) in EMR simulations in our following studies. Because the ultimate purpose of this research is to determine the effects of EMR on a living human body, posture-transformed human phantoms will be made in a future study using the techniques employed herein along with the VK-phantoms of male and female humans [29,44]. The surface models and phantoms of a rhesus monkey in the three positions applied will be useful not only in EMR simulations but also in anatomical and biological experiments.
Author Contributions
C.Y.K.: conceptualization, methodology, resources, writing—original draft preparation, and writing—review and editing. A.-K.L.: software, validation, writing—review and editing, and funding acquisition. H.-D.C.: software, formal analysis, writing—review and editing, and funding acquisition. J.S.P.: conceptualization, resources, writing—original draft preparation, writing—review and editing, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the ICT R&D program of MSIT/IITP) 2019-0-00102, A Study on Public Health and Safety in a Complex EMF Environment.
Institutional Review Board Statement
Review Board (IRB) of National Primate Research Center of Korea Research Institute of Bioscience and Biotechnology (IRB No. KRIBB-AEC-18087).
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.data.go.kr/en/data/15074162/fileData.do (accessed on 10 May 2021).
Conflicts of Interest
The authors have no potential conflict of interest to disclose.
Appendix A

Table A1.
Number of voxels of 177 structures in the monkey phantoms of anatomical, quadrupedal, and sitting positions.
Table A1.
Number of voxels of 177 structures in the monkey phantoms of anatomical, quadrupedal, and sitting positions.
| System | Structure | Density (kg/m3) (A) | Number of Voxels of Each Position | Mass of Each Position (g) | ||||
|---|---|---|---|---|---|---|---|---|
| Anatomical (B) | Quadrupedal (C) | Sitting (D) | Anatomical (A × B) | Quadrupedal (A × C) | Sitting (A × D) | |||
| Skeletal | Cranium | 1908 | 22,051 | 2,761 | 21,814 | 42.07 | 41.52 | 41.62 |
| Mandible | 1908 | 6177 | 6164 | 6096 | 11.79 | 11.76 | 11.63 | |
| 1st CV | 1908 | 622 | 614 | 618 | 1.19 | 1.17 | 1.18 | |
| 2nd CV | 1908 | 933 | 913 | 927 | 1.78 | 1.74 | 1.77 | |
| 3rd CV | 1908 | 724 | 696 | 699 | 1.38 | 1.33 | 1.33 | |
| 4th CV | 1908 | 628 | 632 | 631 | 1.20 | 1.21 | 1.20 | |
| 5th CV | 1908 | 641 | 639 | 642 | 1.22 | 1.22 | 1.22 | |
| 6th CV | 1908 | 739 | 755 | 746 | 1.41 | 1.44 | 1.42 | |
| 7th CV | 1908 | 756 | 777 | 769 | 1.44 | 1.48 | 1.47 | |
| 1st CV | 1908 | 1071 | 1076 | 1083 | 2.04 | 2.05 | 2.07 | |
| 2nd CV | 1908 | 1152 | 1155 | 1137 | 2.20 | 2.20 | 2.17 | |
| 3rd CV | 1908 | 1156 | 1184 | 1177 | 2.21 | 2.26 | 2.25 | |
| 4th CV | 1908 | 1218 | 1229 | 1249 | 2.32 | 2.34 | 2.38 | |
