Strengthening Thermal Stability in V2O5-ZnO-BaO-B2O3-M(PO3)n Glass System (M = Al, Mg) for Laser Sealing Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Glass Fabrication
2.2. Characterization
3. Results and Discussion
3.1. Density and Molar Volume
3.2. Thermal Properties
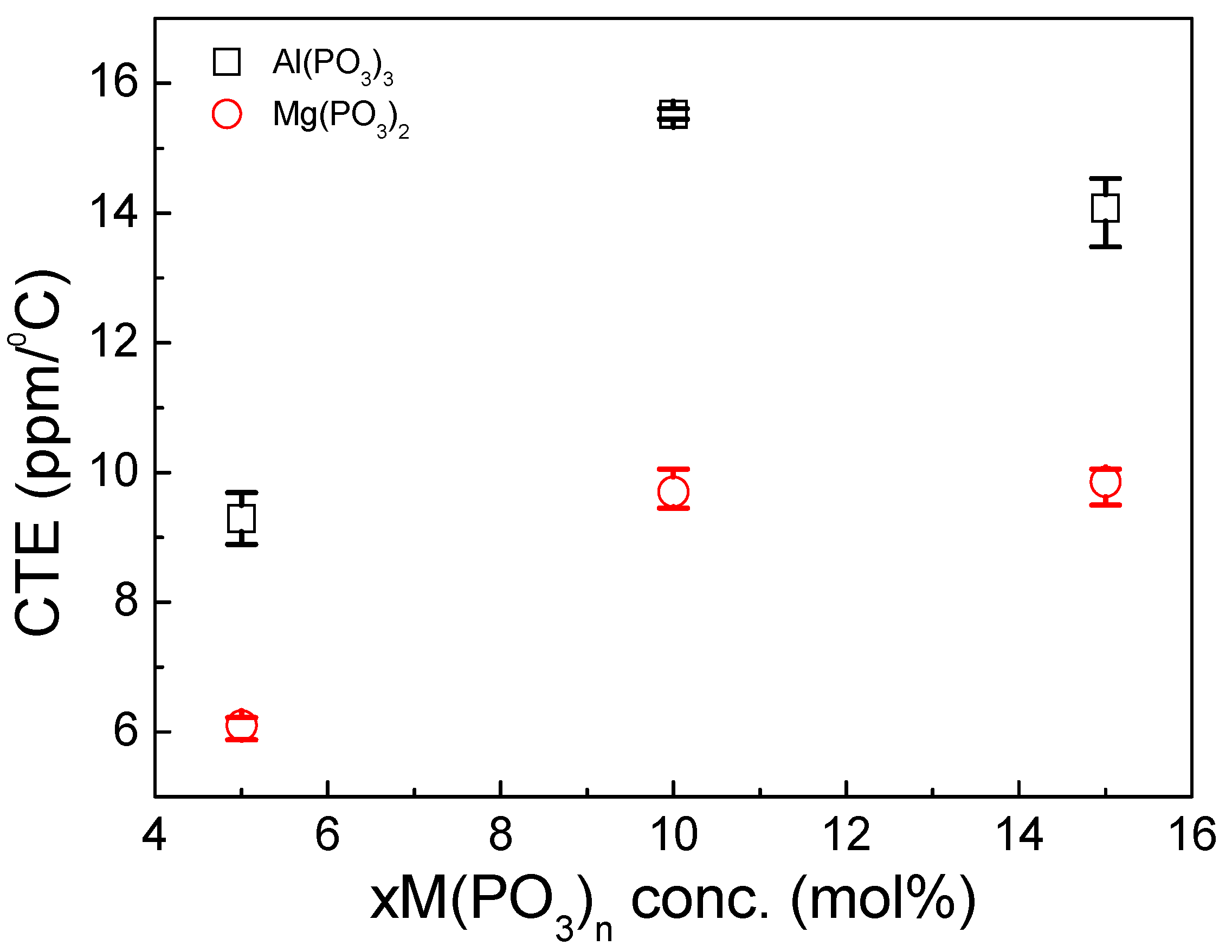
3.3. Thermomechanical Properties
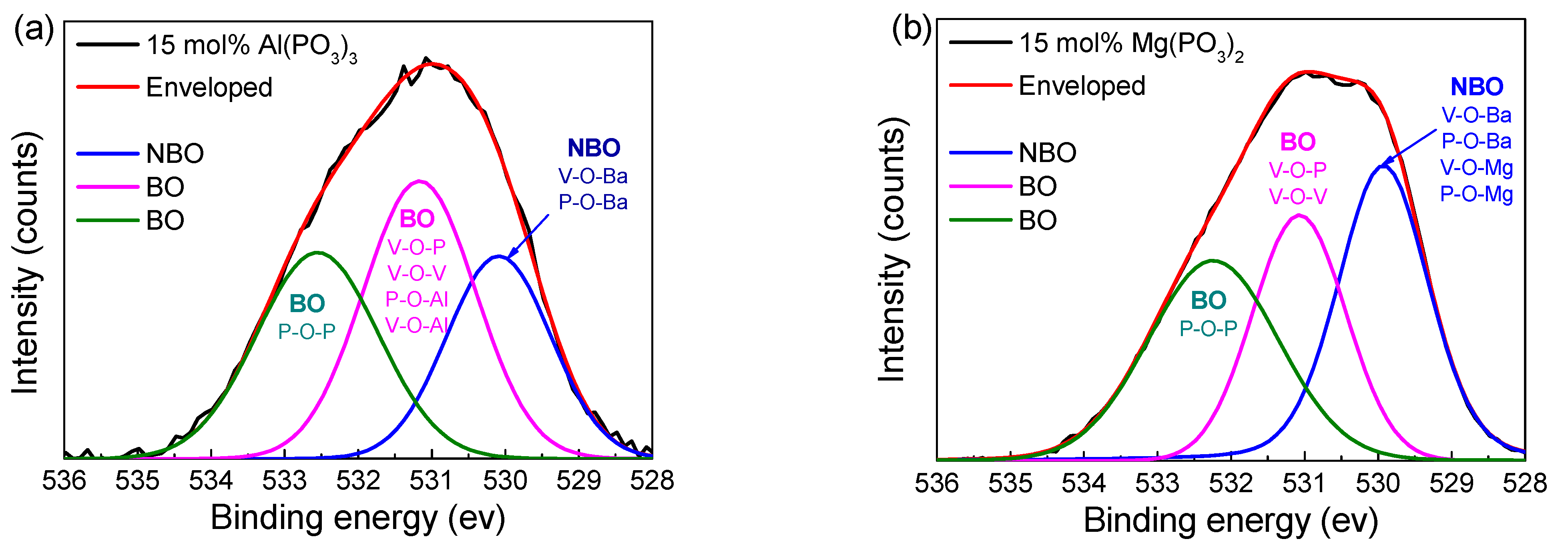
3.4. Structural Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Xiao, Y.; Wu, X.; Zhang, J. Optimization of laser-assisted glass frit bonding process by response surface methodology. Opt. Laser Technol. 2016, 77, 111–115. [Google Scholar] [CrossRef]
- Cruz, R.; da Cruz Ranita, J.A.; Maçaira, J.; Ribeiro, F.; da Silva, A.M.B.; Oliveira, J.M.; Mendes, A. Glass–Glass Laser-Assisted Glass Frit Bonding. IEEE Trans. Compon. Packag. Manuf. Technol. 2012, 2, 1949–1956. [Google Scholar] [CrossRef]
- Lee, K.; Ko, M.J. The Effect of Sealing Technology on the Long-Term Stability of Dye-Sensitized Solar Cell Module. Curr. Photovolt. Res. 2016, 4, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Dietz, R.L. Optical fiber sealing with solder glass. In Photonics North 2004: Photonic Applications in Astronomy, Biomedicine, Imaging, Materials Processing, and Education; International Society for Optics and Photonics: Ottawa, ON, Canada, 2004; pp. 642–651. [Google Scholar]
- Daniel, H.S.; Moore, D.R.; Tekippe, V.J. Glass soldering improves photonic component packaging. Laser Focus World 1994, 30, 94. [Google Scholar]
- Kim, S.Y.; Park, J.; Kim, S.H.; Kadathala, L.; Baek, J.H.; Kim, J.H.; Choi, J.H. The Tuning Capability of CuO and Na2CO3 Dopant on Physical Properties for Laser Sealing Using Fiber Types of Sealant. Appl. Sci. 2020, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Morena, R. Phosphate glasses as alternatives to Pb-based sealing frits. J. Non-Cryst. Solids 2000, 263–264, 382–387. [Google Scholar]
- Sung, W.; Won, J.; Lee, J.; Kim, H. Properties and Structure of P2O5-V2O5-ZnO/B2O3 Glasses. Mol. Cryst. Liq. Cryst. 2009, 499, 234–241. [Google Scholar] [CrossRef]
