Perspectives on Cathodes for Protonic Ceramic Fuel Cells
Abstract
:1. Introduction
- (i)
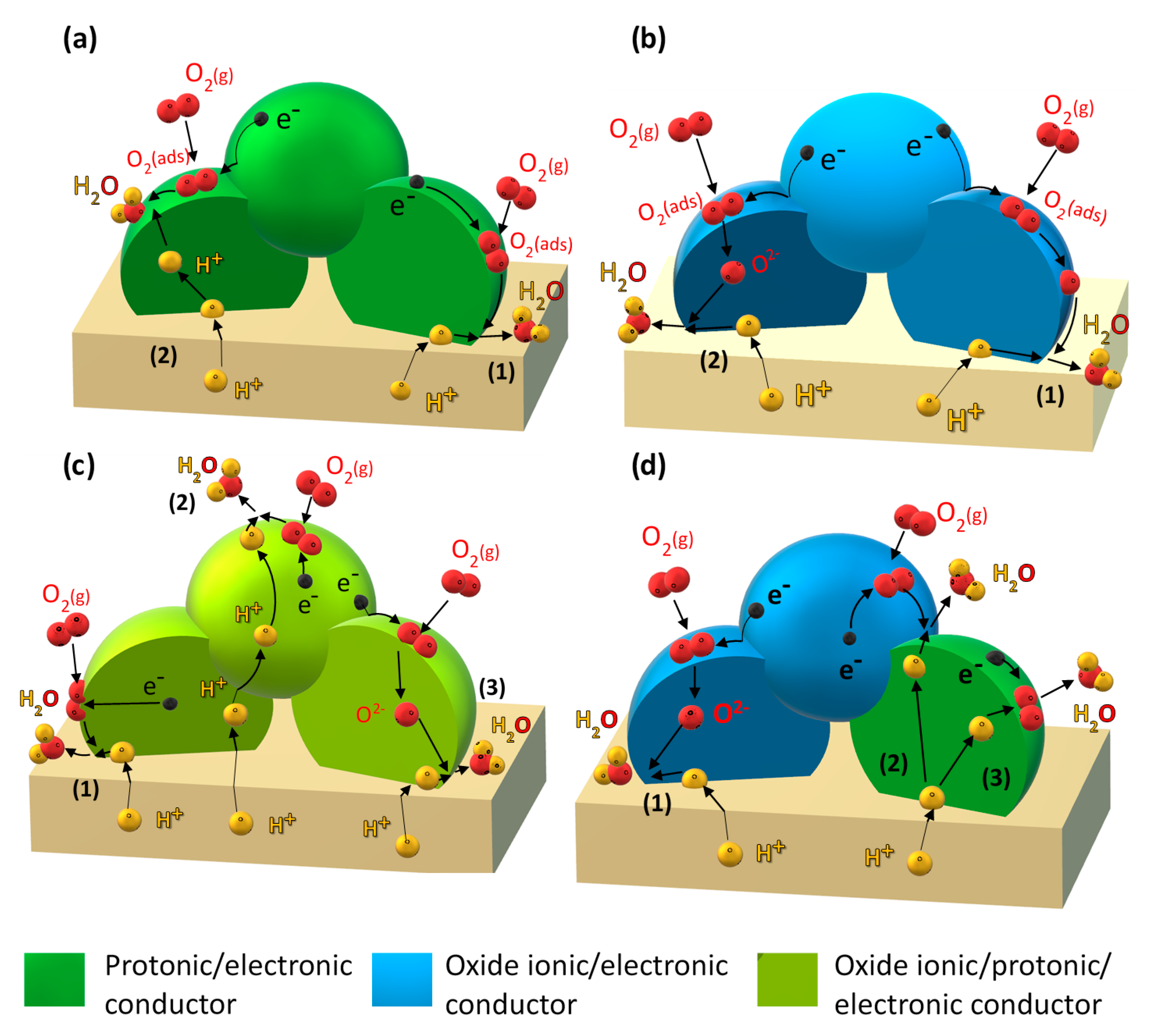
- Improving the electronic conductivity in proton-conducting oxides via substitution of mixed-valence cations (Figure 1a).
- (ii)
- Incorporation of a mixed oxide-ion–electronic conductor (MIEC) analogous to the classical oxide-ion conducting SOFC cathode (Figure 1b).
- (iii)
- Employing so-called triple-conducting oxides (TCOs) with proton, oxide-ion, and electron conductivity (Figure 1c).
- (iv)
- Designing composite electrodes with a proton-conducting oxide (including improving electron conductivity via strategy (i) and an oxide-ion and electron-conducting component (Figure 1d).
2. Mixed Proton–Electron-Conducting Cathodes
3. Mixed Oxide-Ion–Electron-Conducting Cathodes
4. Triple Proton Oxide Ion Electron Hole-Conducting Oxides
5. Composite Cathodes
6. Summarising Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, C.-J. Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrog. Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Coors, W.G. Protonic ceramic fuel cells for high-efficiency operation with methane. J. Power Sources 2003, 118, 150–156. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H. Technological challenges in the application of proton conducting ceramics. Solid State Ion. 1995, 77, 289–298. [Google Scholar] [CrossRef]
- Duan, C.; Huang, J.; Sullivan, N.; O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 2020, 7, 011314. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Medvedev, D. Trends in research and development of protonic ceramic electrolysis cells. Int. J. Hydrog. Energy 2019, 44, 26711–26740. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, J.; Yuan, Z.; Liu, J.; Ni, M.; Chen, F. Progress Report on Proton Conducting Solid Oxide Electrolysis Cells. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Theoretical analysis of reversible solid oxide fuel cell based on proton-conducting electrolyte. J. Power Sources 2008, 177, 369–375. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Schmidt, V.H. Fabrication, Performance, and Model for Proton Conductive Solid Oxide Fuel Cell. J. Electrochem. Soc. 2011, 158, B885. [Google Scholar] [CrossRef]
- Asano, K.; Hibino, T.; Iwahara, H. A Novel Solid Oxide Fuel Cell System Using the Partial Oxidation of Methane. J. Electrochem. Soc. 1995, 142, 3241–3245. [Google Scholar] [CrossRef]
- Luisetto, I.; Di Bartolomeo, E.; D’Epifanio, A.; Licoccia, S. CO2∕CH4 Reforming High Temperature Proton Conductor (HTPC) Fuel Cells. J. Electrochem. Soc. 2011, 158, B1368. [Google Scholar] [CrossRef]
- Uchida, H.; Tanaka, S.; Iwahara, H. Polarization at Pt electrodes of a fuel cell with a high temperature-type proton conductive solid electrolyte. J. Appl. Electrochem. 1985, 15, 93–97. [Google Scholar] [CrossRef]
- Fleig, J. Solid Oxide Fuel Cell Cathodes: Polarization Mechanisms and Modeling of the Electrochemical Performance. Annu. Rev. Mater. Res. 2003, 33, 361–382. [Google Scholar] [CrossRef]
- Zhu, H.; Kee, R.J. Modeling Protonic-Ceramic Fuel Cells with Porous Composite Electrodes in a Button-Cell Configuration. J. Electrochem. Soc. 2017, 164, F1400–F1411. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Electrode materials: A challenge for the exploitation of protonic solid oxide fuel cells. Sci. Technol. Adv. Mater. 2010, 11. [Google Scholar] [CrossRef]
- Mukundan, R.; Davies, P.K.; Worrell, W.L. Electrochemical Characterization of Mixed Conducting Ba(Ce1-(x+y)PryGdx)O3-x/2 Cathodes. MRS Online Proc. Libr. 1996, 453, 573. [Google Scholar] [CrossRef]
- Mukundan, R.; Davies, P.; Worrell, W. Electrochemical Characterization of Mixed Conducting Ba(Ce0.8-yPryGd0.2)O2.9 Cathodes. J. Electrochem. 2001, 148, 82–86. [Google Scholar] [CrossRef]
- Furoy, K.A.; Haugsrud, R.; Hänsel, M.; Magrasó, A.; Norby, T. Role of protons in the electrical conductivity of acceptor-doped BaPrO3, BaTbO3 and BaThO3. Solid State Ion. 2007, 178, 461–467. [Google Scholar] [CrossRef]
- Magrasó, A.; Espiell, F.; Segarra, M.; Irvine, J.T.S. Chemical and electrical properties of BaPr0.7Gd0.3O3−δ. J. Power Sources 2007, 169, 53–58. [Google Scholar] [CrossRef]
- Magrasó, A.; Haugsrud, R.; Segarra, M.; Norby, T. Defects and transport in Gd-doped BaPrO3. J. Electroceramics 2009, 23, 80–88. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Sun, W.; Ding, D.; Lü, Z.; Liu, M. A mixed-conducting BaPr0.8In0.2O3−δ cathode for proton-conducting solid oxide fuel cells. Electrochem. Commun. 2013, 27, 19–21. [Google Scholar] [CrossRef]
- Fabbri, E.; Markus, I.; Bi, L.; Pergolesi, D.; Traversa, E. Tailoring mixed proton-electronic conductivity of BaZrO3 by Y and Pr co-doping for cathode application in protonic SOFCs. Solid State Ion. 2011, 202, 30–35. [Google Scholar] [CrossRef]
- Magrasó, A.; Frontera, C.; Gunnæs, A.E.; Tarancón, A.; Marrero-López, D.; Norby, T.; Haugsrud, R. Structure, chemical stability and mixed proton-electron conductivity in BaZr0.9-xPrxGd0.1O3−δ. J. Power Sources 2011, 196, 9141–9147. [Google Scholar] [CrossRef]
- Heras-Juaristi, G.; Amador, U.; Romero de Paz, J.; Fuentes, R.O.; Chinelatto, A.L.; Ritter, C.; Fagg, D.P.; Pérez-Coll, D.; Mather, G.C. Structures, Phase Fields, and Mixed Protonic–Electronic Conductivity of Ba-Deficient, Pr-Substituted BaZr0.7Ce0.2Y0.1O3−δ. Inorg. Chem. 2018, 57, 15023–15033. [Google Scholar] [CrossRef]
- Heras-Juaristi, G.; Amador, U.; Fuentes, R.O.; Chinelatto, A.L.; Romero De Paz, J.; Ritter, C.; Fagg, D.P.; Pérez-Coll, D.; Mather, G.C. Thermal evolution of structures and conductivity of Pr-substituted BaZr0.7Ce0.2Y0.1O3-δ: Potential cathode components for protonic ceramic fuel cells. J. Mater. Chem. A 2018, 6, 5324–5334. [Google Scholar] [CrossRef]
- Kim, D.Y.; Miyoshi, S.; Tsuchiya, T.; Yamaguchi, S. Defect Chemistry and Electrochemical Properties of BaZrO3 Heavily Doped with Fe. Ecs Trans. 2012, 45, 161–170. [Google Scholar] [CrossRef]