| 5th CV | 1908 | 1255 | 1268 | 1266 | 2.39 | 2.42 | 2.42 | |
| 6th CV | 1908 | 1333 | 1348 | 1366 | 2.54 | 2.57 | 2.61 | |
| 7th CV | 1908 | 1451 | 1439 | 1433 | 2.77 | 2.75 | 2.73 | |
| 8th CV | 1908 | 1653 | 1636 | 1635 | 3.15 | 3.12 | 3.12 | |
| 9th CV | 1908 | 1878 | 1845 | 1818 | 3.58 | 3.52 | 3.47 | |
| 10th TV | 1908 | 2237 | 2259 | 2253 | 4.27 | 4.31 | 4.30 | |
| 11th TV | 1908 | 3208 | 3176 | 3151 | 6.12 | 6.06 | 6.01 | |
| 12th TV | 1908 | 3726 | 3722 | 3731 | 7.11 | 7.10 | 7.12 | |
| 1st LV | 1908 | 4271 | 4236 | 4261 | 8.15 | 8.08 | 8.13 | |
| 2nd LV | 1908 | 4754 | 4784 | 4736 | 9.07 | 9.13 | 9.04 | |
| 3rd LV | 1908 | 4784 | 4774 | 4701 | 9.13 | 9.11 | 8.97 | |
| 4th LV | 1908 | 5140 | 5122 | 5151 | 9.81 | 9.77 | 9.83 | |
| 5th LV | 1908 | 5112 | 5106 | 5085 | 9.75 | 9.74 | 9.70 | |
| 6th LV | 1908 | 5625 | 5589 | 5631 | 10.73 | 10.66 | 10.74 | |
| 7th LV | 1908 | 5816 | 5786 | 5815 | 11.10 | 11.04 | 11.10 | |
| Sacrum | 1908 | 3509 | 3500 | 3472 | 6.70 | 6.68 | 6.62 | |
| Coccyx | 1908 | 4852 | 4860 | 4716 | 9.26 | 9.27 | 9.00 | |
| 1st rib | 1908 | 462 | 475 | 464 | 0.88 | 0.91 | 0.89 | |
| 2nd rib | 1908 | 707 | 699 | 696 | 1.35 | 1.33 | 1.33 | |
| 3rd rib | 1908 | 706 | 706 | 713 | 1.35 | 1.35 | 1.36 | |
| 4th rib | 1908 | 931 | 926 | 920 | 1.78 | 1.77 | 1.76 | |
| 5th rib | 1908 | 1108 | 1107 | 1055 | 2.11 | 2.11 | 2.01 | |
| 6th rib | 1908 | 1138 | 1102 | 1066 | 2.17 | 2.10 | 2.03 | |
| 7th rib | 1908 | 1208 | 1256 | 1190 | 2.30 | 2.40 | 2.27 | |
| 8th rib | 1908 | 1317 | 1334 | 1300 | 2.51 | 2.55 | 2.48 | |
| 9th rib | 1908 | 1286 | 1312 | 1271 | 2.45 | 2.50 | 2.43 | |
| 10th rib | 1908 | 966 | 971 | 965 | 1.84 | 1.85 | 1.84 | |
| 11st rib | 1908 | 690 | 687 | 689 | 1.32 | 1.31 | 1.31 | |
| 12nd rib | 1908 | 496 | 469 | 472 | 0.95 | 0.89 | 0.90 | |
| Costal cartilage | 1100 | 6153 | 6279 | 5806 | 6.77 | 6.91 | 6.39 | |
| Sternum | 1908 | 1636 | 1705 | 1486 | 3.12 | 3.25 | 2.84 | |
| Scapula | 1908 | 6514 | 6529 | 6528 | 12.43 | 12.46 | 12.46 | |
| Clavicle | 1908 | 1176 | 1216 | 1176 | 2.24 | 2.32 | 2.24 | |
| Humerus | 1908 | 24,285 | 24,313 | 24,101 | 46.34 | 46.39 | 45.98 | |
| Radius | 1908 | 8861 | 8951 | 8818 | 16.91 | 17.08 | 16.82 | |
| Ulna | 1908 | 9416 | 9451 | 9404 | 17.97 | 18.03 | 17.94 | |
| Scaphoid | 1908 | 401 | 398 | 405 | 0.77 | 0.76 | 0.77 | |
| Lunate | 1908 | 442 | 449 | 450 | 0.84 | 0.86 | 0.86 | |
| Triquetrum | 1908 | 427 | 424 | 415 | 0.81 | 0.81 | 0.79 | |
| Pisiform | 1908 | 289 | 301 | 297 | 0.55 | 0.57 | 0.57 | |
| Trapezium | 1908 | 235 | 235 | 232 | 0.45 | 0.45 | 0.44 | |
| Trapezoid | 1908 | 364 | 359 | 360 | 0.69 | 0.68 | 0.69 | |
| Capitate | 1908 | 396 | 389 | 391 | 0.76 | 0.74 | 0.75 | |
| Hamate | 1908 | 504 | 503 | 495 | 0.96 | 0.96 | 0.94 | |
| 1st MCB | 1908 | 562 | 557 | 556 | 1.07 | 1.06 | 1.06 | |