- Naito, T.; Matsuda, A.; Shiojiri, D.; Aoyagi, T.; Sawai, Y.; Fujieda, T.; Yoshimoto, M. Yoshimoto. Influence of P2O5/TeO2 composition ratio on the physical properties of V2O5–P2O5–TeO2 glasses for lead-free low-temperature sealing. J. Ceram. Soc. Jpn. 2013, 121, 452–456. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.J.; Kim, K.H.; Choi, Y.G.; Shin, D.W. Compositional Effect on the Electrical Properties of V2O5–P2O5–WO3 System. J. Am. Ceram. Soc. 2010, 93, 2292–2296. [Google Scholar] [CrossRef]
- Stachel, D.; Barz, A. Structure investigations of ultraphosphate glasses with some water content. J. Phys. Chem. Solids 2007, 68, 1021–1023. [Google Scholar] [CrossRef]
- Minami, T.; Mackenzie, J.D. Thermal Expansion and Chemical Durability of Phosphate Glasses. J. Am. Ceram. Soc. 1997, 60, 232–235. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Xu, S.; Wang, G.; Hu, L. Physical and thermal properties of P2O5-Al2O3-BaO-La2O3 glasses. J Mater Sci Technol. 2005, 21, 391–394. [Google Scholar]
- Mošner, P.; Vosejpková, K.; Koudelka, L.; Montagne, L.; Revel, B. Structure and properties of ZnO–B2O3–P2O5–TeO2 glasses. Mater Chem Phys. 2010, 124, 732–737. [Google Scholar] [CrossRef]
- Muñoz-Senovilla, L.; Muñoza, F. Behaviour of viscosity in metaphosphate glasses. J Non-Cryst Solids 2014, 385, 9–16. [Google Scholar] [CrossRef]
- Tsuchida, J.E.; Schneider, J.; Pizani, P.S.; Oliveira, S.L. Lead and Aluminum Bonding in Pb Al Metaphosphate Glasses. Inorg. Chem. 2008, 47, 690–698. [Google Scholar] [CrossRef]
- Sohn, I.; Min, D.J. A Review of the Relationship between Viscosity and the Structure of Calcium-Silicate-Based Slags in Ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]
- Schneider, J.; Oliveira, S.L.; Nunes, L.A.O.; Bonk, F.; Panepucci, H. Panepucc. Short-Range Structure and Cation Bonding in Calcium−Aluminum Metaphosphate Glasses. Inorg. Chem. 2005, 44, 423–430. [Google Scholar] [CrossRef]
- Nascimento, M.L.F.; Souza, L.A.; Ferreira, E.B.; Zanotto, E.D. Can glass stability parameters infer glass forming ability? J. Non. Cryst. Solids 2005, 351, 3296–3308. [Google Scholar] [CrossRef] [Green Version]