- Rao, Y.; Zhong, S.; He, F.; Wang, Z.; Peng, R.; Lu, Y. Cobalt-doped BaZrO3: A single phase air electrode material for reversible solid oxide cells. Int. J. Hydrog. Energy 2012, 37, 12522–12527. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Yang, Y.; Song, W.; Ma, Z.; Chen, H.; Ou, X.; Zhao, L.; Khan, M.; Ling, Y. Investigation of Fe-substituted in BaZr0.8Y0.2O3-δ proton conducting oxides as cathode materials for protonic ceramics fuel cells. J. Alloy. Compd. 2020, 814. [Google Scholar] [CrossRef]
- Tarutina, L.R.; Vdovin, G.K.; Lyagaeva, J.G.; Medvedev, D.A. BaCe0.7–xZr0.2Y0.1FexO3−δ derived from proton-conducting electrolytes: A way of designing chemically compatible cathodes for solid oxide fuel cells. J. Alloy. Compd. 2020, 831. [Google Scholar] [CrossRef]
- Hui, Z.; Michèle, P. Preparation, chemical stability, and electrical properties of Ba(Ce1-xBix)O3 (x = 0.0-0.5). J. Mater. Chem. 2002, 12, 3787–3791. [Google Scholar] [CrossRef]
- Magrasó, A.; Fontaine, M.L.; Larring, Y.; Bredesen, R.; Syvertsen, G.E.; Lein, H.L.; Grande, T.; Huse, M.; Strandbakke, R.; Haugsrud, R.; et al. Development of proton conducting SOFCs based on LaNbO4 electrolyte—Status in norway. Fuel Cells 2011, 11, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Solís, C.; Serra, J.M. Adjusting the conduction properties of La0.995Ca0.005NbO4-δ by doping for proton conducting fuel cells electrode operation. Solid State Ion. 2011, 190, 38–45. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Stability of solid oxide fuel cell components. Solid State Ion. 2001, 143, 39–46. [Google Scholar] [CrossRef]
- Wang, N.; Hinokuma, S.; Ina, T.; Toriumi, H.; Katayama, M.; Inada, Y.; Zhu, C.; Habazaki, H.; Aoki, Y. Incorporation of bulk proton carriers in cubic perovskite manganite driven by interplays of oxygen and manganese redox. Chem. Mater. 2019, 31, 8383–8393. [Google Scholar] [CrossRef]
- Iwahara, H.; Yajima, T.; Hibino, T.; Ushida, H. Performance of Solid Oxide Fuel Cell Using Proton and Oxide Ion Mixed Conductors Based on BaCe1−xSmxO3−δ. J. Electrochem. Soc. 1993, 140, 1687–1691. [Google Scholar] [CrossRef]
- Xie, K.; Yan, R.; Dong, D.; Wang, S.; Chen, X.; Jiang, T.; Lin, B.; Wei, M.; Liu, X.; Meng, G. A modified suspension spray combined with particle gradation method for preparation of protonic ceramic membrane fuel cells. J. Power Sources 2008, 179, 576–583. [Google Scholar] [CrossRef]
- Aguadero, A.; Alonso, J.A.; Escudero, M.J.; Daza, L. Structural and Electrical Characterization of the Novel SrCo0.9Sb0.1O3-δ Perovskite: Evaluation as a Solid Oide Fuel Cell Cathode Material. Chem. Mater. 2007, 19, 6437–6444. [Google Scholar] [CrossRef]
- Ding, H.; Lin, B.; Jiang, Y.; Wang, S.; Fang, D.; Dong, Y.; Tao, S.; Peng, R.; Liu, X.; Meng, G. Low-temperature protonic ceramic membrane fuel cells (PCMFCs) with SrCo0.9Sb0.1O3-δ cubic perovskite cathode. J. Power Sources 2008, 185, 937–940. [Google Scholar] [CrossRef]
- Oda, H.; Yoneda, T.; Sakai, T.; Okuyama, Y.; Matsumoto, H. Preparation of nano-structured cathode for protonic ceramic fuel cell by bead-milling method. Solid State Ion. 2014, 262, 388–391. [Google Scholar] [CrossRef]
- Wang, B.; Bi, L.; Zhao, X.S. Liquid-phase synthesis of SrCo0.9Nb0.1O3-δ cathode material for proton-conducting solid oxide fuel cells. Ceram. Int. 2018, 44, 5139–5144. [Google Scholar] [CrossRef]
- Leonard, K.; Okuyama, Y.; Takamura, Y.; Lee, Y.S.; Miyazaki, K.; Ivanova, M.E.; Meulenberg, W.A.; Matsumoto, H. Efficient intermediate-temperature steam electrolysis with Y:SrZrO3-SrCeO3 and Y:BaZrO3-BaCeO3 proton conducting perovskites. J. Mater. Chem. A 2018, 6, 19113–19124. [Google Scholar] [CrossRef]
- Meng, X.; Yang, N.; Song, J.; Tan, X.; Ma, Z.F.; Li, K. Synthesis and characterization of terbium doped barium cerates as a proton conducting SOFC electrolyte. Int. J. Hydrog. Energy 2011, 36, 13067–13072. [Google Scholar] [CrossRef]
- Robinson, S.; Manerbino, A.; Grover Coors, W.; Sullivan, N.P. Fabrication and Performance of Tubular, Electrode-Supported BaCe0.2Zr0.7Y0.1O3-δ Fuel Cells. Fuel Cells 2013, 13, 584–591. [Google Scholar] [CrossRef]
- Oda, H.; Okuyama, Y.; Sakai, T.; Matsumoto, H. Preparation of Nano-structured La0.6Sr0.4Co0.2Fe0.8O3-δ cathode for protonic ceramic fuel cell by bead-milling method. Mater. Trans. 2014, 55, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Meng, Y.; Lee, S.; Tong, J.; Brinkman, K.S. Effect of Infiltration of Barium Carbonate Nanoparticles on the Electrochemical Performance of La0.6Sr0.4Co0.2Fe0.8O3−δ Cathodes for Protonic Ceramic Fuel Cells. JOM 2019, 71, 90–95. [Google Scholar] [CrossRef]
- Taillades, G.; Battochi, P.; Taillades, M.; Jones, D.; Roziére, J. Chemically Stable Electrolytes and Advanced Electrode Architectures for Efficient Proton Ceramic Fuel Cells. Ecs Trans. 2019, 35, 805–811. [Google Scholar] [CrossRef]
- Dailly, J.; Mauvy, F.; Marrony, M.; Pouchard, M.; Grenier, J.-C. Electrochemical properties of perovskite and A2MO4-type oxides used as cathodes in protonic ceramic half cells. J. Solid State Electrochem. 2011, 15, 245–251. [Google Scholar] [CrossRef]
- Lim, D.-K.; Im, H.-N.; Singh, B.; Song, S.-J. Investigations on Electrochemical Performance of a Proton-Conducting Ceramic-Electrolyte Fuel Cell with La0.8Sr0.2MnO3 Cathode. J. Electrochem. Soc. 2015, 162, F547–F554. [Google Scholar] [CrossRef]
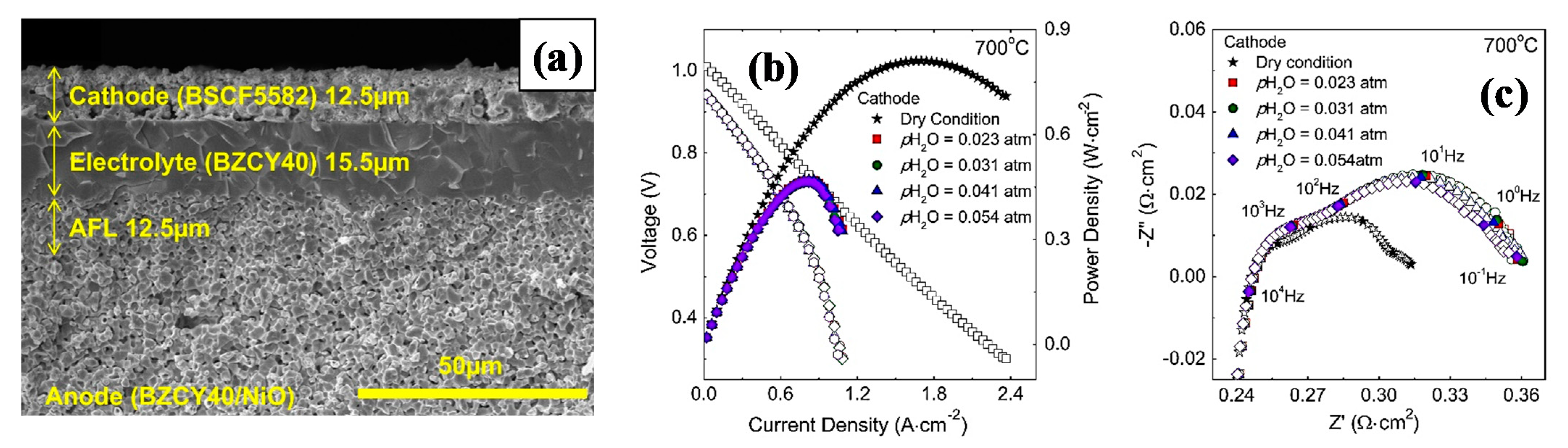
- Lim, D.K.; Kim, J.H.; Chavan, A.U.; Lee, T.R.; Yoo, Y.S.; Song, S.J. Performance of proton-conducting ceramic-electrolyte fuel cell with BZCY40 electrolyte and BSCF5582 cathode. Ceram. Int. 2016, 42, 3776–3785. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Quarez, E.; Solís, C.; Serra, J.M.; Joubert, O. Cathode materials for La0.995Ca0.005NbO4 proton ceramic electrolyte. Int. J. Hydrog. Energy 2011, 36, 13059–13066. [Google Scholar] [CrossRef]
