Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering
Abstract
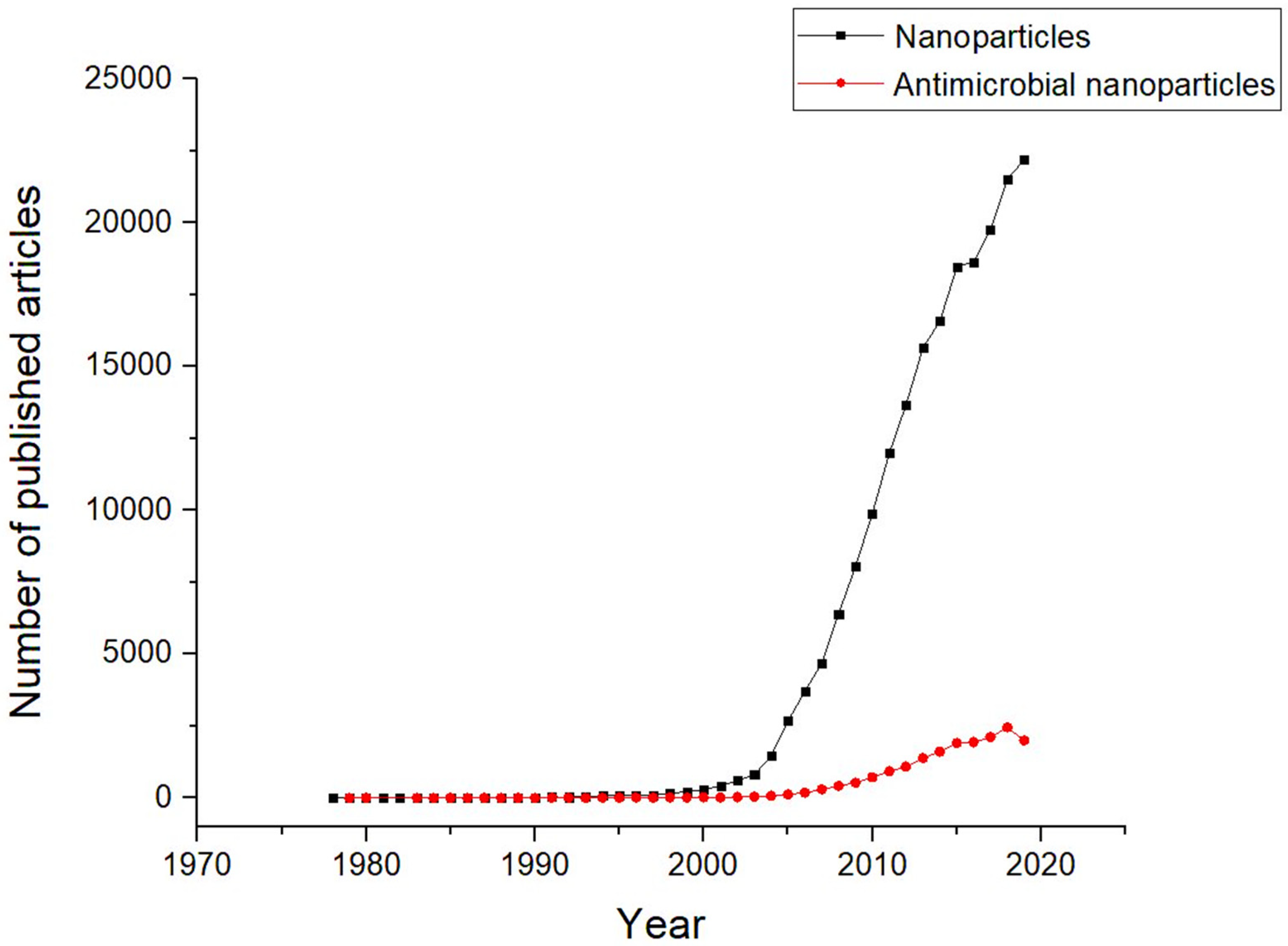
1. Introduction
2. Nanomaterials
2.1. Nanoparticle Synthesis and Processing Methods
2.1.1. Bottom Up
- Gas-phase synthesis: This is when nanoparticles are generated through the interaction of gaseous precursor components over a catalyst or prepared surface. For instance, chemical vapour deposition (CVD) is a method involving the deposition of a thin film of gaseous reactants onto a substrate. A thin film of product is generated on the substrate surface when it is heated at ambient temperature by combining gas molecules [24]. The advantages of CVD are in producing highly pure, uniform, hard and strong nanoparticles. However, CVD requires special equipment, and the gaseous by-products can be toxic [25]. Still, CVD is a widely used technique to deposit metallic nano silver layers on the substrate surface heated to the temperature 573 K at atmospheric pressure. Nano metal layers generated using this method were proved to be active against a wide spectrum of bacterial strains [26]. For example, the antimicrobial property studies of AgNPs and CuNPs deposited on the surface of such biomedical materials, such as titanium, TiAlNb alloy and steel (317 L), confirmed the inhibitory effect against S. aureus [27]. Spange et al. reported wound dressings that were functionalised with Ag NPs using the CVD technique were found to exhibit a strong antibacterial effect against both S. aureus and Klebsiella pneumoniae strains with a low concentration of the silver coating [28].
