The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Man-Made Contaminated Soil
2.3. Selection of Vegetables
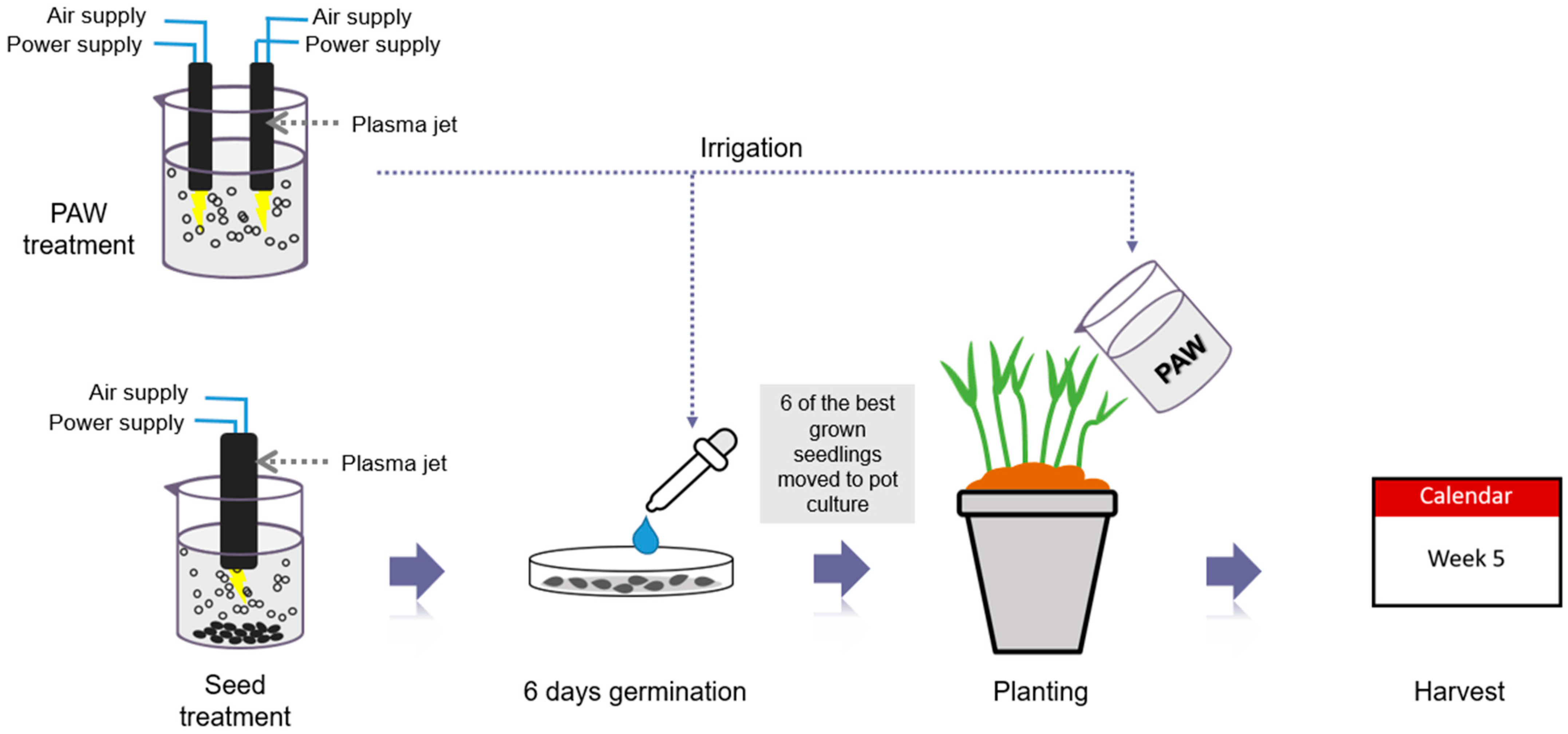
2.4. The Plasma Device and Parameters
2.5. Plasma Treatment of Seeds and Irrigation Water
2.6. Plantation of Water Spinach
2.7. Physiochemical Properties Analysis
2.7.1. Determination of Nitrates and Nitrites of PAW
2.7.2. Determination of pH, Oxidation-Reduction Potential (ORP) and Electrical Conductivity (EC), and H2O2 of PAW
2.8. Metals Analysis
2.8.1. Preparation of Soil Samples
2.8.2. Determination of Heavy Metals in Soils
2.8.3. Preparation of Vegetable Samples
2.8.4. Determination of Heavy Metals in Vegetables
2.8.5. Quality Assurance/Quality Control for Metal Analysis
2.9. Bioconcentration Factor
2.10. Statistical Analysis
3. Results and Discussion
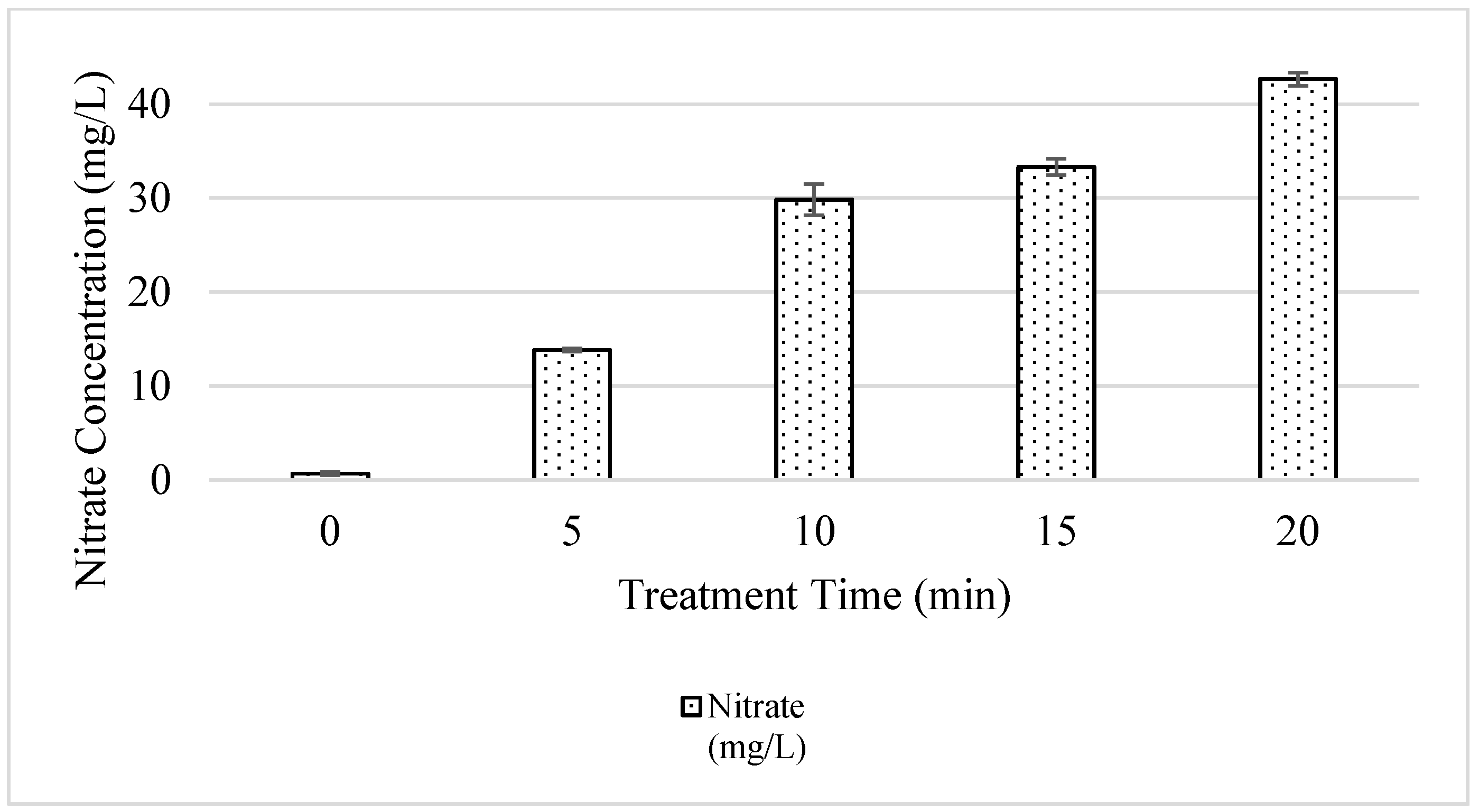
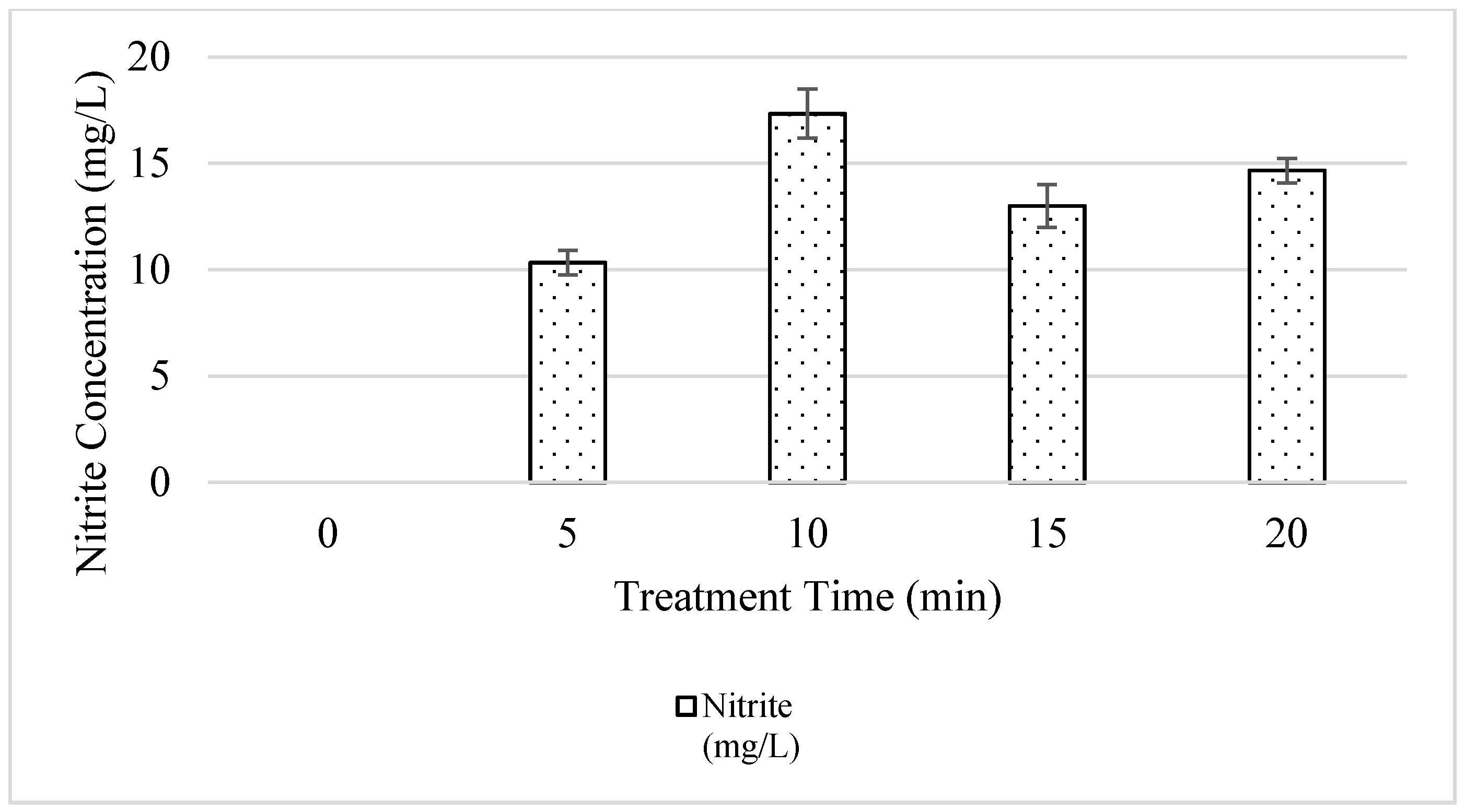
3.1. Physiochemical Properties of Irrigation Water
3.2. Concentration of Heavy Metals in Man-Made Contamination Soil
3.3. Harvested Water Spinach
3.4. Concentration of Heavy Metals in Water Spinach
3.5. BCF of Vegetables Planted in Contaminated Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 1–31. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Nehra, V.; Kumar, A.; Dwivedi, H. Atmospheric non-thermal plasma sources. Int. J. Eng. 2008, 2, 53–68. [Google Scholar]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Q.; Zhang, Q.; He, H.; Chen, Z.; Zhao, Y.; Wei, D.; Kong, M.; Huang, Q. Improved production of polysaccharides in Ganoderma lingzhi mycelia by plasma mutagenesis and rapid screening of mutated strains through infrared spectroscopy. PLoS ONE 2018, 13, e0204266. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Takaki, K.; Yoshida, K.; Saito, T.; Kusaka, T.; Yamaguchi, R.; Takahashi, K.; Sakamoto, Y. Effect of Electrical Stimulation on Fruit Body Formation in Cultivating Mushrooms. Microorganisms 2014, 2, 58–72. [Google Scholar] [CrossRef]
- Pawłat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds’ germination. PLoS ONE 2018, 13, e0194349. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Hanliang, S.; Yuanhua, D. Effects of low-vacuum helium cold plasma treatment on seed germination, plant growth and yield of oilseed rape. Plasma Sci. Technol. 2018, 20, 095502. [Google Scholar]
