Retention of the Antibiotic Cefuroxime onto Agricultural and Forest Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Soils Analyses
2.3. Adsorption and Desorption Experiments
2.4. Chemical Reagents and Quantification of CFX
2.5. Fitting to Adsorption Models and Statistical Treatment
3. Results and Discussion
3.1. Characteristics of the Soil Samples
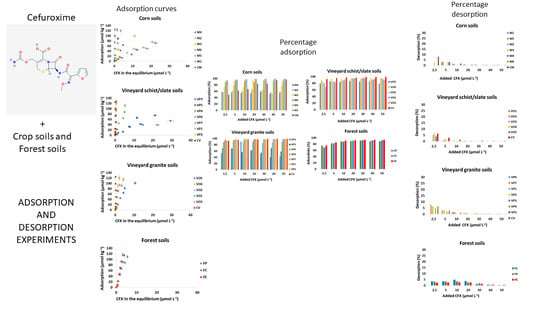
3.2. Adsorption of CFX and Modeling
3.2.1. Adsorption Data
3.2.2. Fitting to Adsorption Models
3.3. Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351–126361. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control); EFSA (European Food Safety Authority); EMA (European Medicines Agency). ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food producing animals—Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA J. 2017, 15, 4872. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control). Antimicrobial Consumption—Annual Epidemiological Report for 2019. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2019 (accessed on 15 April 2021).
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, T.; Lu, S.; Liu, H.; Wang, H.; Li, C.; Liu, X.; Guo, X.; Zhao, X.; Liu, F. Performance and bacterial community dynamics of hydroponically grown Iris pseudacorus L. during the treatment of antibiotic-enriched wastewater at low/normal temperature. Ecotoxicol. Environ. Saf. 2021, 213, 111997. [Google Scholar] [CrossRef]
- Peña-Rodríguez, S.; Bermúdez-Couso, A.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Mercury removal using ground and calcined mussel shell. J. Environ. Sci. 2013, 25, 2476–2486. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Bermúdez-Couso, A.; Garrido-Rodríguez, B.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) retention on soils and forest by-products and other waste materials. Environ. Sci. Pollut. Res. 2013, 20, 6574–6583. [Google Scholar] [CrossRef]
- Kim, S.; Aga, D.S. Potential Ecological and Human Health Impacts of Antibiotics and Antibiotic-Resistant Bacteria from Wastewater Treatment Plants. J. Toxicol. Environ. Health Part B 2007, 10, 559–573. [Google Scholar] [CrossRef]
- Escolà-Casas, M.; Schröter, N.S.; Zammit, I.; Castaño-Trias, M.; Rodriguez-Mozaz, S.; Gago-Ferrero, P.; Corominas, L. Showcasing the potential of wastewater-based epidemiology to track pharmaceuticals consumption in cities: Comparison against prescription data collected at fine spatial resolution. Environ. Int. 2021, 150, 106404. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.A.; Prados-Joya, G.; OcampoPérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Liu, W.; Huang, Y.; Luo, Y.; Christie, P. Dissipation of antibiotics in three different agricultural soils after repeated application of biosolids. Environ. Sci. Poll. Res. 2016, 25, 104–114. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Sabri, N.A.; van Holst, S.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate of antibiotics and antibiotic resistance genes during conventional and additional treatment technologies in wastewater treatment plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Proc. Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Yang, Q.; Sun, L.; Yang, X.; Zhou, M.; Deng, R.; Bi, L. Plant Growth, Antibiotic Uptake, and Prevalence of Antibiotic Resistance in an Endophytic System of Pakchoi under Antibiotic Exposure. Int. J. Environ. Res. Public Health 2017, 14, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, Y.; Shi, M.; Qiu, T.; Gao, M.; Tian, S.; Wang, X. Effect of antibiotic type and vegetable species on antibiotic accumulation in soil-vegetable system, soil microbiota, and resistance genes. Chemosphere 2021, 263, 128099. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Granda, M.D.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Gold, B.; Rodriguez, W.J. Cefuroxime: Mechanisms of Action, Antimicrobial Activity, Pharmacokinetics, Clinical Applications, Adverse Reactions and Therapeutic Indications. Pharmacotherapy 1983, 3, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Latrou, E.I.; Stasinakis, A.S.; Thomaidis, N.S. Consumption-based approach for predicting environmental risk in Greece due to the presence of antimicrobials in domestic wastewater. Environ. Sci. Poll. Res. 2014, 21, 12941–12950. [Google Scholar] [CrossRef]