| 2nd MCB | 1908 | 1062 | 1055 | 1046 | 2.03 | 2.01 | 2.00 | |
| 3rd MCB | 1908 | 1261 | 1254 | 1239 | 2.41 | 2.39 | 2.36 | |
| 4th MCB | 1908 | 983 | 990 | 974 | 1.88 | 1.89 | 1.86 | |
| 5th MCB | 1908 | 725 | 732 | 713 | 1.38 | 1.40 | 1.36 | |
| 1st PP (hand) | 1908 | 215 | 217 | 211 | 0.41 | 0.41 | 0.40 | |
| 2nd PP (hand) | 1908 | 427 | 430 | 435 | 0.81 | 0.82 | 0.83 | |
| 3rd PP (hand) | 1908 | 593 | 588 | 585 | 1.13 | 1.12 | 1.12 | |
| 4th PP (hand) | 1908 | 555 | 551 | 565 | 1.06 | 1.05 | 1.08 | |
| 5th PP (hand) | 1908 | 333 | 338 | 338 | 0.64 | 0.64 | 0.64 | |
| 2nd MP (hand) | 1908 | 136 | 137 | 133 | 0.26 | 0.26 | 0.25 | |
| 3rd MP (hand) | 1908 | 39 | 40 | 33 | 0.07 | 0.08 | 0.06 | |
| 4th MP (hand) | 1908 | 593 | 588 | 585 | 1.13 | 1.12 | 1.12 | |
| 5th MP (hand) | 1908 | 269 | 280 | 276 | 0.51 | 0.53 | 0.53 | |
| 1st DP (hand) | 1908 | 43 | 44 | 42 | 0.08 | 0.08 | 0.08 | |
| 2nd DP (hand) | 1908 | 427 | 430 | 435 | 0.81 | 0.82 | 0.83 | |
| 3rd DP (hand) | 1908 | 136 | 137 | 133 | 0.26 | 0.26 | 0.25 | |
| 4th DP (hand) | 1908 | 39 | 40 | 33 | 0.07 | 0.08 | 0.06 | |
| 5th DP (hand) | 1908 | 593 | 588 | 585 | 1.13 | 1.12 | 1.12 | |
| Sesamoid bone (hand) | 1908 | 46 | 50 | 48 | 0.09 | 0.10 | 0.09 | |
| Pelvis | 1908 | 11,840 | 11,902 | 11,860 | 22.59 | 22.71 | 22.63 | |
| Femur | 1908 | 33,037 | 33,113 | 33,197 | 63.03 | 63.18 | 63.34 | |
| Patella | 1908 | 1586 | 1596 | 1586 | 3.03 | 3.05 | 3.03 | |
| Tibia | 1908 | 19,512 | 19,530 | 19,488 | 37.23 | 37.26 | 37.18 | |
| Fibula | 1908 | 4128 | 4076 | 4086 | 7.88 | 7.78 | 7.80 | |
| Talus | 1908 | 3336 | 3341 | 3300 | 6.37 | 6.37 | 6.30 | |
| Calcaneus | 1908 | 5676 | 5681 | 5667 | 10.83 | 10.84 | 10.81 | |
| Navicular | 1908 | 1228 | 1239 | 1232 | 2.34 | 2.36 | 2.35 | |
| Medial cuneiform | 1908 | 887 | 892 | 884 | 1.69 | 1.70 | 1.69 | |
| Intermediate cuneiform | 1908 | 377 | 382 | 376 | 0.72 | 0.73 | 0.72 | |
| Lateral cuneiform | 1908 | 658 | 671 | 652 | 1.26 | 1.28 | 1.24 | |
| Cuboid | 1908 | 1464 | 1465 | 1463 | 2.79 | 2.80 | 2.79 | |
| 1st MTB | 1908 | 1432 | 1431 | 1455 | 2.73 | 2.73 | 2.78 | |
| 2nd MTB | 1908 | 1397 | 1428 | 1404 | 2.67 | 2.72 | 2.68 | |
| 3rd MTB | 1908 | 1822 | 1811 | 1788 | 3.48 | 3.46 | 3.41 | |
| 4th MTB | 1908 | 1716 | 1733 | 1736 | 3.27 | 3.31 | 3.31 | |
| 5th MTB | 1908 | 1267 | 1264 | 1217 | 2.42 | 2.41 | 2.32 | |
| 1st PP (foot) | 1908 | 420 | 425 | 430 | 0.80 | 0.81 | 0.82 | |
| 2nd PP (foot) | 1908 | 50 | 62 | 74 | 0.10 | 0.12 | 0.14 | |
| 3rd PP (foot) | 1908 | 380 | 368 | 387 | 0.73 | 0.70 | 0.74 | |
| 4th PP (foot) | 1908 | 123 | 138 | 133 | 0.23 | 0.26 | 0.25 | |
| 5th PP (foot) | 1908 | 11 | 17 | 17 | 0.02 | 0.03 | 0.03 | |
| 2nd MP (foot) | 1908 | 123 | 138 | 133 | 0.23 | 0.26 | 0.25 | |
| 3rd MP (foot) | 1908 | 11 | 17 | 17 | 0.02 | 0.03 | 0.03 | |