- Liquid-phase synthesis: Two common processes are used to synthesise nanomaterials via liquid-phase synthesis; they are the ‘sol-gel’ and ‘microwave assist’ methods. Nanomaterials are formed via the sol-gel process. Although often limited to producing metal oxides (i.e., ZnO, TiO2), it is sometimes preferred over using the gas-phase synthesis due to its simplicity and lower processing temperature required to produce nanoparticles at a higher rate [29]. Sol-gel synthesis typically uses metal alkoxides as the precursors or any other reactants that would form a homogeneous medium with the applied solvent. The process first undergoes hydrolysis/polycondensation reactions to form a colloidal suspension (‘sol’). This is then followed by complete solvent evaporation (or calcination) to allow nano products to be formed via precipitation/recrystallisation. By controlling the parameters and drying conditions during the precipitation process, different forms of Nano products, such as gel-film, uniform particle coat, or nano-fibre, can be manufactured using this method. Nanopowder (i.e., iron oxide Fe3O4) can also be obtained using the sol-gel method by simply filtering the precipitated colloidal products [30,31]. Ismail et al. reported how raising the calcination temperature influenced the sizes and agglomeration in the ZnO NPs that were formed using the sol-gel process [32]. Khan et al. claimed to have used the sol-gel method to produce thorn-like ZnO nanoparticles, which showed good antimicrobial and antifungal activities against Bacillus subtilis, Escherichia coli and Candida albicans [33].Research data suggest there has been an increased interest in utilising microwave radiation for nanoparticle synthesis. The electromagnetic energy produced by microwave power enables localised heating to be delivered to the reaction media. [34,35,36,37]. For instance, Yu S-H. and co-workers synthesised environment-friendly Ag NPs using a microwave reactor, where Silver nitrate (AgNO3) was heated and irradiated in water at 150 °C whilst L-Lysine/L-arginine was respectively added as reducing agents and surfactants [38]. This reaction produced uniform and monodispersed Ag NPs with average particle sizes between 26.3 and 26.7 nm in diameter in ten seconds. The manipulation of microwave power and radiation time can also control the morphology of nanoparticles produced. Hasanpoor et al. found that microwave-assisted synthesis can produce needle-like nanoparticles, but when the microwave power was increased, the morphology changed into flower-shaped nanoparticles [36].
- Biological synthesis: Biosynthetic methods can be divided into two classes, the mycosynthesis (utility of fungi) and phytonanotechnology (utility of plants) [39,40]. These eco-friendly methods are also known to produce nanoparticles with active biological function (i.e., antimicrobial). This way, Nanoparticles can be synthesised without utilising toxic chemicals or concerns over generations of harmful by-products. For instance, it is possible to replace the reducing agent used in chemical methods with harmless microorganisms or plant extracts [40]. Well-established biosynthetic methods for NP preparations can also be very cost-effective [41]. The production of antimicrobial Ag NPs via mycosynthesis was reported by Madakka et al., where fungi Aspergillus niger and Fusarium semitectum were utilised [42]. Although there are numerous advantages of using biological methods for nanoparticle synthesis, there are a few drawbacks. First, many of the mechanisms involved in these biosynthetic processes remain unclear. Second, it is not easy to manipulate the constituents in microorganisms or plant extracts to optimise the quality and quantity of nanomaterial productions. Hence, nanoparticles formed using such synthetic methods often result in low production rates and yields.