- Liu, B.; Honnorat, B.; Yang, H.; Arancibia, J.; Rajjou, L.; Rousseau, A. Non-thermal DBD plasma array on seed germination of different plant species. J. Phys. D Appl. Phys. 2018, 52, 025401. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K.K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Herianto, S.; Hou, C.; Lin, C.; Chen, H. Nonthermal plasma-activated water: A comprehensive review of this new tool for enhanced food safety and quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 583–626. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chu, Y.-C.; Hsiao, C.-P.; Wu, J.-S.; Hsieh, C.-W.; Hou, C.-Y. The Optimization of Plasma-Activated Water Treatments to Inactivate Salmonella Enteritidis (ATCC 13076) on Shell Eggs. Foods 2019, 8, 520. [Google Scholar] [CrossRef]
- Hamzah, A.; Hapsari, R.I.; Wisnubroto, E.I. Phytoremediation of cadmium-contaminated agricultural land using indigenous plants. Int. J. Environ. Agric. Res. 2016, 2, 8–14. [Google Scholar]
- Ndeda, L.; Manohar, S. Bio concentration factor and translocation ability of heavy metals within different habitats of hydrophytes in Nairobi Dam, Kenya. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 42–45. [Google Scholar]
- Takarina, N.D.; Pin, T.G. Bioconcentration Factor (BCF) and Translocation Factor (TF) of Heavy Metals in Mangrove Trees of Blanakan Fish Farm. Makara J. Sci. 2017, 21, 77–81. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effect Atmospheric-Pressure N2, He, Air, and O2 Microplasmas on Mung Bean Seed Germination and Seedling Growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Gao, K.; Liu, R.; Liu, R.; Yao, X.; Gong, D.; Su, Z.; Jia, P. Comparison of deionized and tap water activated with an atmospheric pressure glow discharge. Phys. Plasmas 2019, 26, 033507. [Google Scholar] [CrossRef]
- Porto, C.L.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.; Das, U.; Sarkar, U.; Roy, N.C.; Reza, A.; Talukder, M.R.; Uddin, A. Reduction of cadmium toxicity in wheat through plasma technology. PLoS ONE 2019, 14, e0214509. [Google Scholar] [CrossRef] [PubMed]