| 4th MP (foot) | 1908 | 674 | 656 | 699 | 1.29 | 1.25 | 1.33 | |
| 5th MP (foot) | 1908 | 234 | 249 | 243 | 0.45 | 0.48 | 0.46 | |
| 1st DP (foot) | 1908 | 50 | 62 | 74 | 0.10 | 0.12 | 0.14 | |
| 2nd DP (foot) | 1908 | 380 | 368 | 387 | 0.73 | 0.70 | 0.74 | |
| 3rd DP (foot) | 1908 | 123 | 138 | 133 | 0.23 | 0.26 | 0.25 | |
| 4th DP (foot) | 1908 | 11 | 17 | 17 | 0.02 | 0.03 | 0.03 | |
| 5th DP (foot) | 1908 | 674 | 656 | 699 | 1.29 | 1.25 | 1.33 | |
| Intervertebral disc | 1100 | 12,492 | 11,620 | 11,267 | 13.74 | 12.78 | 12.39 | |
| Cartilage | 1100 | 2265 | 2191 | 2169 | 2.49 | 2.41 | 2.39 | |
| Muscular | Muscle | 1090 | 1,699,435 | 1,767,197 | 1,645,241 | 1852.38 | 1926.24 | 1793.31 |
| Alimen-tary | Teeth | 2180 | 5041 | 5164 | 5121 | 10.99 | 11.26 | 11.16 |
| Tongue | 1090 | 6988 | 6986 | 6996 | 7.62 | 7.61 | 7.63 | |
| Esophagus | 1040 | 10,490 | 10,848 | 10,194 | 10.91 | 11.28 | 10.60 | |
| Stomach | 1088 | 468,655 | 467,573 | 390,467 | 509.90 | 508.72 | 424.83 | |
| Small intestine | 1100 | 349,654 | 348,316 | 310,591 | 384.62 | 383.15 | 341.65 | |
| Duodenum | 1102 | 13,417 | 13,297 | 12,039 | 14.79 | 14.65 | 13.27 | |
| Large intestine | 1100 | 472,874 | 472,962 | 449,017 | 520.16 | 520.26 | 493.92 | |
| Liver | 1079 | 137,547 | 137,070 | 126,927 | 148.41 | 147.90 | 136.95 | |
| Gall bladder | 928 | 1550 | 1527 | 1440 | 1.44 | 1.42 | 1.34 | |
| Common bile duct | 1090 | 12 | 23 | 27 | 0.01 | 0.03 | 0.03 | |
| Respira-tory | Trachea | 1080 | 4869 | 5395 | 4741 | 5.26 | 5.83 | 5.12 |
| Bronchus | 1102 | 3411 | 3394 | 3247 | 3.76 | 3.74 | 3.58 | |
| Lungs | 394 | 197,595 | 197,170 | 189,181 | 77.85 | 77.68 | 74.54 | |
| Urinary | Kidney | 1066 | 20,103 | 20,122 | 19,476 | 21.43 | 21.45 | 20.76 |
| Urinary bladder | 928 | 8650 | 8688 | 8644 | 8.03 | 8.06 | 8.02 | |
| Urethra | 1102 | 112 | 106 | 113 | 0.12 | 0.12 | 0.12 | |
| Genital | Ovary | 1048 | 378 | 387 | 391 | 0.40 | 0.41 | 0.41 |
| Uterus | 1105 | 3279 | 3288 | 3293 | 3.62 | 3.63 | 3.64 | |
| Vagina | 1088 | 2936 | 2949 | 2986 | 3.19 | 3.21 | 3.25 | |
| Endo-crine | Thyroid gland | 1071 | 530 | 644 | 562 | 0.57 | 0.69 | 0.60 |
| Adrenal gland | 1071 | 618 | 615 | 587 | 0.66 | 0.66 | 0.63 | |
| Cardio-vascular | Heart | 1071 | 46,668 | 46,607 | 43,353 | 49.98 | 49.92 | 46.43 |
| Common carotid a. | 1050 | 121 | 150 | 130 | 0.13 | 0.16 | 0.14 | |
| External carotid a. | 1050 | 39 | 37 | 48 | 0.04 | 0.04 | 0.05 | |
| Facial a. | 1050 | 22 | 21 | 25 | 0.02 | 0.02 | 0.03 | |
| Superficial temporal a. | 1050 | 46 | 40 | 36 | 0.05 | 0.04 | 0.04 | |
| Alveolar a. | 1050 | 8 | 8 | 10 | 0.01 | 0.01 | 0.01 | |
| Internal carotid a. | 1050 | 47 | 60 | 57 | 0.05 | 0.06 | 0.06 | |
| Middle cerebral a. | 1050 | 31 | 15 | 17 | 0.03 | 0.02 | 0.02 | |
| Basilar a. | 1050 | 7 | 7 | 5 | 0.01 | 0.01 | 0.01 | |