2.1.2. Top-down
2.2. Intrinsic Properties and Characteristics of Nanoparticles
2.3. Nanomaterials with Antimicrobial Properties
2.3.1. Mono-Metallic Nanoparticles
2.3.2. Nanoparticles Combinations with Synergistic Effects
3. Mechanisms of Action of Antibiotics and Nanoparticles Against Bacteria
3.1. Gram-Negative and Gram-Positive Bacteria
3.2. Antibiotic-Resistant Mechanisms in Microorganisms
3.3. The Mechanisms of Actions of Antimicrobial Nanoparticles
- Reactive oxygen species (ROS): ROS include superoxide anion (), hydroxyl radicals (singlet oxygen () and hydrogen peroxide () (by-products of cellular oxidative metabolism) [115]. Typically, redox-active essential metals present in biomolecules act as catalytic cofactors when ROS are either generated or catalysed by cell enzymes. The presence of external metals intensifies reactions producing an excess of ROS that trigger oxidative stress and subsequently lead to cellular programmed death [116,117]. With specific metals, an occurrence of Fenton reactions both increases the formation of ROS and stimulates the electron transport chain to eventually promote bacteria death through the catabolism of the carbon source and the generation of nicotinamide adenine dinucleotide [13].
- Dissolved metal ions: external metal ions are absorbed through the cell membrane and inhibit cellular function or enzyme activity by interacting with the functional groups of proteins and nucleic acids, which eventually affect the normal physiological processes [14].
- Physical interaction: unlike antibiotics, Gram-negative bacteria are more vulnerable to nanoparticle mechanisms of action since their wall structure may assist released ions from the nanoparticle into the cell. In addition, although both bacteria cell walls are dominated by negative charges, Gram-negative bacteria have a higher affinity for the positive ions due to electrostatic attraction [15].
- Internalisation into the cell: smaller sizes of particles are likely to enter the cells via endocytosis. Subsequent, released nanoparticle ions are then up-taken in high intracellular concentration, which leads to oxidative stress [16].
4. Applications of Antimicrobial Nanoparticles
4.1. Fabrics and Fibres
4.2. Surface Films and Coating
4.3. Healthcare Applications
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tolochko, N. History of nanotechnology. In Nanoscience and Nanotechnology Encyclopaedia of Life Support Systems (EOLSS); Developed under the Auspices of the UNESCO; SEolss: Oxford, UK, 2009. [Google Scholar]
- Harden, D.B.; Toynbee, J.M.C. VII.—The Rothschild Lycurgus Cup. Archaeologia 1959, 97, 179–212. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of Nanosilver History: Implications for Policy Makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.P. The mulitfaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. The crisis of no new antibiotics--what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Atlanta, G.; CDC. Antibiotic Resistance Threats in the United States; Call of Duty Control, Ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2019.
- WHO. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Ren, G.; Oxford, J.S.; Reip, P.W.; Lambkin-Williams, R.; Mann, A. Anti-Viral formulations Nanomaterials and Nanoparticles. U.S. Patent 13/691,099, 18 April 2013. [Google Scholar]
- Allaker, R.P.; Ren, G. Potential impact of nanotechnology on the control of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1–2. [Google Scholar] [CrossRef]