- Intawongse, M.; Dean, J.R. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit. Contam. 2006, 23, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Ahn, C.; Gill, J.; Ruzic, D.N. Growth of Plasma-Treated Corn Seeds under Realistic Conditions. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Sajib, S.A.; Rahi, S.; Tahura, S.; Roy, N.C.; Parvez, S.; Reza, A.; Talukder, M.R.; Kabir, A.H. Mechanisms and Signaling Associated with LPDBD Plasma Mediated Growth Improvement in Wheat. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Safari, N.; Iranbakhsh, A.; Ardebili, Z.O. Non-thermal plasma modified growth and differentiation process of Capsicum annuum PP805 Godiva in in vitro conditions. Plasma Sci. Technol. 2017, 19, 055501. [Google Scholar] [CrossRef]
- Shanmugaraj, B.M.; Malla, A.; Ramalingam, S. Cadmium Stress and Toxicity in Plants: An Overview. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Nas, F.; Ali, M. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Eco. Environ. Sci. 2018, 3, 265–268. [Google Scholar]
- Jiang, J.; Jiangang, L.; Yuanhua, D. Effect of cold plasma treatment on seedling growth and nutrient absorption of tomato. Plasma Sci. Technol. 2018, 20, 044007. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Minchong, S.; Jinfeng, H.; Hanliang, S.; Yuanhua, D.; Jiafeng, J. Improving seed germination and peanut yields by cold plasma treatment. Plasma Sci. Technol. 2016, 18, 1027. [Google Scholar]
- Li, L.; Jiang, J.F.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2015, 4, 5859. [Google Scholar]
- Zhang, B.; Li, R.; Yan, J. Study on activation and improvement of crop seeds by the application of plasma treating seeds equipment. Arch. Biochem. Biophys. 2018, 655, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Amelioration of Photosynthesis and Quality of Wheat under Non-thermal Radio Frequency Plasma Treatment. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef]
- Mihai, A.; Dobrin, D.; Magureanu, M.; Popa, M. Positive effect of non-thermal plasma treatment on radish seeds. Rom. Rep. Phys. 2014, 66, 1110–1117. [Google Scholar]
- de Groot, G.J.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Ghoranneviss, M.; Ardebili, Z.O.; Tackallou, S.H.; Nikmaram, H. Non-thermal plasma modified growth and physiology in Triticum aestivum via generated signaling molecules and UV radiation. Biol. Plant. 2017, 61, 702–708. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process. Polym. 2018, 15, 1700064. [Google Scholar] [CrossRef]