- Quarez, E.; Kravchyk, K.V.; Joubert, O. Compatibility of proton conducting La6WO12 electrolyte with standard cathode materials. Solid State Ion. 2012, 216, 19–24. [Google Scholar] [CrossRef]
- Shang, M.; Tong, J.; O’Hayre, R. A promising cathode for intermediate temperature protonic ceramic fuel cells: BaCo0.4Fe0.4Zr0.2O3-δ. RSC Adv. 2013, 3, 15769–15775. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Zhang, S.; Yang, Y. Cerium and Gadolinium co-doped perovskite oxide for a protonic ceramic fuel cell cathode. Int. J. Hydrog. Energy 2019, 44, 27921–27929. [Google Scholar] [CrossRef]
- Tolchard, J.; Grande, T. Physicochemical compatibility of SrCeO3 with potential SOFC cathodes. J. Solid State Chem. 2007, 180, 2808–2815. [Google Scholar] [CrossRef]
- Tolchard, J.R.; Grande, T. Chemical compatibility of candidate oxide cathodes for BaZrO3 electrolytes. Solid State Ion. 2007, 178, 593–599. [Google Scholar] [CrossRef]
- Yamaura, H.; Ikuta, T.; Yahiro, H.; Okada, G. Cathodic polarization of strontium-doped lanthanum ferrite in proton-conducting solid oxide fuel cell. Solid State Ion. 2005, 176, 269–274. [Google Scholar] [CrossRef]
- Yuan, R.H.; He, W.; Zhang, C.; Ni, M. Cobalt free SrFe0.95Nb0.05O3−δ cathode material for proton-conducting solid oxide fuel cells with BaZr0.1Ce0.7Y0.2O3−δ electrolyte. Mater. Lett. 2017, 200, 75–78. [Google Scholar] [CrossRef]
- Ding, H.; Lin, B.; Liu, X.; Meng, G. High performance protonic ceramic membrane fuel cells (PCMFCs) with Ba0.5Sr0.5Zn0.2Fe0.8O3-δ perovskite cathode. Electrochem. Commun. 2008, 10, 1388–1391. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Tarutin, A.; Vdovin, G.; Medvedev, D.; Demin, A.; Tsiakaras, P. Designing a protonic ceramic fuel cell with novel electrochemically active oxygen electrodes based on doped Nd0.5Ba0.5FeO3-δ. Dalt. Trans. 2018, 47, 8149–8157. [Google Scholar] [CrossRef]
- Kim, J.H.; Manthiram, A. Low thermal expansion RBa(Co,M)4O7 cathode materials based on tetrahedral-site cobalt ions for solid oxide fuel cells. Chem. Mater. 2010, 22, 822–831. [Google Scholar] [CrossRef]
- Shao, Q.; Ge, W.; Lu, X.; Chen, Y.; Ding, Y.; Lin, B.; Ling, Y. A promising cathode for proton-conducting intermediate temperature solid oxide fuel cells: Y0.8Ca0.2BaCo4O7+δ. Ceram. Int. 2015, 41, 6687–6692. [Google Scholar] [CrossRef]
- Medvedev, D.; Lyagaeva, J.; Vdovin, G.; Beresnev, S.; Demin, A.; Tsiakaras, P. A tape calendering method as an effective way for the preparation of proton ceramic fuel cells with enhanced performance. Electrochim. Acta 2016, 210, 681–688. [Google Scholar] [CrossRef]
- Li, S.; Funahashi, R.; Matsubara, I.; Ueno, K.; Sodeoka, S.; Yamada, H. Synthesis and thermoelectric properties of the new oxide materials Ca(3-x)Bi(x)Co4O(9-δ) (0.0<x<0.75). Chem. Mater. 2000, 12, 2424–2427. [Google Scholar] [CrossRef]
- Yahia, H.B.; Mauvy, F.; Grenier, J.C. Ca3-xLaxCo4O9+δ (x=0, 0.3): New cobaltite materials as cathodes for proton conducting solid oxide fuel cell. J. Solid State Chem. 2010, 183, 527–531. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Koroleva, M.; Vdovin, G.; Farlenkov, A.; Medvedev, D. Functionality of an oxygen Ca3Co4O9+δ electrode for reversible solid oxide electrochemical cells based on proton-conducting electrolytes. J. Power Sources 2019, 438, 226996. [Google Scholar] [CrossRef]
- Kim, J.H.; Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 22002. [Google Scholar] [CrossRef]
- Pérez-Coll, D.; Heras-Juaristi, G.; Fagg, D.P.; Mather, G.C. Transport-number determination of a protonic ceramic electrolyte membrane via electrode-polarisation correction with the Gorelov method. J. Power Sources 2014, 245, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Frade, J.R. Theoretical behaviour of concentration cells based on ABO3 perovskite materials with protonic and oxygen ion conduction. Solid State Ion. 1995, 78, 87–97. [Google Scholar] [CrossRef]
- Baek, H.-D. Modeling of electrical conductivity in high-temperature proton- conducting oxides. Solid State Ion. 1998, 110, 255–262. [Google Scholar] [CrossRef]
- Heras-Juaristi, G.; Pérez-Coll, D.; Mather, G.C. Temperature dependence of partial conductivities of the BaZr 0.7Ce0.2Y0.1O3-δ proton conductor. J. Power Sources 2017, 364, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, M.L.; Larring, Y.; Haugsrud, R.; Norby, T.; Wiik, K.; Bredesen, R. Novel high temperature proton conducting fuel cells: Production of La0.995Sr0.005NbO4-δ electrolyte thin films and compatible cathode architectures. J. Power Sources 2009, 188, 106–113. [Google Scholar] [CrossRef]
- Taillades, G.; Dailly, J.; Taillades-Jacquin, M.; Mauvy, F.; Essouhmi, A.; Marrony, M.; Lalanne, C.; Fourcade, S.; Jones, D.J.; Grenier, J.C.; et al. Intermediate temperature anode-supported fuel cell based on BaCe0.9Y0.1O3 electrolyte with novel Pr2NiO4 cathode. Fuel Cells 2010, 10, 166–173. [Google Scholar] [CrossRef]
- Dailly, J.; Fourcade, S.; Largeteau, A.; Mauvy, F.; Grenier, J.C.; Marrony, M. Perovskite and A2MO4-type oxides as new cathode materials for protonic solid oxide fuel cells. Electrochim. Acta 2010, 55, 5847–5853. [Google Scholar] [CrossRef]
- Dailly, J.; Marrony, M. BCY-based proton conducting ceramic cell: 1000 h of long term testing in fuel cell application. J. Power Sources 2013, 240, 323–327. [Google Scholar] [CrossRef]
- Grimaud, A.; Mauvy, F.; Bassat, J.M.; Fourcade, S.; Rocheron, L.; Marrony, M.; Grenier, J.C. Hydration Properties and Rate Determining Steps of the Oxygen Reduction Reaction of Perovskite-Related Oxides as H+-SOFC Cathodes. J. Electrochem. Soc. 2012, 159, B683–B694. [Google Scholar] [CrossRef]
- Grimaud, A.; Mauvy, F.; Marc Bassat, J.; Fourcade, S.; Marrony, M.; Claude Grenier, J. Hydration and transport properties of the Pr2−xSrxNiO4+δ compounds as H+-SOFC cathodes. J. Mater. Chem. 2012, 22, 16017. [Google Scholar] [CrossRef]