- Bankier, C.; Cheong, Y.; Mahalingam, S.; Edirisinghe, M.; Ren, G.; Cloutman-Green, E.; Ciric, L. A comparison of methods to assess the antimicrobial activity of nanoparticle combinations on bacterial cells. PLoS ONE 2018, 13, e0192093. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Mahalingam, S.; Wang, K.; Cheong, Y.-K.; Canales, E.; Ren, G.; Cloutman-Green, E.; Edirisinghe, M.; Ciric, L. Gyrospun antimicrobial nanoparticle loaded fibrous polymeric filters. Mater. Sci. Eng. C 2017, 74, 315–324. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Webster, T.J.; Leuba, K.D.; Durmus, N.G.; Taylor, E.N. Short communication: Carboxylate functionalized superparamagnetic iron oxide nanoparticles (SPION) for the reduction of S. aureus growth post biofilm formation. Int. J. Nanomed. 2013, 8, 731. [Google Scholar] [CrossRef][Green Version]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Lai, H.-Z.; Chen, W.-Y.; Wu, C.-Y.; Chen, Y.-C. Potent Antibacterial Nanoparticles for Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 2046–2054. [Google Scholar] [CrossRef]
- Cheong, Y.-K.; Arce, M.P.; Benito, A.; Chen, D.; Crisóstomo, N.L.; Kerai, L.V.; Rodríguez, G.; Valverde, J.L.; Vadalia, M.; Cerpa-Naranjo, A.; et al. Synergistic Antifungal Study of PEGylated Graphene Oxides and Copper Nanoparticles against Candida albicans. Nanomaterials 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Azam, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, P.A. Top-Down and Bottom-Up Approaches to Implementation Research: A Critical Analysis and Suggested Synthesis. J. Public Policy 1986, 6, 21–48. [Google Scholar] [CrossRef]
- Arole, V.M.; Munde, S.V. Fabrication of nanomaterials by top-down and bottom-up approaches—An overview. JAAST Mater. Sci. 2014, 1, 89–93. [Google Scholar]
- Bhaviripudi, S.; Mile, E.; Steiner, S.A.; Zare, A.T.; Dresselhaus, M.S.; Belcher, A.M.; Kong, J. CVD Synthesis of Single-Walled Carbon Nanotubes from Gold Nanoparticle Catalysts. J. Am. Chem. Soc. 2007, 129, 1516–1517. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Tsukui, S.; Okuyama, K. Nanoparticle Synthesis by Ionizing Source Gas in Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2003, 42, L77. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A. Silver Nanoparticles Fabricated Using Chemical Vapor Deposition and Atomic Layer Deposition Techniques: Properties, Applications and Perspectives: Review. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Wan, Y.; Raman, S.; He, F.; Huang, Y. Surface modification of medical metals by ion implantation of silver and copper. Vacuum 2007, 81, 1114–1118. [Google Scholar] [CrossRef]
- Spange, S.; Pfuch, A.; Wiegand, C.; Beier, O.; Hipler, U.C.; Grünler, B. Atmospheric pressure plasma CVD as a tool to functionalise wound dressings. J. Mater. Sci. Mater. Electron. 2015, 26. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Rashid, H.; Mansoor, M.A.; Haider, B.; Nasir, R.; Hamid, S.B.A.; Abdulrahman, A. Synthesis and characterization of magnetite nano particles with high selectivity using in-situ precipitation method. Sep. Sci. Technol. 2019, 55, 1207–1215. [Google Scholar] [CrossRef]
- Raab, C.; Simkó, M.; Fiedeler, U.; Nentwich, M.; Gazsó, A. Production of nanoparticles and nanomaterials. Nano Trust 2011, 6, 4. [Google Scholar]
- Ismail, A.; Menazea, A.; Kabary, H.A.; El-Sherbiny, A.; Samy, A. The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by Sol–Gel method. J. Mol. Struct. 2019, 1196, 332–337. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-M.; Lin, Z.; Yang, X. Numerical simulation of microwave heating on chemical reaction in dilute solution. Prog. Electromagn. Res. 2004, 49, 273–289. [Google Scholar] [CrossRef][Green Version]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process. Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted Synthesis of Zinc Oxide Nanoparticles. Procedia Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, S.-B.; Wang, K.; Zhang, M.; Yu, S.-H. Microwave-Assisted Rapid Facile “Green” Synthesis of Uniform Silver Nanoparticles: Self-Assembly into Multilayered Films and Their Optical Properties. J. Phys. Chem. C 2008, 112, 11169–11174. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.O.; Zamudio-Ojeda, A.; León-Morales, J.M.; López-Velázquez, J.C.; García-Morales, S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J. Sci. Food Agric. 2021, 101, 1270–1287. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Madakka, M.; Jayaraju, N.; Rajesh, N. Mycosynthesis of silver nanoparticles and their characterization. MethodsX 2018, 5, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Yadav, T.; Srivastava, O. An investigation on the transformation of the icosahedral phase in the Al-Fe-Cu system during mechanical milling and subsequent annealing. Philos. Mag. A 2002, 82, 2979–2993. [Google Scholar] [CrossRef]