- Bafoil, M.; Jemmat, A.; Martinez, Y.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS ONE 2018, 13, e0195512. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Alamri, S.A.M.; Sewelam, N.A.; Galal, T.M. Uptake prediction of ten heavy metals by Corchorus olitorius L. cultivated in soil mixed with sewage sludge. Food Energy Secur. 2020, 9, e203. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Liu, A.; Zhou, W.; Liao, K. Translocation analysis and safety assessment in two water spinach cultivars with distinctive shoot Cd and Pb concentrations. Environ. Sci. Pollut. Res. 2014, 21, 11565–11571. [Google Scholar] [CrossRef]
- Saad, F.N.M.; Lim, F.J.; Izhar, T.N.T.; Odli, Z.S.M. Evaluation of phytoremediation in removing Pb, Cd and Zn from contaminated soil using Ipomoea Aquatica and Spinacia Oleracea. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012142. [Google Scholar] [CrossRef]
- Sengar, R.; Gautam, M.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead Stress Effects on Physiobiochemical Activities of Higher Plants. Rev. Environ. Contam. Toxicol. 2008, 196, 73–93. [Google Scholar] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Reeves, R.D. Soil pH Effects on Uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 2006, 281, 325–337. [Google Scholar] [CrossRef]
- Ye, X.; Ma, Y.; Sun, B. Influence of soil type and genotype on Cd bioavailability and uptake by rice and implications for food safety. J. Environ. Sci. 2012, 24, 1647–1654. [Google Scholar] [CrossRef]
| Treatment | n | Cd Concentration | Pb Concentration | ||
|---|---|---|---|---|---|
| Control Soil | Cd-Added Soil | Control Soil | Pb-Added Soil | ||
| NTS + NTW | 3 | ND-<LOQ | 18.18–20.43 | 2.60–3.17 | 2222–2492 |
| NTS + PAW | 3 | <LOQ | 19.06–19.53 | 2.36–3.77 | 2064–2470 |
| PTS + NTW | 3 | ND-<LOQ | 20.93–22.28 | 1.84–2.32 | 2182–2429 |
| PTS + PAW | 3 | ND-<LOQ | 21.17–21.92 | 1.77–2.80 | 2086–2448 |
| Treatment | n | Total Weight (g) of Water Spinach Grown in | ||
|---|---|---|---|---|
| Control Soil | Cd-Added Soil | Pb-Added Soil | ||
| NTS + NTW | 3 | 9.24 | 9.43 | 7.28 |