- Li, J.; Xin, Z.; Zhang, Y.; Chen, J.; Yan, J.; Li, H.; Hu, H. Long-term manure application increased the levels of antibiotics and antibiotic resistance genes in a greenhouse soil. Appl. Soil Ecol. 2017, 121, 193–200. [Google Scholar] [CrossRef]
- Orlewska, K.; Markowicz, A.; Piotrowska-Seget, Z.; Smoleń-Dzirba, J.; Cycoń, M. Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1. Sustainability 2018, 10, 3549. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, A.; Adami, S. Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 1001–1006. [Google Scholar] [CrossRef]
- Figueroa-Diva, R.A.; Vasudevan, D.; MacKay, A. Trends in soil sorption coefficients within common antimicrobial families. Chemosphere 2010, 79, 786–793. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Schmidt, T.C. Determination of acid dissociation constants (pKa) of cephalosporin antibiotics: Computational and experimental approaches. Chemosphere 2017, 169, 524–533. [Google Scholar] [CrossRef]
- Legnoverde, M.S.; Simonetti, S.; Basaldella, E.I. Influence of pH on cephalexin adsorption onto SBA-15 mesoporous silica: Theoretical and experimental study. Appl. Surf. Sci. 2014, 300, 37–42. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Arias-Estévez, M.; Fernández-Calvinho, D.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Adsorption/desorption of three tetracycline antibiotics on different soils in binary competitive systems. J. Environ. Manag. 2020, 262, 110337–110347. [Google Scholar] [CrossRef]
- Yu, H.; Kühne, R.; Ebert, R.U.; Schüürmann, G. Prediction of the Dissociation Constant pKa of Organic Acids from Local Molecular Parameters of Their Electronic Ground State. J. Chem. Inf. Model. 2011, 51, 2336–2344. [Google Scholar] [CrossRef]
- Bhaumik, R.; Mondal, N.K.; Das, B.; Roy, P.K.C. Eggshell powder as an adsorbent for removal of fluoride from aqueous solution: Equilibrium, kinetic and thermodynamic studies. J. Chem. 2012, 9, 1457–1480. [Google Scholar] [CrossRef] [Green Version]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, O. Pharmaceuticals’ sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Proctor, A.; Toro-Vazquez, J.F. The Freundlich isotherm in studying adsorption in oil processing. J. Am. Oil Chem. Soc. 1996, 73, 1627–1633. [Google Scholar] [CrossRef]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.; Qiao, M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical–chemical properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef]
- Tamer, A.; Elbana, H.; Selim, M.; Akrami, N.; Newman, A.; Shaheen, S.M.; Rinklebe, J. Freundlich sorption parameters for cadmium, copper, nickel, lead, and zinc for different soils: Influence of kinetics. Geoderma 2018, 324, 80–88. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Núñez-Delgado, A.; Fernández-Calviño, D.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Competitive adsorption of tetracycline, oxytetracycline and chlortetracycline on soils with different pH value and organic matter content. Environ. Res. 2019, 178, 108669–108679. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Single and simultaneous adsorption of three sulfonamides in agricultural soils: Effects of pH and organic matter content. Sci. Total Environ. 2020, 744, 140872–140886. [Google Scholar] [CrossRef]


| Freundlich | Langmuir | Linear Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | KF | Error | n | Error | R2 | KL | Error | qm | Error | R2 | Kd | Error | R2 |
| M1 | 478.866 | 152.237 | 1.575 | 0.29 | 0.893 | - | - | - | - | - | 247.337 | 29.375 | 0.797 |
| M2 | 5.133 | 0.958 | 0.881 | 0.07 | 0.988 | 0.013 | 0.008 | 341.167 | 174.072 | 0.989 | 3.699 | 0.122 | 0.983 |
| M3 | 22.027 | 11.843 | 0.41 | 0.23 | 0.57 | - | - | - | - | - | 4.735 | 1.169 | 0.328 |
| M4 | 3.234 | 1.002 | 0.983 | 0.11 | 0.979 | - | - | - | - | - | 3.084 | 0.113 | 0.978 |
| M5 | 470.333 | 140.326 | 1.655 | 0.3 | 0.917 | - | - | - | - | - | 233.145 | 26.194 | 0.816 |
| M6 | 32.85 | 8.286 | 1.013 | 0.25 | 0.894 | - | - | - | - | - | 33.257 | 2.798 | 0.894 |
| CM | - | - | - | - | - | - | - | - | - | - | - | - | - |
| VO1 | 36.079 | 9.37 | 0.782 | 0.21 | 0.808 | - | - | - | - | - | 27.873 | 3.461 | 0.779 |