- Mandlule, A.; Döhler, F.; van Wüllen, L.; Kasuga, T.; Brauer, D.S. Changes in structure and thermal properties with phosphate content of ternary calcium sodium phosphate glasses. J Non-Cryst Solids 2014, 392–393, 31–38. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ali, A.A.; Mahmoud, D.A.; El-Fiqi, A.M. Preparation and characterization of antibacterial P2O5-CaO-Na2O-Ag2O glasses. J. Biomed. Mater. Res. A 2011, 98, 132–142. [Google Scholar] [CrossRef]
- Amos, R.T.; Henderson, G.S. The effects of alkali cation mass and radii on the density of alkali germanate and alkali germano-phosphate glasses. J Non-Cryst Solids 2003, 331, 108–121. [Google Scholar] [CrossRef]
- Marzouk, M.A.; ElBatal, F.H.; ElBadry, K.M.; ElBatal, H.A. Optical, structural and thermal properties of sodium metaphosphate glasses containing Bi2O3 with interactions of gamma rays. Spectrochim. Acta A 2017, 171, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hollaway, D.G. The Physical Properties of Glass; Wykeham publications Ltd.: London, UK, 1973; pp. 36–41. [Google Scholar]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed.; The Royal Society of Chemistry United Kingdom: London, UK, 2005; p. 154. [Google Scholar]
- Khan, S.; Singh, K. Effect of MgO on Structural, Thermal and Conducting Properties of V2-xMgxO5-δ (x = 0.05–0.30) Systems. Ceram. Int. 2019, 45, 695–701. [Google Scholar] [CrossRef]
- Walter, G.; Vogel, J.; Hoppe, U.; Hartmann, P. Hartmann. Structural Study of Magnesium Polyphosphate Glasses. J. Non-Cryst. Solids 2003, 320, 210–222. [Google Scholar] [CrossRef]
- Bingham, P.A.; Hand, R.J.; Hannant, O.M.; Forder, S.D.; Kilcoyne, S.H. Effects of modifier additions on the thermal properties, chemical durability, oxidation state and structure of iron phosphate glasses. J. Non-Cryst. Solids 2009, 355, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Brow, R.K. Review: The structure of simple phosphate glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Wang, P.W.; Zhang, L. Structural role of lead in lead silicate glasses derived from XPS spectra. J. Non-Cryst. Solids 1996, 194, 129–134. [Google Scholar] [CrossRef]