| NTS + PAW | 3 | 7.72 | 7.40 | 6.38 |
| PTS + NTW | 3 | 9.29 | 6.48 | 7.38 |
| PTS + PAW | 3 | 5.91 | 7.43 | 8.39 |
| Treatment | Cd Concentration | Pb Concentration | ||
|---|---|---|---|---|
| Control Soil (mg/kg D.W.) | Cd-Added Soil (mg/kg D.W.) | Control Soil (mg/kg D.W.) | Pb-Added Soil (mg/kg D.W.) | |
| NTS + NTW | 0.265 | 15.7 | 0.703 | 7.04 |
| NTS + PAW | 0.182 | 12.9 | 0.246 | 7.00 |
| PTS + NTW | 0.215 | 12.1 | 0.284 | 6.94 |
| PTS + PAW | 0.207 | 15.0 | 0.697 | 11.6 |
| A. Plasma Treatment on Seeds | |||||
| Author | Target | Plasma Generation | Parameters | Outcomes | |
| Seed Germination | Plant Growth | ||||
| Ling et al. [11] | Oilseed rape (Brassica napus L. cv Zhongshuang 9) | Low-vacuum helium cold plasma (Radio frequency discharge) | Helium gas 100 W, 15 s, 13.56 MHz, 150 Pa | Positive | Positive |
| Jiang et al. [32] | Tomato (Solanum lycopersicum L. cv. Shanghai 906) | Inductive helium capacitive coupled plasma (CCP), Computer-controlled plasma treatment apparatus HD-2N (Radiofrequency) | Helium gas 80 W, 15 s, 13.56 MHz, 150 Pa, 3.5 eV (electron temperature) | Positive | Positive |
| Ling et al. [33] | Peanut (Arachis hypogaea L. cv. Eyou 7) | Inductive helium discharge with HD-2N units (Radiofrequency generator) | Helium gas, 0–120 W, 15 s, 13.56 MHz, 150 Pa | Positive | Positive |
| Li et al. [34] | Soybean (Glycine max L. Merr cv. Zhongdou 40) | Inductive helium discharge with computer-controlled plasma treatment apparatus HD-2N units (Radiofrequency generator) | Helium gas 0–120 W, 15 s, 13.56 MHz, 150 Pa | Positive | Positive |
| Zhang et al. [35] | Maize, peppers, wheat, soybeans, tomatoes, eggplants, pumpkins etc. | Electromagnetic shielding and suspension electrode technology; High (glow) radiofrequency discharger produces plasma | Air, 13.56 MHz, 80–180 W, 30–200 Pa, 5–90 s | Positive | Positive |
| Saberi et al. [36] | Wheat (Triticum aestivum L.) | Non-thermal Radio Frequency Plasma | Air, 80 W, 13.56 MHz, 0.1 mbar, 0–240 s | - | Positive |
| Jiang et al. [37] | Wheat (Triticum spp.) | Cold plasma generator | Helium gas, 60–100 W, 15 s, 3 × 109 MHz, 13 eV, 150 Pa | Positive | Positive |
| Safari et al. [29] | Capsicum annum PP805 Godiva | DBD plasma | Argon gas, 23 kHz, 11 kV, 80 W, 94.98 cm2 plasma treatment areas, 0.84 W/cm power density, 0–2 min | - | Positive |