- An, H.; Shin, D.; Ji, H.I. Pr2NiO4+δ for cathode in protonic ceramic fuel cells. J. Korean Ceram. Soc. 2018, 55, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Tarutin, A.; Kasyanova, A.; Lyagaeva, J.; Vdovin, G.; Medvedev, D. Towards high-performance tubular-type protonic ceramic electrolysis cells with all-Ni-based functional electrodes. J. Energy Chem. 2020, 40, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Tarutin, A.P.; Gorshkov, M.Y.; Bainov, I.N.; Vdovin, G.K.; Vylkov, A.I.; Lyagaeva, J.G.; Medvedev, D.A. Barium-doped nickelates Nd2–xBaxNiO4+δ as promising electrode materials for protonic ceramic electrochemical cells. Ceram. Int. 2020, 46, 24355–24364. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Vdovin, G.K.; Medvedev, D.A.; Yaremchenko, A.A. Fluorine-containing oxygen electrodes of the nickelate family for proton-conducting electrochemical cells. Electrochim. Acta 2020, 337. [Google Scholar] [CrossRef]
- Miao, L.; Hou, J.; Gong, Z.; Jin, Z.; Liu, W. A high-performance cobalt-free Ruddlesden-Popper phase cathode La1.2Sr0.8Ni0.6Fe0.4O4+δ for low temperature proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2019, 44, 7531–7537. [Google Scholar] [CrossRef]
- Gu, C.Y.; Wu, X.S.; Cao, J.F.; Hou, J.; Miao, L.N.; Xia, Y.P.; Liu, W. High performance Ca-containing La2-xCaxNiO4+δ(0≤x≤0.75) cathode for proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2020, 45, 23422–23432. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Huan, D.; Sun, S.; Wang, G.; Fu, Z.; Zhang, W.; Zheng, X.; Pan, H.; Peng, R.; et al. Tailoring the activity via cobalt doping of a two-layer Ruddlesden-Popper phase cathode for intermediate temperature solid oxide fuel cells. J. Power Sources 2017, 371, 41–47. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Wang, T.; Chen, G.; Wu, K.; Cheng, Y. Decreasing the polarization resistance of LaSrCoO4 cathode by Fe substitution for Ba(Zr0.1Ce0.7Y0.2)O3-based protonic ceramic fuel cells. J. Alloy. Compd. 2016, 689, 581–586. [Google Scholar] [CrossRef]
- Tang, H.; Jin, Z.; Wu, Y.; Liu, W.; Bi, L. Cobalt-free nanofiber cathodes for proton conducting solid oxide fuel cells. Electrochem. Commun. 2019, 100, 108–112. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Li, J. A novel composite cathode for intermediate temperature solid oxide fuel cell. J. Power Sources 2014, 269, 723–726. [Google Scholar] [CrossRef]
- Fan, L.; Su, P.C. Layer-structured LiNi0.8Co0.2O2: A new triple (H+/O2-/e-) conducting cathode for low temperature proton conducting solid oxide fuel cells. J. Power Sources 2016, 306, 369–377. [Google Scholar] [CrossRef]
- Batocchi, P.; Mauvy, F.; Fourcade, S.; Parco, M. Electrical and electrochemical properties of architectured electrodes based on perovskite and A2MO4-type oxides for Protonic Ceramic Fuel Cell. Electrochim. Acta 2014, 145, 1–10. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, S.; Zhang, L.; Bi, L.; Ding, H.; Liu, X.; Gao, J.; Meng, G. Prontonic ceramic membrane fuel cells with layered GdBaCo2O5+δ cathode prepared by gel-casting and suspension spray. J. Power Sources 2008, 177, 330–333. [Google Scholar] [CrossRef]
- Lin, B.; Dong, Y.; Yan, R.; Zhang, S.; Hu, M.; Zhou, Y.; Meng, G. In situ screen-printed BaZr0.1Ce0.7Y0.2O3-δ electrolyte-based protonic ceramic membrane fuel cells with layered SmBaCo2O5+δ cathode. J. Power Sources 2009, 186, 446–449. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X.; Liu, X.; Meng, G. A novel layered perovskite cathode for proton conducting solid oxide fuel cells. J. Power Sources 2010, 195, 775–778. [Google Scholar] [CrossRef]
- Ling, Y.; Lin, B.; Zhao, L.; Zhang, X.; Yu, J.; Peng, R.; Meng, G.; Liu, X. Layered perovskite LaBaCuMO5+δ (M = Fe, Co) cathodes for intermediate-temperature protonic ceramic membrane fuel cells. J. Alloy. Compd. 2010, 493, 252–255. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kwon, G.; Ding, D.; Shin, J.; Liu, M.; Kim, G. Triple-Conducting Layered Perovskites as Cathode Materials for Proton-Conducting Solid Oxide Fuel Cells. ChemSusChem 2014, 7, 2811–2815. [Google Scholar] [CrossRef]
- Strandbakke, R.; Cherepanov, V.A.; Zuev, A.Y.; Tsvetkov, D.S.; Argirusis, C.; Sourkouni, G.; Prünte, S.; Norby, T. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 2015, 278, 120–132. [Google Scholar] [CrossRef]
- Brieuc, F.; Dezanneau, G.; Hayoun, M.; Dammak, H. Proton diffusion mechanisms in the double perovskite cathode material GdBaCo2O5.5: A molecular dynamics study. Solid State Ion. 2017, 309, 187–191. [Google Scholar] [CrossRef] [Green Version]
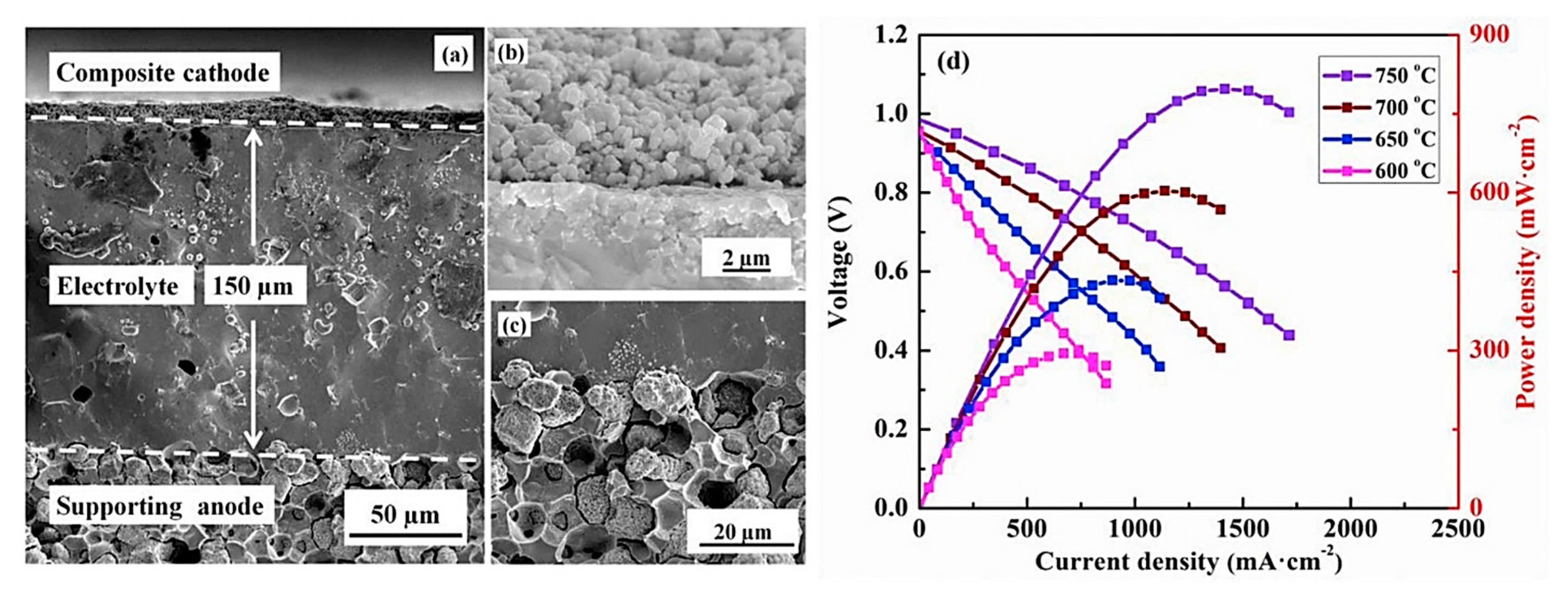
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: Exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ. Sci. 2019, 12, 206–215. [Google Scholar] [CrossRef]
- Kang, E.H.; Choi, H.R.; Park, J.S.; Kim, K.H.; Kim, D.H.; Bae, K.; Prinz, F.B.; Shim, J.H. Protonic ceramic fuel cells with slurry-spin coated BaZr0.2Ce0.6Y0.1Yb0.1O3-δ thin-film electrolytes. J. Power Sources 2020, 465, 228254. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Rioja-Monllor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.A.; Grande, T. Effect of cation ordering on the performance and chemical stability of layered double perovskite cathodes. Materials 2018, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Ding, H.; Bian, W.; Wu, W.; Li, W.; Liu, X.; Gomez, J.Y.; Regalado Vera, C.Y.; Zhou, M.; Ding, D. Understanding of A-site deficiency in layered perovskites: Promotion of dual reaction kinetics for water oxidation and oxygen reduction in protonic ceramic electrochemical cells. J. Mater. Chem. A 2020, 8, 14600–14608. [Google Scholar] [CrossRef]
