Environmental Remediation of Metribuzin Herbicide by Mesoporous Carbon—Rich from Wheat Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization
2.3. Adsorption Experiments
2.4. Analytical Determination of Metribuzin
3. Results and Discussion
3.1. Biochar Properties
3.2. Morphology Analysis
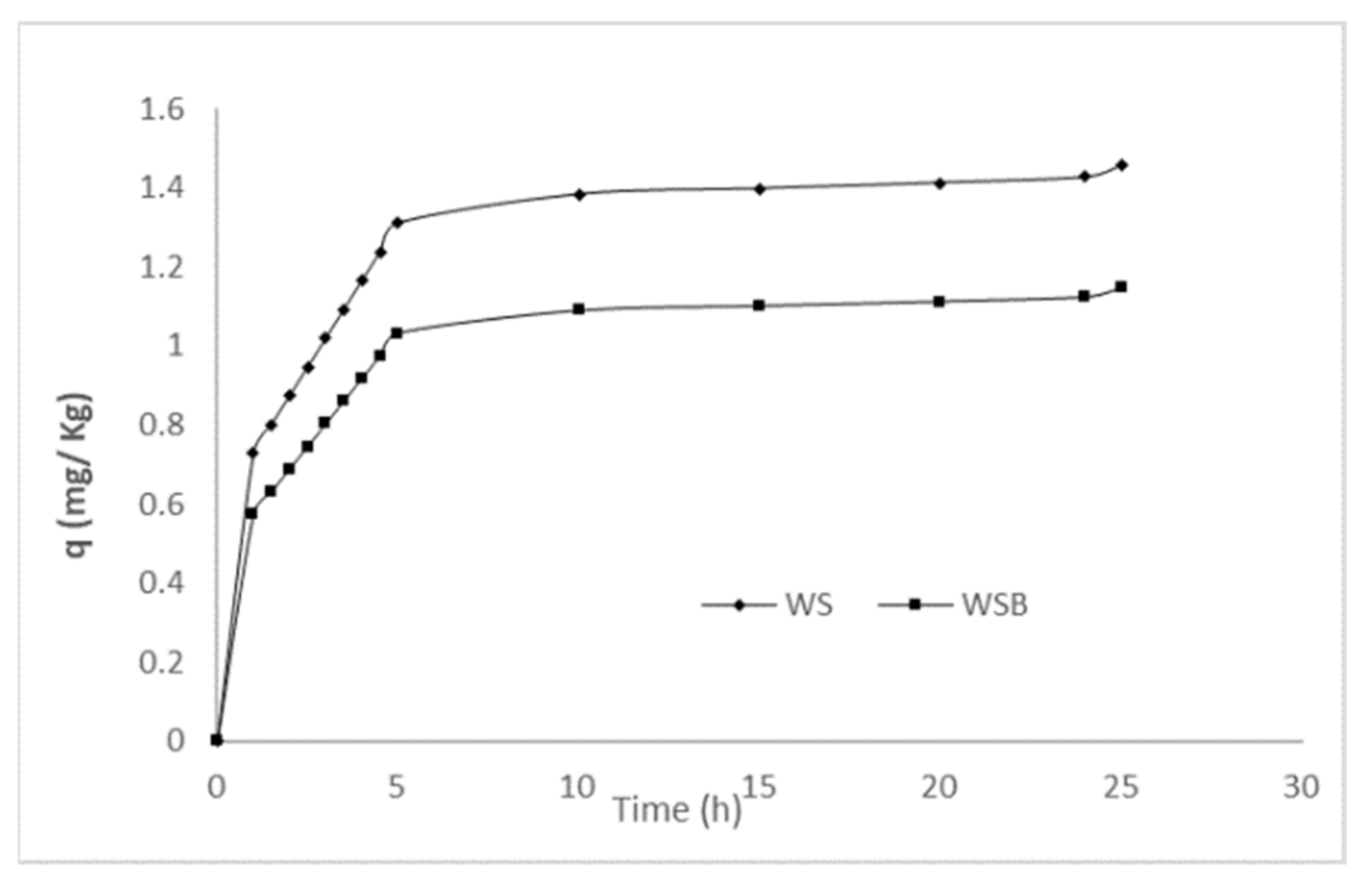
3.3. Adsorption Kinetic Modeling
3.4. Adsorption Performance
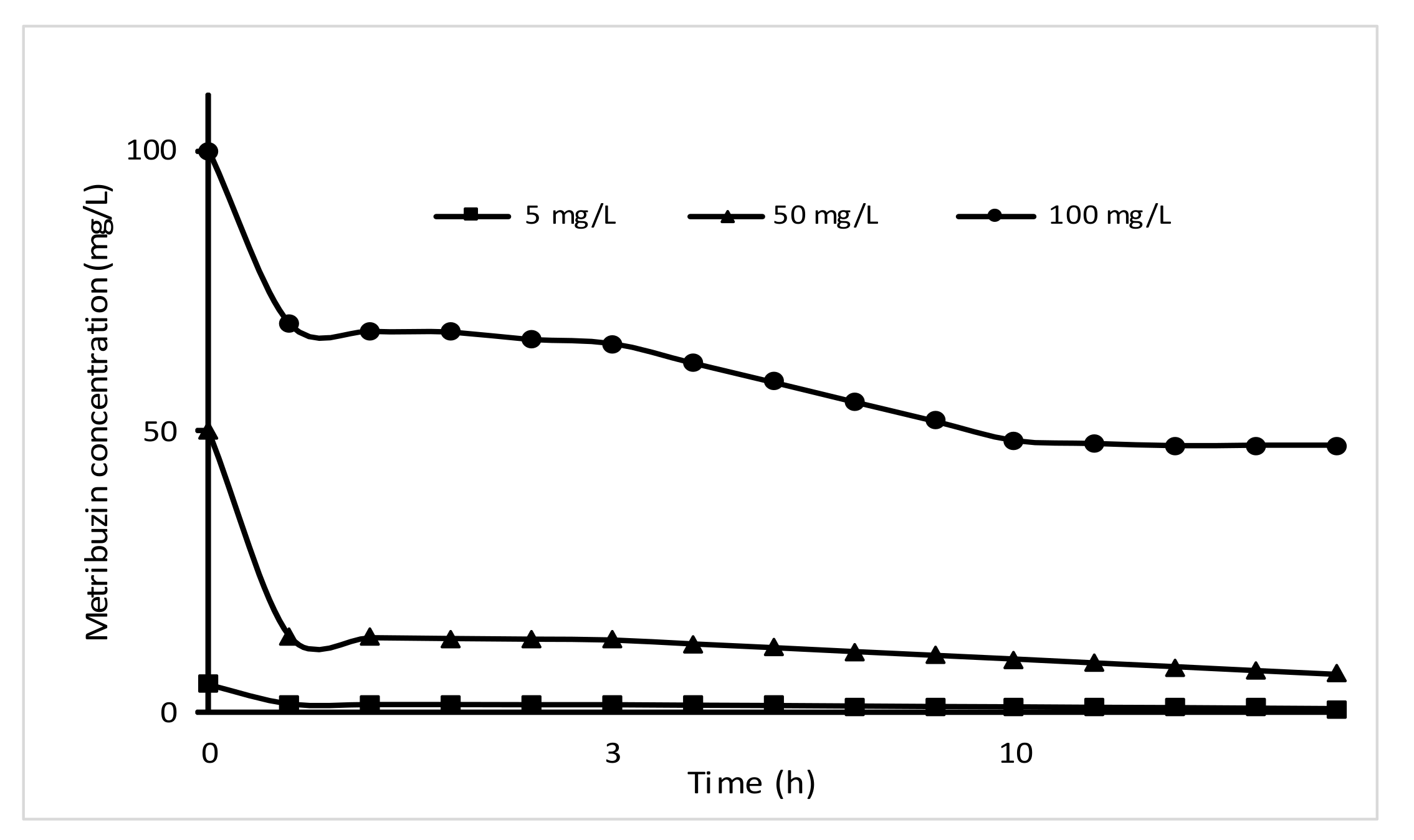
3.4.1. The Effect of Metribuzin Concentration and Time
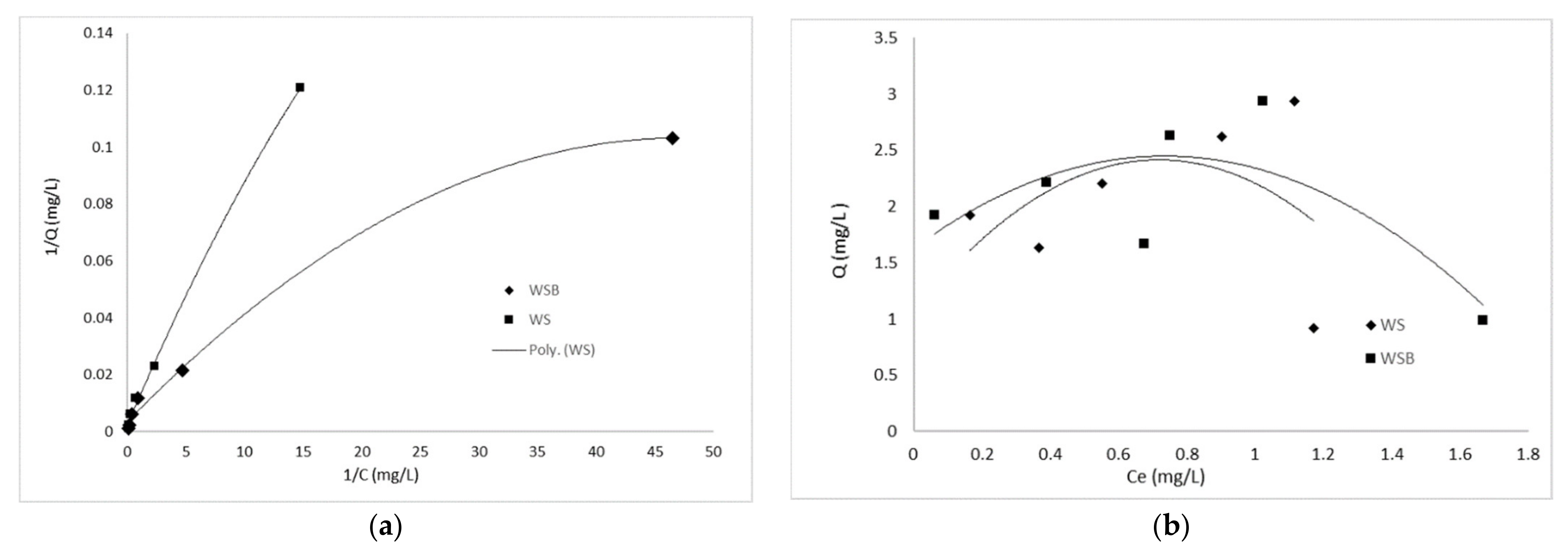
3.4.2. Isotherm Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, S.; Muhammad, S.; Behzard, M.; Irshad, B.N.; Muhannad, A.N.; Nabeel, K.N. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef] [PubMed]
- Minuț, M.; Roșca, M.; Hlihor, R.-M.; Cozma, P.; Gavrilescu, M. Modelling of Health Risk associated with the intake of Pesticides from Romanian fruits and vegetables. Sustainability 2020, 12, 10035. [Google Scholar] [CrossRef]
- Lee, Y.G.; Shin, J.; Kwak, J.; Kim, S.; Son, C.; Kim, G.Y.; Lee, C.H.; Chon, K. Enhanced adsorption capacities of fungicides using peanuts shell biochar via successive chemical modification with KMnO4 and KOH. Separations 2021, 8, 52. [Google Scholar] [CrossRef]
- Hamsan, H.; Ho, Y.B.; Zaidon, S.Z.; Hashim, Z.; Saari, N.; Karami, A. Occurrence of commonly used pesticides in personal air samples and their associated health risk among paddy farmers. Sci. Total Environ. 2017, 603, 381–389. [Google Scholar] [CrossRef]
- Lee, K.M.; Park, S.-Y.; Lee, K.; Oh, S.-S.; Ko, S.B. Pesticide metabolites and oxidative stress in male farmers exposed to pesticides. Ann. Occupat. Environ. Med. 2017, 29, 5. [Google Scholar] [CrossRef] [Green Version]
- Mossa, A.-T.H.; Swelam, E.S.; Mohafrash, S.M. Sub-chronic exposure to npronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015, 2, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Reeves, W.R.; McGuire, M.K.; Stokes, M.; Vicini, J.L. Assessing the safety of pesticides in food: How current regulations protect human health. Adv. Nutr. 2019, 10, 80–88. [Google Scholar] [CrossRef]
- Gautam, K.R.; Goswami, M.; Tech, M.; Mishra, R.K.; Chaturvedi, P.; Awashthi, M.K.; Singh, R.S.; Giri, B.S.; Pandey, A. Biochar for remediation of agrochemicals and synthetic organic dyes from environmental samples: A review. Chemosphere 2021, 272, 129917. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Poll. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S.; Sahni, D.; Sehgal, M.; Srivastava, D.; Singh, A. Pesticide use and its effect on soil bacteria and fungal populations, microbial biomass carbon and enzymatic activity. Curr. Med. Sci. 2019, 116, 643–649. [Google Scholar] [CrossRef]