| Mihai et al. [38] | Radish seed | Non-thermal plasma-surface discharge | Air, 15 kV, 2.7 W, 20 min, Gas flow rate = 1 L/min | No Change | No Change |
| de Groot et al. [39] | Cotton seeds variety Sicot 74BRF | Cold atmospheric-pressure plasma (CAP) | Air/Argon gas; Flow rate = 1 L/min, AC power supply, 1 kHz, sine wave with 38 kVpp for air and 11 kVpp for argon, treatment time: 0, 3, 27 min with dry air and 81 min with argon gas | Positive | Not significant but positive for Air-27 min and Ar-81 min, and negative for Air-3 min |
| Iranbakhsh et al. [40] | Wheat (Triticum aestivum L. cv. Parsi) | Dielectric barrier discharge (DBD) | Nitrogen and Helium gas, 20 kHz, 15 kV, 100 W, 254.3 cm2; 0.4 W/cm; 100 Pa, 15, 30, 60, 120 s, repetition with 1, 2, 4 times with 24 h intervals | - | Positive |
| Pawlat et al. [41] | Lavatera thuringiaca L. | Gliding arc reactor | Nitrogen gas (8 L/min), 680 V, 50 Hz, 33 mA, 40 W, 1, 2, 5, 10, 15 min | Positive | - |
| Pawlat et al. [10] | Lavatera thuringiaca L. | Dielectric barrier discharge (DBD) plasma jet | Helium: 1.6 dm3/min, Nitrogen: 0.03 dm3/min, 3.7 kV; 17 kHz; mean of 6 W, 1, 2, 5, 10, 15 min | Positive | - |
| Rahman et al. [28] | Wheat (BARI Gom 22) | Low pressure dielectric barrier discharge (DBD) | Ar60%/Air40%; Ar60%/Oxygen40%, 5 kV, 4.5 kHz, ~45 W, 90 s | Positive | - |
| B. Plasma Treatment on Seeds through Aqueous Media | |||||
| Author | Target | Plasma Generation | Parameters | Outcomes | |
| Seed Germination | Plant Growth | ||||
| Zhou et al. [21] | Mung bean seeds (Vigna radiata Linn. Wilczek) | Atmospheric pressure microplasma array | He, N2, artificial Air, O2, (2 standard liters per min), 36 microplasma jet units, 4.5 kV, 9.0 kHz, 25 W, 10 min | Positive | Positive |
| Liu et al. [12] | Tomato, Lettuce, Mung bean, Sticky bean, Radish, Dianthus, Mustard, Wheat | Dielectric barrier discharge (DBD) | N2, O2, Synthetic Air, 1.5 L/min, 0–18 kV, 500 Hz, Power consumption: ~2.5 W, Vpp = 20 kV, 2, 4, 6 min | Positive | Positive |
| C. Combination: Plasma Treatment on Seeds and Water | |||||
| Author | Target | Plasma Generation | Parameters | Outcomes | |
| Seed Germination | Plant Growth | ||||