- Khattak, G.D.; Salim, M.A.; Wenger, L.E.; Gilani, A.H. X-ray photoelectron spectroscopy (XPS) and magnetic susceptibility studies of copper-vanadium phosphate glasses. J. Non-Cryst. Solids 2000, 262, 66–79. [Google Scholar] [CrossRef]
- Khattak, G.D.; Mekki, A.; Wenger, L.E. X-ray photoelectron spectroscopy (XPS) and magnetic susceptibility studies of vanadium phosphate glasses. J. Non-Cryst. Solids 2009, 355, 2148–2155. [Google Scholar] [CrossRef]
- Majjane, A.; Chahine, A.; Et-tabirou, M.; Echchahed, B.; Do, T.O.; Mc Breen, P. X-ray photoelectron spectroscopy (XPS) and FTIR studies of vanadium barium phosphate glasses. Mater. Chem. Phys. 2014, 143, 779–787. [Google Scholar] [CrossRef]
- Brow, R.K.; Kirkpatrick, R.J.; Turner, G.L. Local Structure of xAl2O3·(I − x)NaPO3 Glasses: An NMR and XPS Study. J. Am. Ceram Soc. 1990, 73, 2293–2300. [Google Scholar] [CrossRef]
- Brow, R.K. Nature of Alumina in Phosphate Glass: I, Properties of Sodium Aluminophosphate Glass. J. Am. Ceram Soc. 1993, 76, 913–918. [Google Scholar] [CrossRef]
- Brow, R.K.; Kirkpatrick, R.J.; Turner, G.L. Nature of Alumina in Phosphate Glass: II, Structure of Sodium Aluminophosphate Glass. J. Am. Ceram Soc. 1993, 76, 919–928. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, M.; Chen, J.H. (Eds.) Glass Making Techniques; Chemical Industry Press: Beijing, China, 2006; Chapter 4. [Google Scholar]





| Glasses | V2O5 | ZnO | BaO | Al(PO3)3 | B2O3 |
| VZBB | 70 | 5 | 22 | 0 | 3 |
| AP2.5 | 67.5 | 5 | 22 | 2.5 | 3 |
| AP5.0 | 65 | 5 | 22 | 5 | 3 |
| AP7.5 | 62.5 | 5 | 22 | 7.5 | 3 |
| AP10.0 | 60 | 5 | 22 | 10 | 3 |
| AP12.5 | 57.5 | 5 | 22 | 12.5 | 3 |
| AP15.0 | 55 | 5 | 22 | 15 | 3 |
| Glasses | V2O5 | ZnO | BaO | Mg(PO3)2 | B2O3 |
| MP2.5 | 67.5 | 5 | 22 | 2.5 | 3 |
| MP5.0 | 65 | 5 | 22 | 5 | 3 |
| MP7.5 | 62.5 | 5 | 22 | 7.5 | 3 |
| MP10.0 | 60 | 5 | 22 | 10 | 3 |
| MP12.5 | 57.5 | 5 | 22 | 12.5 | 3 |
| MP15.0 | 55 | 5 | 22 | 15 | 3 |
| Glasses | V2O5 | ZnO | BaO | NaPO3 | B2O3 |
| NP2.5 | 67.5 | 5 | 22 | 2.5 | 3 |
| NP5.0 | 65 | 5 | 22 | 5 | 3 |
| NP7.5 | 62.5 | 5 | 22 | 7.5 | 3 |
| NP10.0 | 60 | 5 | 22 | 10 | 3 |
| NP12.5 | 57.5 | 5 | 22 | 12.5 | 3 |