- Ali, N.; Khan, S.; Yao, H.; Wang, J. Biochar reduced the bioaccessibillity and (bio) uptake of orhanochlorine pesticides and changed the microbial community dynamics in agricultural soils. Chemosphere 2019, 224, 805–815. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Effects of biochars on the earthworm (Wisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci. Total Environ. 2019, 671, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Pi, F.W.; Wang, Y.F.; Xu, H.; Zhang, Y.Z.; Sun, X.L. Photocatalytic degradation of acephate, amethoate, and methyl parathion by Fe3O4 SiO2 TiO2 nanomicrospheres. J. Hazard. Mater. 2016, 315, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Charru, A.B.; Weng, C.H.; Yuan, X.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Cacioli, P.; Bultler, P.; Kyratzis, I.L. Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzym. Microb. Technol. 2014, 54, 38–44. [Google Scholar] [CrossRef]
- Garcia-Jaramillo, M.; Cox, L.; Cornejo, J.; Hermosin, M.C. Effect of soil organic amendments on the behavior of bentazone and tricyclazole. Sci. Total Environ. 2014, 466–467, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Kang, S.-W.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–87. [Google Scholar] [CrossRef]
- Ignat, M.; Sacarescu, L.; Fortuna, M.E.; Cool, P.; Harabagiu, V. Effect of synthesis parameters on sorptive properties of glycol-derived mesoporous carbon. Environ. Eng. Manag. J. 2019, 18, 59–69. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolized wood and rice rusks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. J. Sci. Technol. 2015, 45, 5580–5586. [Google Scholar]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm studies of Ni2+ and Pb2+Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability 2021, 13, 3785. [Google Scholar] [CrossRef]
- Drake, J.A.; Carrucan, A.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Biochar application during reforestation alters species present and soil chemistry. Sci. Total Environ. 2015, 514, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, H.; Yang, S.; Wang, Y. Impact of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. Field Crop. Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent advances in biochar application in agricultural soils: Benefits and environmental implication. Clean Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, S.; Verheijen, F.G.A.; Van der Velde, M.; Bastos, A.C. A quantitative review of the effect of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Peake, L.R.; Reid, B.J.; Tang, X. Quantifying the influence of biochar on the physical and hydrological properties of dissimilars soils. Geoderma 2014, 235, 182–190. [Google Scholar] [CrossRef]
- Lu, S.G.; Sun, F.f.; Zong, Y.T. Effect of rice husk biochar and coal fly ash on some physical properties of expansive clayey soil (Vertisol). Catena 2014, 114, 37–44. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 84, 687–711. [Google Scholar] [CrossRef] [Green Version]
- Agegnehu, G.; Srivastava, A.K.; Bird, A.K. The role of biochar and biochar compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Oguntunde, P.; Fosu, M.; Ajayi, A.; Giesen, N. Effect of charcoal production in maize yield, chemical properties and texture of soil. Biol. Fertil. Soils 2004, 39, 295–299. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Jien, S.H.; Chen, W.C.; Ok, Y.S.; Awad, Y.M.; Liao, C.S. Short-term biochar application induced variations in C and N mineralization in compost-amended tropical soil. Environ. Sci. Poll. Res. 2017, 25, 25715–25725. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyvaluoma, J. How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenerg. 2018, 119, 346–353. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, J.; Sebestyen, V.; Juzsakova, T.; Cretescu, I.; Domokos, E.; Redey, A. Study of the glyphosate-amine pesticide mineralization in wastewater by ozonation treatment. Environ. Eng. Manag. J. 2019, 9, 1867–1873. [Google Scholar] [CrossRef]
- Singh, R.; Nidheesh, P.V.; Sivasankar, T. Integrating ultrasound with activated carbon prepared from mangosteen fruit peel for reactive black 5 removal. Environ. Eng. Manag. J. 2019, 18, 2335–2342. [Google Scholar]
- Boni, M.R.; Chiavola, A.; Marzeddu, S. Remediation of Lead-contamined water by virgin coniferous wood biochar adsorbent: Batch and column application. Water Air Soil Pollut. 2020, 231, 171. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore water concentrations of polycyclic aromatic hydrocarbons (pahs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Subratti, A.; Vidal, J.L.; Lalgee, L.J.; Kerton, F.M.; Jalsa, N.K. Preparation and characteriazation of biochar derived from the fruit seed of Cedrela odorata L and evaluation of its adsorption capacity with methylene blue. Sustain. Chem. Pharm. 