| Vertebral a. | 1050 | 208 | 237 | 212 | 0.22 | 0.25 | 0.22 | |
| the rest arteries | 1050 | 6395 | 6364 | 6130 | 6.71 | 6.68 | 6.44 | |
| Internal jugular v. | 1050 | 570 | 622 | 613 | 0.60 | 0.65 | 0.64 | |
| Retromandibular v. | 1050 | 370 | 369 | 355 | 0.39 | 0.39 | 0.37 | |
| Superficial temporal v. | 1050 | 5 | 3 | 2 | 0.01 | 0.003 | 0.002 | |
| External jugular v. | 1050 | 1048 | 1163 | 1035 | 1.10 | 1.22 | 1.09 | |
| Anterior jugular v. | 1050 | 365 | 440 | 379 | 0.38 | 0.46 | 0.40 | |
| Transverse sinus | 1050 | 50 | 57 | 78 | 0.05 | 0.06 | 0.08 | |
| Confluence of sinuses | 1050 | 30 | 31 | 31 | 0.03 | 0.03 | 0.03 | |
| Sigmoid sinus | 1050 | 82 | 80 | 67 | 0.09 | 0.08 | 0.07 | |
| Superior sagittal sinus | 1050 | 130 | 129 | 142 | 0.14 | 0.14 | 0.15 | |
| Straight sinus | 1050 | 35 | 30 | 28 | 0.04 | 0.03 | 0.03 | |
| the rest veins | 1050 | 13,501 | 13,816 | 13,327 | 14.18 | 14.51 | 13.99 | |
| Lymph-oid | Bone marrow | 1029 | 121,055 | 121,287 | 121,546 | 124.57 | 124.80 | 125.07 |
| Spleen | 1089 | 2655 | 2672 | 2561 | 2.89 | 2.91 | 2.79 | |
| Central nervous | Gray matter of CB | 1046 | 59,217 | 59,180 | 59,271 | 61.94 | 61.90 | 62.00 |
| White matter of CB | 1041 | 20,257 | 20,368 | 20,315 | 21.09 | 21.20 | 21.15 | |
| Dura mater | 1908 | 3873 | 3746 | 3722 | 7.39 | 7.15 | 7.10 | |
| Cerebrospinal fluid | 1007 | 2102 | 2250 | 2164 | 2.12 | 2.27 | 2.18 | |
| Spinal cord | 1075 | 8328 | 8439 | 8496 | 8.95 | 9.07 | 9.13 | |
| Medulla oblongata | 1075 | 1396 | 1392 | 1380 | 1.50 | 1.50 | 1.48 | |
| Pons | 1046 | 1759 | 1731 | 1778 | 1.84 | 1.81 | 1.86 | |
| Midbrain | 1046 | 1530 | 1507 | 1438 | 1.60 | 1.58 | 1.50 | |
| Cerebellum | 1045 | 4929 | 4952 | 4961 | 5.15 | 5.17 | 5.18 | |
| Thalamus | 1045 | 1736 | 1712 | 1693 | 1.81 | 1.79 | 1.77 | |
| Hypothalamus | 1045 | 46 | 41 | 35 | 0.05 | 0.04 | 0.04 | |
| Cingulate gyrus | 1046 | 892 | 917 | 965 | 0.93 | 0.96 | 1.01 | |
| Hippocampus | 1045 | 281 | 262 | 258 | 0.29 | 0.27 | 0.27 | |
| Sensory | Sclera | 911 | 419 | 439 | 463 | 0.38 | 0.40 | 0.42 |
| Cornea | 1051 | 18 | 22 | 19 | 0.02 | 0.02 | 0.02 | |
| Lens cortex | 1076 | 74 | 79 | 85 | 0.08 | 0.09 | 0.09 | |
| Lens nucleus | 1076 | 78 | 84 | 75 | 0.08 | 0.09 | 0.08 | |
| Vitreous humor | 1005 | 5507 | 5512 | 5493 | 5.53 | 5.54 | 5.52 | |
| Integum-entary | Skin | 1109 | 376,101 | 374,396 | 379,802 | 417.10 | 415.21 | 421.20 |
| Breast fat | 911 | 230 | 234 | 236 | 0.21 | 0.21 | 0.21 | |
| Fat | 911 | 675,922 | 732,245 | 644,889 | 615.76 | 667.08 | 587.49 | |
| Total | 5,054,022 | 5,174,453 | 4,803,886 | 5473.69 | 5595.16 | 5211.24 | ||
CV, cervical vertebra; TV, thoracic vertebra; LV, lumbar vertebra; MCA, metacarpal bone; PP, proximal phalanx; MP, middle phalanx; DP, distal phalanx; MTB, metatarsal bone; a., artery; v., vein; CB, cerebrum.