| NP15.0 | 55 | 5 | 22 | 15 | 3 |
| Cation (M) | Charge (Z) | Ionic Radius (α) | Ratio (Z/α) | Cation Field Strength (Z/α2) |
|---|---|---|---|---|
| Na | 1 | 1.02 Å | 0.980 Å−1 | 0.961 Å−2 |
| Mg | 2 | 0.72 Å | 2.778 Å−1 | 3.858 Å−2 |
| Al | 3 | 0.53 Å | 4.615 Å−1 | 10.679 Å−2 |
| Raman Vibrational Bands (cm−1) | Attributed Vibrational Modes | |||
|---|---|---|---|---|
| VZBB | VA#1-6 | VM#1-6 | VN#1-6 | |
| 291 | 275 | 289 | 279 | Bending modes of PO4 tetrahedra units or bending vibrations of V3–O, V–O–V, and V=O bonds |
| 399 | 491 | 494 | 496 | Bending modes of PO4 tetrahedra units or stretching vibration of V3–O bonds |
| 665 | - | 626 | 633 | Stretching vibrations of V–O–V, V–O–P, P–O–P bonds |
| 799 | 706 | 685 | 689 | Stretching vibrations of V–O–V, V–O–P, P–O–P bonds |
| 1154 | 991 | 984 | 979 | V=O vibrations in VO4 tetragonal pyramid or PO4 vibrations of Q0 units |
| - | 1120 | - | 1127 | PO2 symmetric stretching in O–P–O groups of corner sharing PO4 tetrahedra |
| - | 1326 | 1327 | Symmetric stretching modes of P=O bonds of Q3 units | |
| 15 mol% Al(PO3)3 | 15mol% Mg(PO3)2 | |||
|---|---|---|---|---|
| Area (%) | Centroid (eV) | Area (%) | Centroid (eV) | |
| NBO | 27.3 | 530.09 | 36.49 | 529.97 |
| BO | 40.24 | 531.17 | 29.55 | 531.07 |
| BO | 32.46 | 532.55 | 33.95 | 532.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Han, K.; Kim, S.; Kadathala, L.; Kim, J.; Choi, J. Strengthening Thermal Stability in V2O5-ZnO-BaO-B2O3-M(PO3)n Glass System (M = Al, Mg) for Laser Sealing Applications. Appl. Sci. 2021, 11, 4603. https://doi.org/10.3390/app11104603
Kim S, Han K, Kim S, Kadathala L, Kim J, Choi J. Strengthening Thermal Stability in V2O5-ZnO-BaO-B2O3-M(PO3)n Glass System (M = Al, Mg) for Laser Sealing Applications. Applied Sciences. 2021; 11(10):4603. https://doi.org/10.3390/app11104603
Chicago/Turabian StyleKim, Soyoung, Karam Han, Seonhoon Kim, Linganna Kadathala, Jinhyeok Kim, and Juhyeon Choi. 2021. "Strengthening Thermal Stability in V2O5-ZnO-BaO-B2O3-M(PO3)n Glass System (M = Al, Mg) for Laser Sealing Applications" Applied Sciences 11, no. 10: 4603. https://doi.org/10.3390/app11104603