2021, 21, 100421. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameeda, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixedbed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameeda, B.H. Insight into co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Wang, D.; Mukome, F.N.; Yan, D.; Wang, H.; Scow, K.M.; Parikh, S.J. Phenylurea herbicide sorption to biochars and agricultural soils. J. Environ. Sci. Health. Part B 2015, 50, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soil on sorption, desorption and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Yang, Y.; Huang, M.; Yang, K. Influence of pH on pesticide sorption by soil containing wheat residues-derived char. Environ. Pollut. 2005, 134, 457–463. [Google Scholar] [CrossRef]
- Lopez-Pineiro, A.; Pena, D.; Albarran, A.; Becerra, D.; Sanchez-Llerena, J. Sorption, leaching and persistence of metribuzin in Mediterranean soil amended with olive mill waste of different degrees of organic matter maturity. J. Environ. Manag. 2013, 122, 76–84. [Google Scholar] [CrossRef]
- Borja-Urzola, A.; Garcia-Gomez, R.S.; Bernal-Gonzales, M.; Duran-Dominguez-de-Bazua, M. Chitosan-calcite from shrimp residues: A low cost adsorbent for three triazines removal from aqueous media. Mater. Today Comunn. 2021, 26, 102131. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and suitable adsorbent—Critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Yi, S.; Gao, B.; Sun, Y.; Wu, J.; Shi, X.; Wu, B.; Hu, X. Removal of levofloxacin from aqueous solution using rice husk and wood chip biochars. Chemosphere 2016, 150, 694–701. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y. Effects of feedstock type, production methods and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.W.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Siedt, M.; Schaffer, A.; Smith, K.E.C.; Nabel, M.; Rob-Nickoll, M.; van Dongen, J.T. Comparing straw, compost and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Guo, W.; Hua, S.; Feng, J.; Lu, X. Adsorption of perfluorooctane sulfonate (PFOS) on corn straw derived biochar prepared at different pyrolitic temperatures. J. Taiwan Instit. Chem. Eng. 2017, 78, 265–271. [Google Scholar] [CrossRef]