| Bafoil et al. [42] | Arabidopsis thaliana (Early stage) | Floating electrode dielectric-barrier discharge (FE-DBD) | Ambient air, 10 kV for the voltage, 9.7 kHz for the frequency and 1 us for the pulse duration, 15 min | Positive | - |
| Distilled water and tap water (Later stage) | Plasma jet, the floating electrode-dielectric barrier discharges (FE-DBD) | Helium gas, 10 kV, 9.7 kHz, 1µs pulse duration, 3 L/min, 15 min, 30 mL water used in each treatment | - | Positive | |
| Sivachandiran et al. [26] | Water | Cylindrical double DBD reactor in air under atmospheric pressure and room temp | Synthetic Air (Air Liquide), Flow rate = 1 L/min, Pulse width: 120 ns; 21 kV; 2.4 A; 400 Hz; Max energy: 7 mJ, 250 mL DI water activated for 15 min and 30 min | Positive when treat on dry seeds, no significant influences on wet seeds | Negative for stem length on P-10 min seeds and P-20 min seed + PAW-30 min |
| Radish, Tomato, Sweet Pepper seeds | Plate-to-plate double DBD reactor in air under atmospheric pressure and room temp | Synthetic Air (Air Liquide), Flow rate = 1 L/min, Pulse width: 120 ns; 21 kV; 18 A; 200 Hz; Max energy: 57 mJ, 10 min; Plasma discharge volume: 130 W/cm | |||
| Treatment | n | Cd | Pb |
|---|---|---|---|
| NTS + NTW | 3 | 0.819 ± 0.048 | 0.003 ± 0.000 |
| NTS + PAW | 3 | 0.669 ± 0.008 | 0.003 ± 0.000 |
| PTS + NTW | 3 | 0.564 ± 0.019 | 0.003 ± 0.000 |
| PTS + PAW | 3 | 0.695 ± 0.012 | 0.005 ± 0.000 |
| p-value | 0.016 * | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.-Y.; Kong, T.-K.; Lin, C.-M.; Chen, H.-L. The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Appl. Sci. 2021, 11, 5304. https://doi.org/10.3390/app11115304
Hou C-Y, Kong T-K, Lin C-M, Chen H-L. The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Applied Sciences. 2021; 11(11):5304. https://doi.org/10.3390/app11115304
Chicago/Turabian StyleHou, Chih-Yao, Ting-Khai Kong, Chia-Min Lin, and Hsiu-Ling Chen. 2021. "The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach" Applied Sciences 11, no. 11: 5304. https://doi.org/10.3390/app11115304
APA StyleHou, C.-Y., Kong, T.-K., Lin, C.-M., & Chen, H.-L. (2021). The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Applied Sciences, 11(11), 5304. https://doi.org/10.3390/app11115304