References
- Nagaoka, T.; Watanabe, S.; Sakurai, K.; Kunieda, E.; Watanabe, S.; Taki, M.; Yamanaka, Y. Development of realistic high-resolution whole-body voxel models of Japanese adult males and females of average height and weight, and application of models to radio-frequency electromagnetic-field dosimetry. Phys. Med. Biol. 2004, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dimbylow, P. Development of the female voxel phantom, NAOMI, and its application to calculations of induced current densities and electric fields from applied low frequency magnetic and electric fields. Phys. Med. Biol. 2005, 50, 1047–1070. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family-development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 2009, 55, N23–N38. [Google Scholar] [CrossRef] [PubMed]
- Segars, W.P.; Sturgeon, G.; Mendonca, S.; Grimes, J.; Tsui, B.M.W. 4D XCAT phantom for multimodality imaging research. Med. Phys. 2010, 37, 4902–4915. [Google Scholar] [CrossRef]
- Gosselin, M.-C.; Neufeld, E.; Moser, H.; Huber, E.; Farcito, S.; Gerber, L.; Jedensjo, M.; Hilber, I.; Di Gennaro, F.; Lloyd, B.; et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: The Virtual Population 3.0. Phys. Med. Biol. 2014, 59, 5287–5303. [Google Scholar] [CrossRef]
- Yeom, Y.S.; Jeong, J.H.; Kim, C.H.; Han, M.C.; Ham, B.K.; Cho, K.W.; Hwang, S.B. HDRK-Woman: Whole-body voxel model based on high-resolution color slice images of Korean adult female cadaver. Phys. Med. Biol. 2014, 59, 3969–3984. [Google Scholar] [CrossRef]
- Lee, A.-K.; Hong, S.-E.; Kwon, J.-H.; Choi, H.-D.; Cardis, E. Mobile phone types and SAR characteristics of the human brain. Phys. Med. Biol. 2017, 62, 2741–2761. [Google Scholar] [CrossRef]
- Kim, C.H.; Yeom, Y.S.; Nguyen, T.T.; Han, M.C.; Choi, C.; Lee, H.; Han, H.; Shin, B.; Lee, J.K.; Kim, H.S.; et al. New mesh-type phantoms and their dosimetric applications, including emergencies. Ann. ICRP 2018, 47, 45–62. [Google Scholar] [CrossRef]
- Yeom, Y.S.; Han, H.; Choi, C.; Nguyen, T.T.; Shin, B.; Lee, C.; Kim, C.H. Posture-dependent dose coefficients of mesh-type ICRP reference computational phantoms for photon external exposures. Phys. Med. Biol. 2019, 64, 075018. [Google Scholar] [CrossRef]
- Lee, A.K.; Park, J.S.; Hong, S.E.; Taki, M.; Wake, K.; Wiart, J.; Choi, H.-D. Brain SAR of average male Korean child to adult models for mobile phone exposure assessment. Phys. Med. Biol. 2019, 64, 045004. [Google Scholar] [CrossRef]
- Davids, M.; Guerin, B.; Vom Endt, A.; Schad, L.R.; Wald, L.L. Prediction of peripheral nerve stimulation thresholds of MRI gradient coils using coupled electromagnetic and neurodynamic simulations. Magn. Reson. Med. 2019, 81, 686–701. [Google Scholar] [CrossRef]
- Yang, B.; Tam, F.; Davidson, B.; Wei, P.S.; Hamani, C.; Lipsman, N.; Chen, C.H.; Graham, S.J. Technical Note: An anthropomorphic phantom with implanted neurostimulator for investigation of MRI safety. Med. Phys. 2020, 47, 3745–3751. [Google Scholar] [CrossRef]
- Yeni-Komshian, G.H.; Benson, D.A. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees, and rhesus monkeys. Science 1976, 192, 387–389. [Google Scholar] [CrossRef]