| VO2 | 40.898 | 10.846 | 0.83 | 0.28 | 0.746 | - | - | - | - | - | 35.283 | 4.958 | 0.728 |
| VO3 | 32.17 | 13.084 | 0.855 | 0.35 | 0.603 | - | - | - | - | - | 27.53 | 4.932 | 0.593 |
| VO4 | 42.584 | 6.548 | 1.378 | 0.26 | 0.943 | - | - | - | - | - | 51.391 | 3.805 | 0.917 |
| VO5 | 21.681 | 4.085 | 0.685 | 0.1 | 0.948 | 0.113 | 0.037 | 193.418 | 37.659 | 0.957 | 11.48 | 1.085 | 0.863 |
| VP1 | 228.797 | 25.578 | 1.589 | 0.18 | 0.971 | - | - | - | - | - | 157.328 | 13.55 | 0.888 |
| VP2 | 327.646 | 63.461 | 1.54 | 0.23 | 0.941 | - | - | - | - | - | 201.923 | 19.545 | 0.861 |
| VP3 | 10.877 | 3.319 | 0.458 | 0.1 | 0.895 | 0.15 | 0.095 | 58.473 | 11.744 | 0.876 | 2.018 | 0.266 | 0.693 |
| VP4 | 20.2 | 5.829 | 0.944 | 0.19 | 0.947 | - | - | - | - | - | 18.56 | 1.088 | 0.947 |
| VP5 | 10.842 | 3.289 | 0.459 | 0.1 | 0.896 | 0.15 | 0.094 | 58.46 | 11.649 | 0.878 | 2.017 | 0.265 | 0.696 |
| VP6 | 207.294 | 19.519 | 1.451 | 0.15 | 0.975 | - | - | - | - | - | 157.181 | 11.182 | 0.923 |
| VP7 | 164.232 | 5.17 | 1.099 | 0.05 | 0.996 | - | - | - | - | - | 156.031 | 3.176 | 0.993 |
| CV | - | - | - | - | - | - | - | - | - | - | - | - | - |
| FP | 21.07 | 3.307 | 0.978 | 0.11 | 0.971 | - | - | - | - | - | 20.43 | 0.876 | 0.971 |
| FE | 24.905 | 2.618 | 1.323 | 0.1 | 0.986 | - | - | - | - | - | 33.435 | 1.836 | 0.954 |
| FC | 20.149 | 3.368 | 1.251 | 0.14 | 0.971 | - | - | - | - | - | 26.738 | 1.548 | 0.949 |
| C0 (µmol L−1) | |||||||
|---|---|---|---|---|---|---|---|
| Soil | 2.5 | 5 | 10 | 20 | 30 | 40 | 50 |
| M1 | 0 (0) | 0.37 (1.5) | 0.37 (0.8) | 0.39 (0.8) | 0.51 (0.7) | 0.51 (0.5) | 0.53 (0.4) |
| M2 | 0 (0) | 0.36 (3.0) | 0.35 (1.4) | 0.36 (0.7) | 0.39 (0.5) | 0.36 (0.4) | 0.48 (0.4) |
| M3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.11 (2.7) |
| M4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| M5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| M6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| M7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VO1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VO2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VO3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VO4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VO5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VP1 | 0.39 (6.64) | 0.41 (3.35) | 0.43 (1.77) | 0.50 (1.02) | 0.49 (0.67) | 0.59 (0.60) | 0.59 (0.48) |
| VP2 | 0.38 (6.45) | 0.38 (3.15) | 0.41 (1.67) | 0.46 (0.92) | 0.66 (0.89) | 0.65 (0.66) | 0.73 (0.59) |
| VP3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VP4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VP5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VP6 | 0 (0) | 0 (0) | 0.37 (1.55) | 0.34 (0.69) | 0.47 (0.64) | 0.42 (0.43) | 0.49 (0.40) |
| VP7 | 0.44 (7.98) | 0.42 (3.07) | 0.39 (1.66) | 0.33 (0.67) | 0.27 (0.37) | 0.30 (0.33) | 0.51 (0.40) |
| VP8 | 0.36 (6.27) | 0.35 (2.90) | 0.33 (1.34) | 0.11 (0.23) | 0.32 (0.43) | 0.31 (0.31) | 0.46 (0.37) |
| FP | 0.14 (3.12) | 0.33 (3.22) | 0.73 (3.33) | 1.13 (2.53) | 1.22 (1.81) | 0.41 (0.47) | 0 (0) |
| FE | 0.11 (2.32) | 0.26 (2.42) | 0.58 (2.58) | 1.17 (2.54) | 0.96 (1.38) | 0.66 (0.72) | 0.49 (0.42) |
| FC | 0.15 (3.53) | 0.35 (3.50) | 1.05 (4.85) | 1.87 (3.79) | 0.77 (1.11) | 0.58 (0.64) | 0.33 (0.29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cela-Dablanca, R.; Nebot, C.; López, L.R.; Ferández-Calviño, D.; Arias-Estévez, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Retention of the Antibiotic Cefuroxime onto Agricultural and Forest Soils. Appl. Sci. 2021, 11, 4663. https://doi.org/10.3390/app11104663
Cela-Dablanca R, Nebot C, López LR, Ferández-Calviño D, Arias-Estévez M, Núñez-Delgado A, Álvarez-Rodríguez E, Fernández-Sanjurjo MJ. Retention of the Antibiotic Cefuroxime onto Agricultural and Forest Soils. Applied Sciences. 2021; 11(10):4663. https://doi.org/10.3390/app11104663
Chicago/Turabian StyleCela-Dablanca, Raquel, Carolina Nebot, Lucia Rodríguez López, David Ferández-Calviño, Manuel Arias-Estévez, Avelino Núñez-Delgado, Esperanza Álvarez-Rodríguez, and María J. Fernández-Sanjurjo. 2021. "Retention of the Antibiotic Cefuroxime onto Agricultural and Forest Soils" Applied Sciences 11, no. 10: 4663. https://doi.org/10.3390/app11104663