- Zhou, C.; Sunarso, J.; Song, Y.; Dai, J.; Zhang, J.; Gu, B.; Zhou, W.; Shao, Z. New reduced-temperature ceramic fuel cells with dual-ion conducting electrolyte and triple-conducting double perovskite cathode. J. Mater. Chem. A 2019, 7, 13265–13274. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Hu, X.; Sun, L.; Ling, Y. High Performance Proton-Conducting Solid Oxide Fuel Cells with a Layered Perovskite GdBaCuCoO5+δ Cathode. Electron. Mater. Lett. 2018, 14, 147–153. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, J.; Fan, Y.; Xi, X.A.; Li, J.; Lu, Y.; Huo, G.; Shao, L.; Fu, X.Z.; Luo, J.L. Pr2BaNiMnO7-δ double-layered Ruddlesden-Popper perovskite oxides as efficient cathode electrocatalysts for low temperature proton conducting solid oxide fuel cells. J. Mater. Chem. A 2020, 8, 7704–7712. [Google Scholar] [CrossRef]
- Grimaud, A.; Bassat, J.M.; Mauvy, F.; Pollet, M.; Wattiaux, A.; Marrony, M.; Grenier, J.C. Oxygen reduction reaction of PrBaCo2-xFexO5+δ compounds as H+-SOFC cathodes: Correlation with physical properties. J. Mater. Chem. A 2014, 2, 3594–3604. [Google Scholar] [CrossRef]
- Téllez Lozano, H.; Druce, J.; Cooper, S.J.; Kilner, J.A. Double perovskite cathodes for proton-conducting ceramic fuel cells: Are they triple mixed ionic electronic conductors? Sci. Technol. Adv. Mater. 2017, 18, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Malyshkin, D.; Novikov, A.; Ivanov, I.; Sereda, V.; Tsvetkov, D.; Zuev, A. The origin of triple conductivity and water uptake in layered double perovskites: A case study on lanthanum-substituted GdBaCo2O6−δ. J. Alloy. Compd. 2020, 845, 156309. [Google Scholar] [CrossRef]
- Leonard, K.; Druce, J.; Thoreton, V.; Kilner, J.A.; Matsumoto, H. Exploring mixed proton/electron conducting air electrode materials in protonic electrolysis cell. Solid State Ion. 2018, 319, 218–222. [Google Scholar] [CrossRef]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, W.; Jiang, C.; Ding, Y.; Bian, W.; Hu, B.; Singh, P.; Orme, C.J.; Wang, L.; Zhang, Y.; et al. Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; OHayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Duan, C.; Hook, D.; Chen, Y.; Tong, J.; O’Hayre, R. Zr and Y co-doped perovskite as a stable, high performance cathode for solid oxide fuel cells operating below 500 °C. Energy Environ. Sci. 2017, 10, 176–182. [Google Scholar] [CrossRef]
- Dubois, A.; Ricote, S.; Braun, R.J. Benchmarking the expected stack manufacturing cost of next generation, intermediate-temperature protonic ceramic fuel cells with solid oxide fuel cell technology. J. Power Sources 2017, 369, 65–77. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Xu, C.; Sun, W.; Qiao, J.; Rooney, D.W.; Sun, K. Tuning the defects of the triple conducting oxide BaCo0.4Fe0.4Zr0.1Y0.1O3-δ perovskite toward enhanced cathode activity of protonic ceramic fuel cells. J. Mater. Chem. A 2019, 7, 18365–18372. [Google Scholar] [CrossRef]
- He, F.; Liang, M.; Wang, W.; Ran, R.; Yang, G.; Zhou, W.; Shao, Z. High-Performance Proton-Conducting Fuel Cell with B-Site-Deficient Perovskites for All Cell Components. Energy Fuels 2020, 34, 11464–11471. [Google Scholar] [CrossRef]
- Li, J.; Hou, J.; Lu, Y.; Wang, Q.; Xi, X.; Fan, Y.; Fu, X.Z.; Luo, J.L. Ca-containing Ba0.95Ca0.05Co0.4Fe0.4Zr0.1Y0.1O3-δ cathode with high CO2-poisoning tolerance for proton-conducting solid oxide fuel cells. J. Power Sources 2020, 453, 227909. [Google Scholar] [CrossRef]
- Liang, M.; He, F.; Zhou, C.; Chen, Y.; Ran, R.; Yang, G. Nickel-doped BaCo0.4Fe0.4Zr0.1Y0.1O3-δ as a new high-performance cathode for both oxygen-ion and proton conducting fuel cells. Chem. Eng. J. 2020, 127717. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3-δ and comparison to protonic electrolyte materials. Solid State Ion. 2017, 299, 64–69. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Raimondi, G.; Maier, J. Mixed-Conducting Perovskites as Cathode Materials for Protonic Ceramic Fuel Cells: Understanding the Trends in Proton Uptake. Adv. Funct. Mater. 2018, 28, 1801241. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, T.; Wang, P.; Brinkman, K.; Tong, J.; Cheng, J. Investigate the proton uptake process of proton/oxygen ion/hole triple conductor BaCo0.4Fe0.4Zr0.1Y0.1O3-δ by electrical conductivity relaxation. J. Power Sources 2019, 440, 227122. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Z.; Wang, H.; Gong, Z.; Lv, H.; Peng, R.; Liu, W.; Bi, L. A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance. J. Mater. Chem. A 2019, 7, 16136–16148. [Google Scholar] [CrossRef]
- Dusastre, V.; Kilner, J.A. Optimisation of composite cathodes for intermediate temperature SOFC applications. Solid State Ion. 1999, 126, 163–174. [Google Scholar] [CrossRef]
- Wu, T.; Peng, R.; Xia, C. Sm0.5Sr0.5CoO3-δ-BaCe0.8Sm0.2O3-δ composite cathodes for proton-conducting solid oxide fuel cells. Solid State Ion. 2008, 179, 1505–1508. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X.; Liu, X.; Meng, G. High performance protonic ceramic membrane fuel cells (PCMFCs) with Sm0.5Sr0.5CoO3-δ perovskite cathode. J. Alloy. Compd. 2010, 494, 233–235. [Google Scholar] [CrossRef]
- Dailly, J.; Taillades, G.; Ancelin, M.; Pers, P.; Marrony, M. High performing BaCe0.8Zr0.1Y0.1O3-δ-Sm0.5Sr0.5CoO3-δ based protonic ceramic fuel cell. J. Power Sources 2017, 361, 221–226. [Google Scholar] [CrossRef]
- Dai, H.; Da’as, E.H.; Shafi, S.P.; Wang, H.; Bi, L. Tailoring cathode composite boosts the performance of proton-conducting SOFCs fabricated by a one-step co-firing method. J. Eur. Ceram. Soc. 2018, 38, 2903–2908. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Baek, H.; Shin, D. Cell performance enhancement facilitated by mixed ionic and electronic conductor fiber for protonic ceramic fuel cells. J. Electroceramics 2020, 11–13. [Google Scholar] [CrossRef]