- Lagergren, S. Lagergren Zur theorie der sogenannten adsorption gelöster stroffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Khanday, W.A.; Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. Single step pyrolysis of phosphoric acid activated chitin for efficient adsorption of cephalexin antibiotic. Bioresour. Technol. 2019, 280, 255–259. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.-S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Han, Q.; Yang, Y.; Wang, R.; Zhang, K.; Liu, N.; Hong, M. Biochar derived from agricultural wastes as a means of facilitating the degradation of azo dyes by sulfides. Catalysts 2021, 11, 434. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Galvis, E.A.S.; Porras, J.; Florez, E.; Torres-Palma, R.A.; Acelas, N. Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 2021, 326, 124753. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments of biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Nufida, B.A.; Ismail, N.M.; Zakaria, Z.Y.; Jagaba, A.H.; Ghaleb, A.A.S.; Al-Dhawi, B.N.S. Modeling and optimization of biochar based adsorbent derived from Kenaf using response surface methodology on adsorption of Cd2+. Water 2021, 13, 999. [Google Scholar] [CrossRef]
- Manya, J.J.; Ortigosa, M.A.; Laguarta, S.; Manso, J.A. Experimetal study on the effect of pyrolysis pressure, peak temperature, and particle size on the potential stability of vine shoots-derived biochar. Fuel 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameeda, B.H. Adsorption behaviour of salicylic acid on biochar as derived from the thermal pyrolysis of barley straw. J. Clean. Prod. 2018, 195, 1162–1169. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameeda, B.H. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Biores. Tech. 2019, 278, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature. A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Moslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy metals ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Kim, J.E.; Song, H.J.; Oh, K.B.; Jo, J.W.; Yang, Y.-H.; Lee, S.H.; Kang, G.; Kim, H.J.; Choi, Y.K. Assessment of adsorptive behaviors and properties of grape pomace-derived biochar as adsorbent for removal of cymoxanil pesticide. Environ. Technol. Innov. 2021, 21, 101242. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef]
- Li, Q.; Yu, W.; Guo, L.; Wang, Y.; Zhao, S.; Zhou, L.; Jiang, X. Sorption of Sulfamethoxazole on Inorganic acid solution etched Biochar derived from Alfalfa. Materials 2021, 14, 1033. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.-H.; Wang, X.-M. Adsorption behaviour and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; Gonzalez-Delgado, A.; Herrera, A.; De la Voz, A.V. Efficient sulfate on Modified Adsorbents prepared from Zea Mays Stems. Appl. Sci. 2021, 11, 1596. [Google Scholar] [CrossRef]