- Kammler, H.K.; Mädler, L.; Pratsinis, S.E. Flame Synthesis of Nanoparticles. Chem. Eng. Technol. 2001, 24, 583–596. [Google Scholar] [CrossRef]
- D’Amato, R.; Falconieri, M.; Gagliardi, S.; Popovici, E.; Serra, E.; Terranova, G.; Borsella, E. Synthesis of ceramic nanoparticles by laser pyrolysis: From research to applications. J. Anal. Appl. Pyrolysis 2013, 104, 461–469. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Müllen, K. A bottom-up approach from molecular nanographenes to unconventional carbon materials. J. Mater. Chem. 2008, 18, 1472–1484. [Google Scholar] [CrossRef]
- Park, J.; Ham, S.; Jang, M.; Lee, J.; Kim, S.; Kim, S.; Lee, K.; Park, D.; Kwon, J.; Kim, H.; et al. Spatial-Temporal Dispersion of Aerosolized Nanoparticles During the Use of Consuemr Spray Products and Estimates of Inhalation Exposure. Environ. Sci. Technol. 2017, 51, 7624–7638. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Ramalingam, G. Quantum Confinement; IntechOpen: London, UK, 2020. [Google Scholar]
- Kim, N.H.; Kim, J.-Y.; Ihn, K.J. Preparation of Silver Nanoparticles Having Low Melting Temperature Through a New Synthetic Process without Solvent. J. Nanosci. Nanotechnol. 2007, 7, 3805–3809. [Google Scholar] [CrossRef]
- Loulijat, H.; Zerradi, H.; Mizani, S.; Achhal, E.M.; Dezairi, A.; Ouaskit, S. The behavior of the thermal conductivity near the melting temperature of copper nanoparticle. J. Mol. Liq. 2015, 211, 695–704. [Google Scholar] [CrossRef]
- Rostami-Charati, F.; Akbari, R. ZnO-nanoparticles as an Efficient Catalyst for the Synthesis of Functionalized Benzenes: Multicomponent Reactions of Sulfonoketenimides. Comb. Chem. High Throughput Screen. 2017, 20, 781–786. [Google Scholar] [CrossRef]
- Kumar, B.V.; Naik, H.S.B.; Girija, D. ZnO nanoparticle as catalyst for efficient green one-pot synthesis of coumarins through Knoevenagel condensation. J. Chem. Sci. 2011, 123, 615–621. [Google Scholar] [CrossRef]
- Khan, M.S.; Bhaisare, M.L.; Gopal, J.; Wu, H.-F. Highly efficient gold nanorods assisted laser phototherapy for rapid treatment on mice wound infected by pathogenic bacteria. J. Ind. Eng. Chem. 2016, 36, 49–58. [Google Scholar] [CrossRef]
- Millenbaugh, N.J.; Baskin, J.B.; DeSilva, M.N.; Elliott, W.R.; Glickman, R.D. Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int. J. Nanomed. 2015, 10, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Weber, G.; Talackine, J.; Millenbaugh, N.J. Targeted laser therapy synergistically enhances efficacy of antibiotics against multi-drug resistant Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102018. [Google Scholar] [CrossRef] [PubMed]
- Evanoff, D.D.; Chumanov, G. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, F.; Liu, C.; Zhou, B.; Wei, Q.; Li, W.; Zhong, D.; Li, Y.; Zhou, M. Near-Infrared Laser-Excited Nanoparticles To Eradicate Multidrug-Resistant Bacteria and Promote Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 193–206. [Google Scholar] [CrossRef]
- Parak, W.J.; Gerion, D.; Pellegrino, T.; Zanchet, D.; Micheel, C.; Williams, S.C.; Boudreau, R.; Le Gros, M.A.; Larabell, C.A.; Alivisatos, A.P. Biological applications of colloidal nanocrystals. Nanotechnology 2003, 14, R15–R27. [Google Scholar] [CrossRef]
- Lea, M.C. ART. L.—On Allotropic Forms of Silver. Am. J. Sci. 1889, 37, 476. [Google Scholar] [CrossRef]