- Bi, L.; Shafi, S.P.; Da’as, E.H.; Traversa, E. Tailoring the Cathode–Electrolyte Interface with Nanoparticles for Boosting the Solid Oxide Fuel Cell Performance of Chemically Stable Proton-Conducting Electrolytes. Small 2018, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, T.; Peng, R.; Xia, C. Cathode reaction models and performance analysis of Sm0.5Sr0.5CoO3-δ-BaCe0.8Sm0.2O3-δ composite cathode for solid oxide fuel cells with proton conducting electrolyte. J. Power Sources 2009, 194, 263–268. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Y.; Ding, J.; Jiang, G. New approaches for the determination of electrochemical parameters in the model of proton-conducting solid oxide fuel cell. Electrochim. Acta 2019, 318, 560–571. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Y.; Ding, J.; He, M. Modeling investigation of Sm0.5Sr0.5CoO3−δ–Ce0.8Sm0.2O2−δ cathode for proton-conducting ceramic fuel cell. J. Solid State Electrochem. 2020, 24, 1487–1495. [Google Scholar] [CrossRef]
- Lv, X.; Chen, H.; Zhou, W.; Li, S.D.; Shao, Z. A CO2-tolerant SrCo0.8Fe0.15Zr0.05O3−δ cathode for proton-conducting solid oxide fuel cells. J. Mater. Chem. A 2020, 8, 11292–11301. [Google Scholar] [CrossRef]
- Bu, Y.; Joo, S.; Zhang, Y.; Wang, Y.; Meng, D.; Ge, X.; Kim, G. A highly efficient composite cathode for proton-conducting solid oxide fuel cells. J. Power Sources 2020, 451, 227812. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Lenrick, F.; Wallenberg, R. LaCoO3: Promising cathode material for protonic ceramic fuel cells based on a BaCe0.2Zr0.7Y0.1O3−δ electrolyte. J. Power Sources 2012, 218, 313–319. [Google Scholar] [CrossRef]
- Matsui, T.; Manriki, K.; Miyazaki, K.; Muroyama, H.; Eguchi, K. A new oxygen reduction electrocatalyst of barium lanthanide cobaltate for composite cathodes of proton-conducting ceramic fuel cells. J. Mater. Chem. A 2018, 6, 14188–14194. [Google Scholar] [CrossRef]
- Rioja-Monllor, L.; Bernuy-Lopez, C.; Fontaine, M.L.; Grande, T.; Einarsrud, M.A. Processing of high performance composite cathodes for protonic ceramic fuel cells by exsolution. J. Mater. Chem. A 2019, 7, 8609–8619. [Google Scholar] [CrossRef]
- Rioja-Monllor, L.; Bernuy-Lopez, C.; Fontaine, M.L.; Grande, T.; Einarsrud, M.A. Compositional engineering of a La1-xBaxCoO3-δ-(1-a) BaZr0.9Y0.1O2.95 (a = 0.6, 0.7, 0.8 and x = 0.5, 0.6, 0.7) nanocomposite cathodes for protonic ceramic fuel cells. Materials 2019, 12, 3441. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Si, F.; Fu, X.Z.; Luo, J.L. Stable SrCo0.7Fe0.2Zr0.1O3-δ cathode material for proton conducting solid oxide fuel cell reactors. Int. J. Hydrog. Energy 2018, 43, 7511–7514. [Google Scholar] [CrossRef]
- Fabbri, E.; Licoccia, S.; Traversa, E.; Wachsman, E.D. Composite cathodes for proton conducting electrolytes. Fuel Cells 2009, 9, 128–138. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Yun, D.S.; Park, S.Y.; Park, J.; Joo, J.H.; Park, H.; Kwak, M.; Yu, J.H. Investigation of Electrochemical Properties of Model Lanthanum Strontium Cobalt Ferrite-Based Cathodes for Proton Ceramic Fuel Cells. Electrocatalysis 2016, 7, 280–286. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Wee, S.; Baek, H.W.; Shin, D. One-dimensional structured La0.6Sr0.4Co0.2Fe0.8O3−δ-BaCe0.5Zr0.35Y0.15O3−δ composite cathode for protonic ceramic fuel cells. Solid State Ion. 2018, 320, 347–352. [Google Scholar] [CrossRef]
- Cui, J.; Wang, J.; Zhang, X.; Li, G.; Wu, K.; Cheng, Y.; Zhou, J. Enhanced oxygen reduction reaction through Ca and Co Co-doped YFeO3 as cathode for protonic ceramic fuel cells. J. Power Sources 2019, 413, 148–157. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, Y.; Sumi, H.; Mizutani, Y. Performance Comparison of Perovskite Composite Cathodes with BaZr0.1Ce0.7Y0.1Yb0.1O3−δ in Anode-Supported Protonic Ceramic Fuel Cells. J. Electrochem. Soc. 2020, 167, 124506. [Google Scholar] [CrossRef]
- Lin, B.; Ding, H.; Dong, Y.; Wang, S.; Zhang, X.; Fang, D.; Meng, G. Intermediate-to-low temperature protonic ceramic membrane fuel cells with Ba0.5Sr0.5Co0.8Fe0.2O3-δ–BaZr0.1Ce0.7Y0.2O3-δ composite cathode. J. Power Sources 2009, 186, 58–61. [Google Scholar] [CrossRef]
- Taillades, G.; Pers, P.; Batocchi, P.; Taillades, M. Advanced Electrodes for Intermediate Temperature Proton Conducting Fuel Cell. Ecs Trans. 2013, 57, 1289–1296. [Google Scholar] [CrossRef]
- Taillades, G.; Pers, P.; Mao, V.; Taillades, M. High performance anode-supported proton ceramic fuel cell elaborated by wet powder spraying. Int. J. Hydrog. Energy 2016, 41, 12330–12336. [Google Scholar] [CrossRef]
- Marrony, M.; Dailly, J. Advanced Proton Conducting Ceramic Cell as Energy Storage Device. J. Electrochem. Soc. 2017, 164, F988–F994. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Fronzi, M.; Wang, X.; Bi, L.; Traversa, E. Tailoring cations in a perovskite cathode for proton-conducting solid oxide fuel cells with high performance. J. Mater. Chem. A 2019, 7, 20624–20632. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Nanostructured BaCo0.4Fe0.4Zr0.1Y0.1O3-δ cathodes with different microstructural architectures. Nanomaterials 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Zhang, S.; Lin, B.; Fang, S.; Xia, C.; Liu, W. Screen-printed BaCe0.8Sm0.2O3-δ thin membrane solid oxide fuel cells with surface modification by spray coating. J. Alloy. Compd. 2009, 473, 48–52. [Google Scholar] [CrossRef]
- Song, S.H.; Yoon, S.E.; Choi, J.; Kim, B.K.; Park, J.S. A high-performance ceramic composite anode for protonic ceramic fuel cells based on lanthanum strontium vanadate. Int. J. Hydrog. Energy 2014, 39, 16534–16540. [Google Scholar] [CrossRef]
- Choi, J.; Kim, B.; Song, S.H.; Park, J.S. A composite cathode with undoped LaFeO3 for protonic ceramic fuel cells. Int. J. Hydrog. Energy 2016, 41, 9619–9626. [Google Scholar] [CrossRef]
- Shin, E.K.; Anggia, E.; Parveen, A.S.; Park, J.S. Optimization of the protonic ceramic composition in composite electrodes for high-performance protonic ceramic fuel cells. Int. J. Hydrog. Energy 2019, 44, 31323–31332. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Wang, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Tailoring the Oxygen Vacancy to Achieve Fast Intrinsic Proton Transport in a Perovskite Cathode for Protonic Ceramic Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 4914–4922. [Google Scholar] [CrossRef]