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar and vermicompost. J. Environ. Sci. Health Part B Pest Food Contamin. Agric. Waste 2019, 54, 728–735. [Google Scholar] [CrossRef]
- Bettayeb, A.; Reguig, B.A.; Mouchaal, Y.; Yahiaoui, A.; Chehimi, M.M.; Berredjem, Y. Adsorption of metribuzin herbicide on raw maghnite and acid treated maghnite in aqueous solutions. Desalin. Water Treat. 2019, 145, 262–272. [Google Scholar] [CrossRef]
- Salimi, M.; Salehi, Z.; Heidari, H.; Vahabzadeh, F. Production of activated biochar from Luffa cylindrica and its application for adsorption of 4-Nitrophenol. J. Environ. Chem. Eng. 2021, 9, 105403. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Singh, N. Biocompost from sugar distillery effluent: Effect on metribuzin degradation, sorption and mobility. Pest. Manag. Sci. 2008, 46, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Suo, F.; Liu, X.; Li, C.; Yuan, M.; Zhang, B.; Wang, J.; Ma, Y.; Lai, Z.; Mingshan, J. Mesoporous activated carbon from starch for superior rapid pesticides removal. Int. J. Biol. Macromol. 2019, 121, 806–813. [Google Scholar] [CrossRef]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of tetracycline and sulfamethoxazole on crop residues derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Njoku, V.O.; Azharul Islam, M.; Asif, M.; Hameed, B.H. Preparation of mesoporous activated carbon from coconut frond for the adsorption of carbofuran insecticide. J. Anal. Appl. Pyrol. 2014, 110, 172–180. [Google Scholar] [CrossRef]
- Jin, J.; Kang, M.; Sun, K.; Pan, Z.; Wu, F.; Xing, B. Properties of biochar amended soils and their sorption of imidacloprid, isoproturon and atrazine. Sci. Total Environ. 2016, 550, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Ighalo, J.; Adeniyi, A.G.; Adelodun, A. Recent advances on the adsorption of herbicides and pesticides from plluted waters: Performance evaluation via physical attributes. J. Ind. Eng. Chem. 2021, 93, 117–137. [Google Scholar] [CrossRef]
- Kitous, O.; Abdi, H.; Lounici, H.; Grib, H.; Drouiche, N.; Benyoussef, E.; Mameri, N. Modeling of the adsorption of metribuzin pesticide onto electro-activated granular carbon. Desalin. Water Treat. 2016, 57, 1865. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.L.; Liu, W.T.; Zhou, Q.X. Biochar: An effective amedment for remediating contaminated soil. Rev. Environ. Contam. Toxicol. 2014, 228, 83–99. [Google Scholar] [PubMed]