- Frens, G.; Overbeek, J.T.G. Carey Lea’s colloidal silver. Kolloid Z. Z. Polym. 1969, 233, 922–929. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape Control of Silver Nanoparticles by Stepwise Citrate Reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Hashim, A.F.; Alghuthaymi, M.A. Bimetallic nanoparticles as antimicrobials. J. Nanotechnol. Mater. Sci. 2016, 3, 1–2. [Google Scholar] [CrossRef][Green Version]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; De La Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; Van Loveren, H.; De Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial Properties of a Novel Silver-Silica Nanocomposite Material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Mandal, S.; Raj, C. Antimicrobial activity of fluorescent Ag nanoparticles. Lett. Appl. Microbiol. 2014, 58, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núñez, N.V.; Turrent, L.D.C.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2009, 26, 615–621. [Google Scholar] [CrossRef]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthus amarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.; Barwinska-Sendra, A.; Tarrant, E.; Waldron, K.J. Mechanism of action and applications of the antimicrobial properties of copper. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center: Norristown, PA, USA, 2013; pp. 468–479. [Google Scholar]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Tkeshelashvili, L.K.; McBride, T.; Spence, K.; Loeb, L.A. Mutation spectrum of copper-induced DNA damage. J. Biol. Chem. 1991, 266, 6401–6406. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Yoon, K.-Y.; Byeon, J.H.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- El Zowalaty, M.; Ibrahim, N.A.; Salama, M.; Shameli, K.; Usman, M.; Zainuddin, N. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, O.; Bhagat, M.; Gopalakrishnan, C.; Arunachalam, K.D. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J. Exp. Nanosci. 2008, 3, 185–193. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Saraf, R. Cost effective and Monodispersed Zinc Oxide Nanoparticles Synthesis and their Characterization. Int. J. Adv. Appl. Sci. 2013, 2, 85–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids–A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Zhang, W.; Zhang, L.; Shang, E. Influence of Aqueous Media on the ROS-Mediated Toxicity of ZnO Nanoparticles toward Green Fluorescent Protein-Expressing Escherichia coli under UV-365 Irradiation. Langmuir 2014, 30, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Vargas-Reus, M.A.; Memarzadeh, K.; Huang, J.; Ren, G.G.; Allaker, R.P. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 2012, 40, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, K.; Nazari, P.; Nabavi, M.; Golkar, M.; Almasirad, A.; Shahverdi, A.R. Hydroxyl capped silver-gold alloy nanoparticles: Characterization and their combination effect with different antibiotics against Staphylococcus aureus. Nanomed. J. 2014, 1, 155–161. [Google Scholar]
- Zhao, Y.; Ye, C.; Liu, W.; Chen, R.; Jiang, X. Tuning the Composition of AuPt Bimetallic Nanoparticles for Antibacterial Application. Angew. Chem. Int. Ed. 2014, 53, 8127–8131. [Google Scholar] [CrossRef]
- Li, H.F.; Qiu, K.J.; Zhou, F.Y.; Li, L.; Zheng, Y.F. Design and development of novel antibacterial Ti-Ni-Cu shape memory alloys for biomedical application. Sci. Rep. 2016, 6, 37475. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Jia, X.; Han, Q.; Yan, X.; Yang, R.; Wang, C. Porous Pt/Ag nanoparticles with excellent multifunctional enzyme mimic activities and antibacterial effects. Nano Res. 2017, 1, 2056–2069. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Dzidic, S.; Suskovic, J.; Kos, B. Antibiotic Resistance Mechanisms in Bacteria: Biochemical and Genetic Aspects. Food Technol. Biotechnol. 2008, 46, 11–21. [Google Scholar]
- Lambert, P.A. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef]
- Hamilton, S.M.; Alexander, J.A.N.; Choo, E.J.; Basuino, L.; Da Costa, T.M.; Severin, A.; Chung, M.; Aedo, S.; Strynadka, N.C.J.; Tomasz, A.; et al. High-Level Resistance of Staphylococcus aureus to β-Lactam Antibiotics Mediated by Penicillin-Binding Protein 4 (PBP4). Antimicrob. Agents Chemother. 2017, 61, e02727-16. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.M. Gebe Cassettes in Brenner’s Encyclopedia of Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 177–180. [Google Scholar]