- Lei, L.; Tao, Z.; Wang, X.; Lemmon, J.P.; Chen, F. Intermediate-temperature solid oxide electrolysis cells with thin proton-conducting electrolyte and a robust air electrode. J. Mater. Chem. A 2017, 5, 22945–22951. [Google Scholar] [CrossRef]
- Shin, E.K.; Shin, M.; Lee, H.; Park, J.S. Catalysts for composite cathodes of protonic ceramic fuel cells. Ceram. Int. 2018, 44, 8423–8426. [Google Scholar] [CrossRef]
- Sun, W.; Fang, S.; Yan, L.; Liu, W. Proton-Blocking Composite Cathode for Proton-Conducting Solid Oxide Fuel Cell. J. Electrochem. Soc. 2011, 158, B1432. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Z.; Jiang, Y.; Shi, Z.; Yan, L.; Liu, W. Optimization of BaZr0.1Ce0.7Y0.2O3−δ-based proton-conducting solid oxide fuel cells with a cobalt-free proton-blocking La0.7Sr0.3FeO3−δ–Ce0.8Sm0.2O2−δ composite cathode. Int. J. Hydrog. Energy 2011, 36, 9956–9966. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, Z.; Qian, J.; Liu, W. A new cobalt-free proton-blocking composite cathode La2NiO4+δ-LaNi0.6Fe0.4O3-δ for BaZr0.1Ce0.7Y0.2O3-δ-based solid oxide fuel cells. J. Power Sources 2014, 264, 67–75. [Google Scholar] [CrossRef]
- Gong, Z.; Hou, J.; Wang, Z.; Cao, J.; Zhang, J.; Liu, W. A new cobalt-free composite cathode Pr0.6Sr0.4Cu0.2Fe0.8O3-δ-Ce0.8Sm0.2O2-δ for proton-conducting solid oxide fuel cells. Electrochim. Acta 2015, 178, 60–64. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Ma, J.; Liu, W.; Wang, X.; Fronzi, M.; Bi, L. Impressive performance of proton-conducting solid oxide fuel cells using a first-generation cathode with tailored cations. J. Mater. Chem. A 2019, 7, 18792–18798. [Google Scholar] [CrossRef]
- Vert, V.B.; Solís, C.; Serra, J.M. Electrochemical Properties of PSFC-BCYb Composites as Cathodes for Proton Conducting Solid Oxide Fuel Cells. Fuel Cells 2011, 11, 81–90. [Google Scholar] [CrossRef]
- Shi, H.; Ding, Z.; Ma, G. Electrochemical Performance of Cobalt-free Nd0.5Ba0.5Fe1-xNixO3-δ Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells. Fuel Cells 2016, 16, 258–262. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, J.; Zou, M.; Mu, S.; Huang, H.; Meng, Y.; He, K.; Brinkman, K.S.; Tong, J. Novel twin-perovskite nanocomposite of Ba–Ce–Fe–Co–O as a promising triple conducting cathode material for protonic ceramic fuel cells. J. Power Sources 2020, 450, 227609. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Ding, Y.; Lin, B. A cobalt-free Sm0.5Sr0.5FeO3-δ-BaZr0.1Ce0.7Y0.2O3-δ composite cathode for proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2012, 37, 8630–8634. [Google Scholar] [CrossRef]
- Liu, J.; Ding, J.; Miao, L.; Gong, Z.; Li, K.; Liu, W. High performance Ba0.95Ca0.05Fe0.9-xSnxY0.1O3-δ-SDC as cobalt-free cathode for intermediate-temperature proton-conducting solid oxide fuel cells with BaZr0.1Ce0.7Y0.2O3-δ electrolyte. J. Alloy. Compd. 2019, 786, 163–168. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Wu, C.; Lu, F.; He, H.; Su, J.; Cai, B. Physical properties of (SrBa)1-xPrx(CuTi)0.2Fe0.8O3-δ (x = 0–1.0) and its application in H-SOFCs. Solid State Ion. 2020, 348. [Google Scholar] [CrossRef]
- Lin, B.; Wang, S.; Liu, X.; Meng, G. Stable proton-conducting Ca-doped LaNbO4 thin electrolyte-based protonic ceramic membrane fuel cells by in situ screen printing. J. Alloy. Compd. 2009, 478, 355–357. [Google Scholar] [CrossRef]
- Solís, C.; Vert, V.B.; Fabuel, M.; Serra, J.M. Electrochemical properties of composite cathodes for La0.995Ca0.005NbO4-δ-based proton conducting fuel cells. J. Power Sources 2011, 196, 9220–9227. [Google Scholar] [CrossRef] [Green Version]
- Solís, C.; Navarrete, L.; Roitsch, S.; Serra, J.M. Electrochemical properties of composite fuel cell cathodes for La5.5WO12-δ proton conducting electrolytes. J. Mater. Chem. 2012, 22, 16051–16059. [Google Scholar] [CrossRef]
- Solís, C.; Navarrete, L.; Bozza, F.; Bonanos, N.; Serra, J.M. Catalytic Surface Promotion of Composite Cathodes in Protonic Ceramic Fuel Cells. ChemElectroChem 2015, 2, 1106–1110. [Google Scholar] [CrossRef]
- Strandbakke, R.; Dyrlie, O.; Hage, F.S.; Norby, T. Reaction Kinetics of Protons and Oxide Ions in LSM/Lanthanum Tungstate Cathodes with Pt Nanoparticle Activation. J. Electrochem. Soc. 2016, 163, F507–F515. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Choi, M.B.; Lim, D.K.; Singh, B.; Song, S.J. Effect of humidification on the performance of intermediate-temperature proton conducting ceramic fuel cells with ceramic composite cathodes. J. Power Sources 2013, 232, 224–233. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Q.; Li, J.; Lu, Y.; Wang, L.; Fu, X.Z.; Luo, J.L. Rational design of an in-situ co-assembly nanocomposite cathode La0.5Sr1.5MnO4+δ-La0.5Sr0.5MnO3-δ for lower-temperature proton-conducting solid oxide fuel cells. J. Power Sources 2020, 466, 228240. [Google Scholar] [CrossRef]
- Bausá, N.; Serra, J.M. Robust catalytically-activated LSM-BCZY-based composite steam electrodes for proton ceramic electrolysis cells. Rsc Adv. 2019, 9, 20677–20686. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Zhang, J.; Li, Y.; Li, S.; Kui, X.; Irvine, J.T.S. Composite Oxygen Electrode Based on LSCF and BSCF for Steam Electrolysis in a Proton-Conducting Solid Oxide Electrolyzer. J. Electrochem. Soc. 2012, 160, F763–F767. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Zhao, H.; Shen, Y.; Du, Z.; Zhang, C. Electrochemical properties of BaZr0.1Ce0.7Y0.1Yb0.1O3-δ-Nd1.95NiO4+δ composite cathode for protonic ceramic fuel cells. Int. J. Hydrog. Energy 2015, 40, 2800–2807. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Ricote, S.; Sullivan, N.P. Characterization of ionic transport through BaCe0.2Zr0.7Y0.1O3-δ membranes in galvanic and electrolytic operation. Int. J. Hydrog. Energy 2015, 40, 9278–9286. [Google Scholar] [CrossRef]