- Frey, S.; Pandya, D.N.; Chakravarty, M.M.; Bailey, L.; Petrides, M.; Collins, D.L. An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). Neuroimage 2011, 55, 1435–1442. [Google Scholar] [CrossRef]
- De Schotten, M.T.; Dell’Acqua, F.; Valabregue, R.; Catani, M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 2012, 48, 82–96. [Google Scholar] [CrossRef]
- Takemura, H.; Pestilli, F.; Weiner, K.S.; Keliris, G.A.; Landi, S.M.; Sliwa, J.; Ye, F.Q.; Barnett, M.A.; Leopold, D.A.; Freiwald, W.A.; et al. Occipital White Matter Tracts in Human and Macaque. Cereb. Cortex 2017, 27, 3346–3359. [Google Scholar] [CrossRef]
- Mars, R.B.; Sotiropoulos, S.N.; Passingham, R.E.; Sallet, J.; Verhagen, L.; Khrapitchev, A.A.; Sibson, N.; Jbabdi, S. Whole brain comparative anatomy using connectivity blueprints. Elife 2018, 7, e35237. [Google Scholar] [CrossRef]
- Capogrosso, M.; Milekovic, T.; Borton, D.; Wagner, F.; Moraud, E.M.; Mignardot, J.B.; Buse, N.; Gandar, J.; Barraud, Q.; Xing, D.; et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016, 539, 284–288. [Google Scholar] [CrossRef]
- Findlay, R.P.; Lee, A.K.; Dimbylow, P.J. FDTD calculations of SAR for child voxel models in different postures between 10 MHz and 3 GHz. Radiat. Prot. Dosim. 2009, 135, 226–231. [Google Scholar] [CrossRef]
- Lee, A.-K.; Choi, H.-D. Determining the influence of Korean population variation on whole-body average SAR. Phys. Med. Biol. 2012, 57, 2709–2725. [Google Scholar] [CrossRef]
- Xie, T.; Park, J.S.; Zhuo, W.; Zaidi, H. Development of a nonhuman primate computational phantom for radiation dosimetry. Med. Phys. 2020, 47, 736–744. [Google Scholar] [CrossRef]
- Chung, B.S.; Jeon, C.-Y.; Huh, J.W.; Jeong, K.-J.; Har, D.; Kwack, K.-S.; Park, J.S. Rise of the Visible Monkey: Sectioned images of rhesus monkey. J. Korean Med. Sci. 2019, 34, e66. [Google Scholar] [CrossRef]
- Kawai, M.; Mito, U. Quantitative study of activity patterns and postures of Formosan monkeys by the radio-telemetrical technique. Primates 1973, 14, 179–194. [Google Scholar] [CrossRef]
- Hunt, K.D.; Cant, J.G.H.; Gebo, D.L.; Rose, M.D.; Walker, S.E.; Youlatos, D. Standardized descriptions of primate locomotor and postural modes. Primates 1996, 37, 363–387. [Google Scholar] [CrossRef]
- Rose, M.D. Positional behaviour of olive baboons (Papio anubis) and its relationship to maintenance and social activities. Primates 1977, 18, 59–116. [Google Scholar] [CrossRef]
- Chivers, D.J. Malayan Forest Primates: Ten Years’ Study in Tropical Rain Forest; Springer: New York, NY, USA, 2013; pp. 198–199. [Google Scholar]
- Kim, C.Y.; Lee, A.-K.; Choi, H.-D.; Park, J.S. Dawn of the Visible Monkey: Segmentation of the rhesus monkey for 2D and 3D applications. J. Korean Med. Sci. 2020, 35, e100. [Google Scholar] [CrossRef]
- Han, M.; Lee, A.-K.; Choi, H.-D.; Jung, Y.W.; Park, J.S. Averaged head phantoms from magnetic resonance images of Korean children and young adults. Phys. Med. Biol. 2018, 63, 035003. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jung, Y.W.; Choi, H.-D.; Lee, A.-K. VK-phantom male with 583 structures and female with 459 structures, based on the sectioned images of a male and a female, for computational dosimetry. J. Radiat. Res. 2018, 59, 338–380. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef]