- Dzidic, S.; Bedeković, V. Horizontal gene transfer-emerging multidrug resistance in hospital bacteria. Acta Pharmacol. Sin. 2003, 24, 519–526. [Google Scholar] [PubMed]
- Sun, D. Pull in and Push Out: Mechanisms of Horizontal Gene Transfer in Bacteria. Front. Microbiol. 2018, 9, 2154. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes—Generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef]
- Sintubin, L.; De Windt, W.; Dick, J.; Mast, J.; Van Der Ha, D.; Verstraete, W.; Boon, N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 2009, 84, 741–749. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Efect of the size and shape of silver nanoparticles on bacterial growth and metabolism by monitoring optical density and fluorescence intensity. Biotechnol. Bioprocess Eng. 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Deng, X.; Huang, Z.; Wang, W.; Davé, R.N. Investigation of nanoparticle agglomerates properties using Monte Carlo simulations. Adv. Powder Technol. 2016, 27, 1971–1979. [Google Scholar] [CrossRef]
- Fortescue-Brickdale, J.M. Collargol: A Review of Some of Its Clinical Applications, with Experiments on Its Antiseptic Action. Bristol Med. Chir. J. 1903, 21, 337–344. [Google Scholar] [PubMed]
- Fung, M.C.; Bowen, D.L. Silver Products for Medical Indications: Risk-Benefit Assessment. J. Toxicol. Clin. Toxicol. 1996, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Lem, K.W.; Choudhury, A.; Lakhani, A.A.; Kuyate, P.; Haw, J.R.; Lee, D.S.; Iqbal, Z.; Brumlik, C.J. Use of nanosilver in consumer products. Recent Pat. Nanotechnol. 2012, 6, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, C.; De Maeseneire, E.; Kerckhof, F.-M.; Verliefde, A.; Van De Wiele, T.; Boon, N. Microbial Odor Profile of Polyester and Cotton Clothes after a Fitness Session. Appl. Environ. Microbiol. 2014, 80, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef]
- Troccaz, M.; Starkenmann, C.; Niclass, Y.; van de Waal, M.; Clark, A.J. 3-Methly-3-sulfanylhexan-1-ol as a Major Descriptor for the Human Axilla-Sweat Odour Profile. Chem. Biodivers. 2004, 1, 1022–1035. [Google Scholar] [CrossRef]
- Ozeki, C.; Moro, O. A study of the suppression of body odour in elderly subjects by anti-fungal agents. Int. J. Cosmet. Sci. 2016, 38, 312–318. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Gerber, L.C.; Mohn, D.; Fortunato, G.; Astasov-Frauenhoffer, M.; Imfeld, T.; Waltimo, T.; Zehnder, M.; Stark, W.J. Incorporation of reactive silver-tricalcium phosphate nanoparticles into polyamide 6 allows preparation of self-disinfecting fibers. Polym. Eng. Sci. 2011, 51, 71–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hu, Q. Colourless antibacterial cotton fabrics based on silver nanoparticles and chitosan complexes. Int. J. Cloth. Sci. Technol. 2012, 24, 118–128. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Muzslay, M.; Ali, S.; Wilson, P. 1382Antimicrobial efficacy of Corning® Gorilla® Glass 3 under laboratory conditions. Open Forum Infect. Dis. 2014, 1, S363. [Google Scholar] [CrossRef][Green Version]
- Hodges, K.; Gill, R. Infectious diarrhea. Gut Microbes 2010, 1, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polyactide Films and Bimetallic Ag-Cu Nanoparticles and Essential Oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control. 2010, 22, 408–413. [Google Scholar] [CrossRef]