- Zhao, K.; Ouyang, W.; Hao, F.; Lin, C.; Wang, F.; Han, S.; Geng, X. Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour. Technol. 2013, 147, 338–344. [Google Scholar] [CrossRef] [PubMed]



| Material | Basic Properties | Consequent Analysis (wt%) | Atomic Ratios (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ash | Moisture | C | H | O | N | H/C | O/C | (O + N)/C | |
| WS | 7.98 | 3.45 | 47.15 | 6.56 | 43.98 | 2.05 | 0.139 | 0.932 | 0.976 |
| WSB | 5.68 | 5.13 | 73.25 | 2.61 | 19.67 | 2.79 | 0.035 | 0.268 | 0.306 |
| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| qe exp (mg·g−1) | qe est (mg·g−1) | K1 (mg g−1·min−1) | R2 | qe est (mg·g−1) | K2 (mg·g−1·min−1) | R2 | |
| WS | 1.453 | 5.901 | 0.005 | 0.726 | 1.431 | 0.956 | 0.996 |
| WSB | 1.565 | 6.083 | 0.009 | 0.531 | 1.541 | 0.735 | 0.998 |
| Isotherm | Product | Constants | R2 | |
|---|---|---|---|---|
| qL (mg·g−1) | KL (L·g−1) | |||
| Langmuir | WS | 289 | 0.46 | 0.997 |
| WSB | 596 | 0.52 | 0.995 | |
| Freundlich | Kf (L·g−1) | n | ||
| WS | 98.21 | 0.68 | 0.848 | |
| WSB | 154.5 | 0.65 | 0.976 | |
| Raw Material | Pesticide |
Pyrolysis Temperature (°C) | Concentration | Q (mg g−1) | Reference |
|---|---|---|---|---|---|
| Wheat straw | atrazine | 450 | 20% | 220.57 | [82] |
| Rice straw | imidacloprid | 600 | 20% | 152.10 | [82] |
| Wheat straw | metribuzin | 800 | 1–100 mg L | 596 | This study |
| Rice straw | isoproturon | 600 | 10% | 406.5 | [82] |
| Corn straw | atrazine | 450 | 11.566 | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cara, I.G.; Filip, M.; Bulgariu, L.; Raus, L.; Topa, D.; Jitareanu, G. Environmental Remediation of Metribuzin Herbicide by Mesoporous Carbon—Rich from Wheat Straw. Appl. Sci. 2021, 11, 4935. https://doi.org/10.3390/app11114935
Cara IG, Filip M, Bulgariu L, Raus L, Topa D, Jitareanu G. Environmental Remediation of Metribuzin Herbicide by Mesoporous Carbon—Rich from Wheat Straw. Applied Sciences. 2021; 11(11):4935. https://doi.org/10.3390/app11114935
Chicago/Turabian StyleCara, Irina Gabriela, Manuela Filip, Laura Bulgariu, Lucian Raus, Denis Topa, and Gerard Jitareanu. 2021. "Environmental Remediation of Metribuzin Herbicide by Mesoporous Carbon—Rich from Wheat Straw" Applied Sciences 11, no. 11: 4935. https://doi.org/10.3390/app11114935
APA StyleCara, I. G., Filip, M., Bulgariu, L., Raus, L., Topa, D., & Jitareanu, G. (2021). Environmental Remediation of Metribuzin Herbicide by Mesoporous Carbon—Rich from Wheat Straw. Applied Sciences, 11(11), 4935. https://doi.org/10.3390/app11114935