- Lafon, Y.; Smith, F.W.; Beillas, P. Combination of a model-deformation method and a positional MRI to quantify the effects of posture on the anatomical structures of the trunk. J. Biomech. 2010, 43, 1269–1278. [Google Scholar] [CrossRef]
- Montagna, W. The Skin of Nonhuman Primates. Am. Zool. 1972, 12, 109–124. [Google Scholar] [CrossRef]
- Aiello, L.C.; Wheeler, P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr. Anthr. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, S.H.; Jeong, J.H.; Lee, C.; Chung, M.S. HDRK-Man: A whole-body voxel model based on high-resolution color slice images of a Korean adult male cadaver. Phys. Med. Biol. 2008, 53, 4093–4106. [Google Scholar] [CrossRef] [PubMed]
- Yeom, Y.S.; Han, M.C.; Kim, C.H.; Jeong, J.H. Conversion of ICRP male reference phantom to polygon-surface phantom. Phys. Med. Biol. 2013, 58, 6985–7007. [Google Scholar] [CrossRef]
- Yeom, Y.S.; Jeong, J.H.; Han, M.C.; Kim, C.H. Tetrahedral-mesh-based computational human phantom for fast Monte Carlo dose calculations. Phys. Med. Biol. 2014, 59, 3173–3185. [Google Scholar] [CrossRef]
- Bottauscio, O.; Cassara, A.M.; Hand, J.W.; Giordano, D.; Zilberti, L.; Borsero, M.; Chiampi, M.; Weidemann, G. Assessment of computational tools for MRI RF dosimetry by comparison with measurements on a laboratory phantom. Phys. Med. Biol. 2015, 60, 5655–5680. [Google Scholar] [CrossRef]
- Kim, H.S.; Yeom, Y.S.; Nguyen, T.T.; Choi, C.; Han, M.C.; Lee, J.K.; Kim, C.H.; Zankl, M.; Petoussi-Henss, N.; Bolch, W.E.; et al. Inclusion of thin target and source regions in alimentary and respiratory tract systems of mesh-type ICRP adult reference phantoms. Phys. Med. Biol. 2017, 62, 2132–2152. [Google Scholar] [CrossRef]
- Spitzer, V.; Ackerman, M.J.; Scherzinger, A.L.; Whitlock, D. The visible human male: A technical report. J. Am. Med. Inform. Assoc. 1996, 3, 118–130. [Google Scholar] [CrossRef]
- Park, J.S.; Chung, M.S.; Hwang, S.B.; Lee, Y.S.; Har, D.H.; Park, H.S. Visible Korean human: Improved serially sectioned images of the entire body. IEEE Trans. Med. Imaging 2005, 24, 352–360. [Google Scholar] [CrossRef]
- Zhang, S.X.; Heng, P.A.; Liu, Z.J. Chinese visible human project. Clin. Anat. 2006, 19, 204–215. [Google Scholar] [CrossRef]
- Park, J.S.; Chung, M.S.; Shin, D.S.; Har, D.H.; Cho, Z.H.; Kim, Y.B.; Han, J.Y.; Chi, J.G. Sectioned images of the cadaver head including the brain and correspondences with ultrahigh field 7.0 T MRIs. Proc. IEEE 2009, 97, 1988–1996. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, D.H.; Park, J.S. Improved sectioned images and surface models of the whole female body. Int. J. Morphol. 2015, 33, 1323–1332. [Google Scholar] [CrossRef]
- Park, J.S. Neuroman: Voxel phantoms from surface models of 300 head structures including 12 pairs of cranial nerves. Health Phys. 2020, 119, 192–205. [Google Scholar] [CrossRef]
- Yanamadala, J.; Noetscher, G.M.; Rathi, V.K.; Maliye, S.; Win, H.A.; Tran, A.L.; Jackson, X.J.; Htet, A.T.; Kozlov, M.; Nazarian, A.; et al. New VHP-Female v. 2.0 full-body computational phantom and its performance metrics using FEM simulator ANSYS HFSS. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 3237–3241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).