- An, J.; Zhang, M.; Wang, S.; Tang, J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT 2008, 41, 1100–1107. [Google Scholar] [CrossRef]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G.C. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Gunell, M.; Haapanen, J.; Brobbey, K.J.; Saarinen, J.J.; Toivakka, M.; Mäkelä, J.M.; Huovinen, P.; Eerola, E. Antimicrobial characterization of silver nanoparticle-coated surfaces by “touch test” method. Nanotechnol. Sci. Appl. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, J.; Reid, M.; Whatley, V.; Dancer, S. Disastrous performance of NanoCote/Aqua Based antimicrobial paint in a hospital setting. J. Biol. Phys. Chem. 2016, 16, 131–136. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Wang, H.; Peng, J.; Wong, K.K. Silver nanoparticle-coated suture effectively reduces inflammation and improves mechanical strength at intestinal anastomosis in mice. J. Pediatr. Surg. 2014, 49, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, C.; Visai, L.; Santin, M.; Cigada, A.; Candiani, G.; Pezzoli, D.; Arciola, C.L.; Imbriani, M.; Chiesa, R. A novel antibacterial modification treatment of titanium capable to improve osseointegration. Int. J. Artif. Organs 2012, 35, 864–875. [Google Scholar] [CrossRef]
- Xia, W.; Grandfield, K.; Hoess, A.; Ballo, A.; Cai, Y.; Engqvist, H. Mesoporous titanium dioxide coating for metallic implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 82–93. [Google Scholar] [CrossRef]
- Samuel, U.; Guggenbichler, J. Prevention of catheter-related infections: The potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents 2004, 23, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Galiano, K.; Pleifer, C.; Engelhardt, K.; Brössner, G.; Lackner, P.; Huck, C.; Lass-Flörl, C.; Obwegeser, A. Silver segregation and bacterial growth of intraventricular catheters impregnated with silver nanoparticles in cerebrospinal fluid drainages. Neurol. Res. 2008, 30, 285–287. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile coating of urinary catheter with bio–inspired antibacterial coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl. Nanosci. 2016, 6, 337–344. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
| 1 | N/A |




| Monometallic/Metal Oxide Nanoparticles | Mean Size (Shape) | Bacteria/Fungi Tested | Reference |
|---|---|---|---|
| Silver (Ag) | 21 nm (cuboctahedron icosahedron and decahedron) | E. coli V. cholerae S. Typhi P. aeruginosa | [73] |
| 12.4 nm | E. coli | [75] | |
| 13.5 nm (spherical) | Yeast E. coli S. aureus | [78] | |
| 39 nm (spherical); 16 nm (rod) | E. coli | [79] | |
| 1.5–10 nm (spherical, triangular and polyhedron) | S. epidermidis B. megaterium P. aeruginosa E. coli C. albicans A. niger | [80] | |
| Copper (Cu) | 100 nm (spherical) | E. coli B. subtilis | [88] |
| 9 nm (quasi-sphere) | E. coli S. aureus | [89] | |
| 2–350 nm | S. aureus B. subtilis P. aeruginosa S. choleraesuis C. albicans | [90] | |
| 80–160 nm | K. pneumoniae P. aeruginosa S. paratyphi Shigella | [91] | |
| Copper oxide (CuO) | 20–95 nm (rod and rectangle) | EMRSA MRSA S. aureus S. sepidermidis E. coli Proteus spp. P. aeruginosa | [47] |
| 23 nm | E. coli E. faecalis K. pneumonia | [92] | |
| 20–28.9 nm | E. coli P. aeruginosa B. subtilis S. aureus | [21] | |
| Zinc oxide (ZnO) | 20 nm | E. coli P. aeruginosa S. aureus B. subtilis | [95] |
| 20 nm | B. subtilis E. coli P. fluorescens | [100] | |
| 20–40 nm | E. coli | [101] | |
| Intermetallic Nanoparticles | Mean Size (nm) | Bacteria/Fungi Tested | Reference |
| Silver-Gold alloy (Ag-Au) | <200 nm (spherical) | S. aureus | [103] |
| Titanium-Nickel-Copper alloy (Ti-Ni-Cu) | N/A | S. aureus E. coli | [105] |
| Platinum-Silver alloy (Pt-Ag) | N/A | E. coli S. aureus | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.-K. Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Appl. Sci. 2021, 11, 4520. https://doi.org/10.3390/app11104520
Yang X, Chung E, Johnston I, Ren G, Cheong Y-K. Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Applied Sciences. 2021; 11(10):4520. https://doi.org/10.3390/app11104520
Chicago/Turabian StyleYang, XiuYi, Etelka Chung, Ian Johnston, Guogang Ren, and Yuen-Ki Cheong. 2021. "Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering" Applied Sciences 11, no. 10: 4520. https://doi.org/10.3390/app11104520
APA StyleYang, X., Chung, E., Johnston, I., Ren, G., & Cheong, Y.-K. (2021). Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Applied Sciences, 11(10), 4520. https://doi.org/10.3390/app11104520