- Li, G.; Jin, H.; Cui, Y.; Gui, L.; He, B.; Zhao, L. Application of a novel (Pr0.9La0.1)2(Ni0.74Cu0.21Nb0.05)O4+δ-infiltrated BaZr0.1Ce0.7Y0.2O3-δ cathode for high performance protonic ceramic fuel cells. J. Power Sources 2017, 341, 192–198. [Google Scholar] [CrossRef]
- Tarutin, A.; Danilov, N.; Lyagaeva, J.; Medvedev, D. One-step fabrication of protonic ceramic fuel cells using a convenient tape calendering method. Appl. Sci. 2020, 10, 2481. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Guan, B.; Ma, L.; Hu, S.; Zhang, N.; Liu, X. High performing triple-conductive Pr2NiO4+δ anode for proton-conducting steam solid oxide electrolysis cell. J. Mater. Chem. A 2018, 6, 18057–18066. [Google Scholar] [CrossRef]
- Tang, H.; Gong, Z.; Wu, Y.; Jin, Z.; Liu, W. Electrochemical performance of nanostructured LNF infiltrated onto LNO cathode for BaZr0.1Ce0.7Y0.2O3-δ –based solid oxide fuel cell. Int. J. Hydrog. Energy 2018, 43, 19749–19756. [Google Scholar] [CrossRef]
- Pikalova, E.; Kolchugin, A.; Bogdanovich, N.; Medvedev, D.; Lyagaeva, J.; Vedmid’, L.; Ananyev, M.; Plaksin, S.; Farlenkov, A. Suitability of Pr2–xCaxNiO4+δ as cathode materials for electrochemical devices based on oxygen ion and proton conducting solid state electrolytes. Int. J. Hydrog. Energy 2020, 45, 13612–13624. [Google Scholar] [CrossRef]
- Huan, D.; Shi, N.; Zhang, L.; Tan, W.; Xie, Y.; Wang, W.; Xia, C.; Peng, R.; Lu, Y. New, Efficient, and Reliable Air Electrode Material for Proton-Conducting Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2018, 10, 1761–1770. [Google Scholar] [CrossRef]
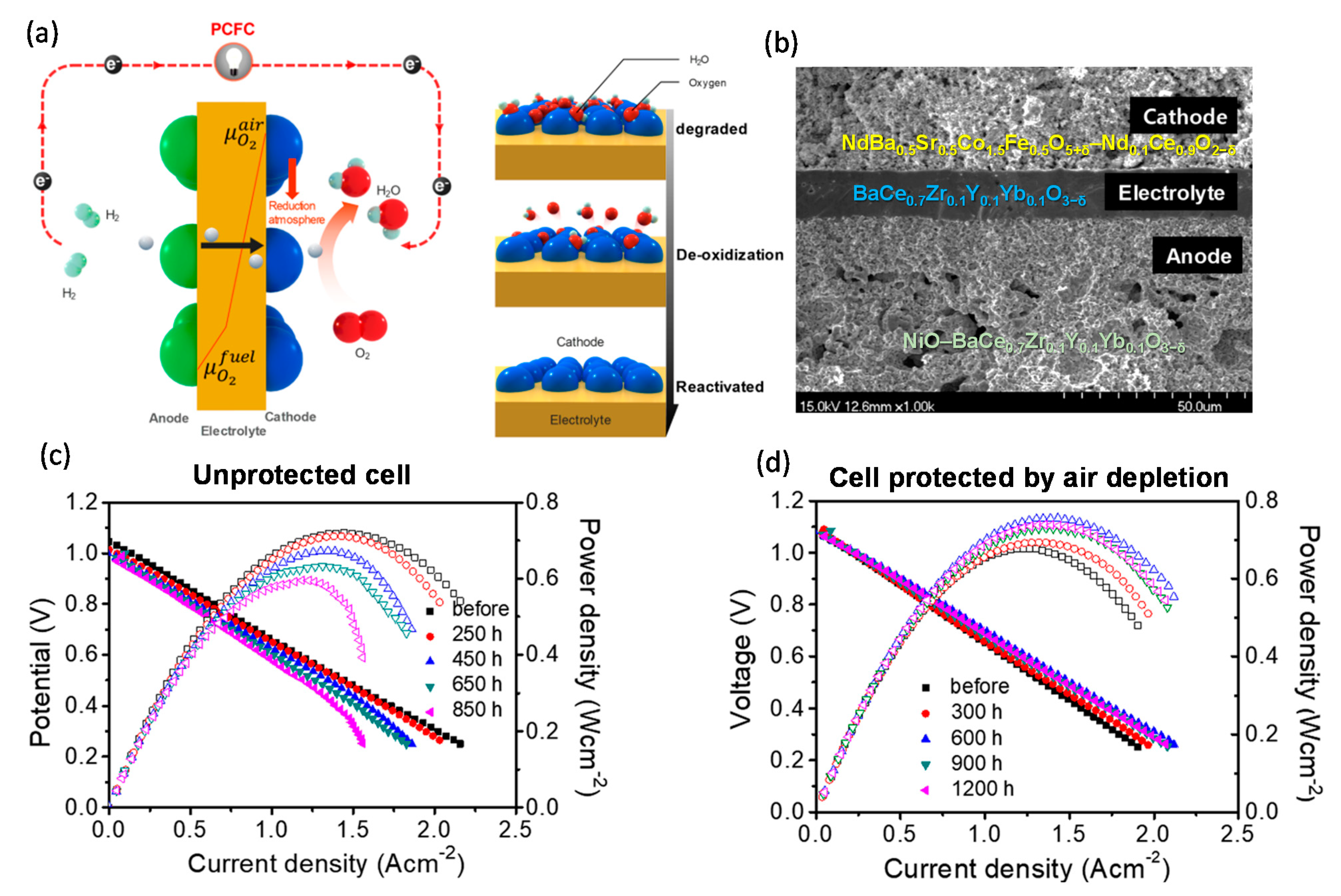
- Park, K.Y.; Kim, Y.D.; Lee, J.I.; Saqib, M.; Shin, J.S.; Seo, Y.; Kim, J.H.; Lim, H.T.; Park, J.Y. Operation Protocols to Improve Durability of Protonic Ceramic Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 457–468. [Google Scholar] [CrossRef]
- Xie, D.; Ling, A.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. A comparative study on the composite cathodes with proton conductor and oxygen ion conductor for proton-conducting solid oxide fuel cell. Electrochim. Acta 2020, 344, 136143. [Google Scholar] [CrossRef]
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.H.; Kim, G. Hybrid-solid oxide electrolysis cell: A new strategy for efficient hydrogen production. Nano Energy 2018, 44, 121–126. [Google Scholar] [CrossRef]
- Liu, B.; Jia, L.; Chi, B.; Pu, J.; Li, J. A novel PrBaCo2O5+δ-BaZr0.1Ce0.7Y0.1Yb0.1O3 composite cathode for proton-conducting solid oxide fuel cells. Compos. Part. B Eng. 2020, 191, 107936. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Y.; Lv, H.; Miao, L.; Bi, L.; Liu, W. PrBaCo2-xTaxO5+δ based composite materials as cathodes for proton-conducting solid oxide fuel cells with high CO2 resistance. Int. J. Hydrog. Energy 2020, 45, 31017–31026. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. Novel PrBa0.9Ca0.1Co2-xZnxO5+δ double-perovskite as an active cathode material for high-performance proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2020, 45, 31009–31016. [Google Scholar] [CrossRef]
- Wei, K.; Li, N.; Wu, Y.; Song, W.; Wang, X.; Guo, L.; Khan, M.; Wang, S.; Zhou, F.; Ling, Y. Characterization and optimization of highly active and Ba-deficient BaCo0.4Fe0.4Zr0.1Y0.1O3-δ-based cathode materials for protonic ceramics fuel cells. Ceram. Int. 2019, 45, 18583–18591. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Yang, L.; Guan, R.; Jiang, J.; Jin, F.; He, T. Ba0.95La0.05Fe0.8Zn0.2O3-δ cobalt-free perovskite as a triple-conducting cathode for proton-conducting solid oxide fuel cells. Ceram. Int. 2020, 46, 18216–18223. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Q.; Lu, L.; Periasamy, V.; Tade, M.O.; Shao, Z. Multi scale and physics models for intermediate and low temperatures H+-solid oxide fuel cells with H+/e-/O2- mixed conducting properties: Part A, generalized percolation theory for LSCF-SDC-BZCY 3-component cathodes. J. Power Sources 2016, 303, 305–316. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Ling, Y.; He, B.; Xu, J.; Zhao, L. Probing novel triple phase conducting composite cathode for high performance protonic ceramic fuel cells. Int. J. Hydrog. Energy 2016, 41, 5074–5083. [Google Scholar] [CrossRef]
- Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells: Stability and cation interdiffusion. Energies 2019, 12, 417. [Google Scholar] [CrossRef] [Green Version]






| Basic Concept | Abbreviation | Basic Concept | Abbreviation |
|---|---|---|---|
| Area Specific Resistance | ASR | Protonic Ceramic Fuel Cells | PCFCs |
| Distribution of Relaxation Times | DRT | Ruddlesden–Popper | RP |
| Electrode Polarization Resistance | Rp | Secondary Ion Mass Spectroscopy | SIMS |
| Electrochemical Impedance Spectroscopy | EIS | Solid Oxide Fuel Cells | SOFCs |
| Maximum Power Density | MPD | Thermogravimetric Analysis | TGA |
| Mixed Oxide-Ion Electron Conductors | MIECs | Triple Phase Boundary | TPB |
| Oxygen Reduction Reaction | ORR | Triple Protonic Oxide-Ionic Electron Hole Conducting Oxides | TCOs |
| Protonic Ceramic Electrolysis Cells | PCECs |
| Composition | Abbreviation | Composition | Abbreviation |
|---|---|---|---|
| BaCe0.8Gd0.2O2.9 | BCG20 | Ce0.5La0.5O1.75 | 50LDC |
| BaCe0.8Sm0.2O3−δ | BCS20 | Ce0.8Sm0.2O2−δ | 20SDC |
| BaCe0.9Sm0.1O3−δ | BCS10 | GdBaCo2O5+δ | GBCO |
| BaCe0.8Y0.2O3−δ | BCY20 | LaCoO3−δ | LCO |
| BaCe0.85Y0.15O3−δ | BCY15 | LaFeO3 | LF |
| BaCe0.9Y0.1O3−δ | BCY10 | La2NiO4±δ | LNO |
| BaCe0.9Yb0.1O3−δ | BCYb10 | LaNi0.6Fe0.4O3−δ | LNF |
| BaCe0.2Zr0.7Y0.1O3−δ | BCZY27 | La0.5Sr0.5CoO3−δ | LSC55 |
| BaCe0.4Zr0.4Y0.2O3−δ | BCZY44 | La0.6Sr0.4CoO3−δ | LSC64 |
| BaCe0.5Zr0.3Y0.2O3−δ | BCZY53 | La0.6Sr0.4Co0.2Fe0.8O3−δ | LSCF |
| BaCe0.6Zr0.2Y0.2O3−δ | BCZY62 | La0.75Sr0.25Cr0.5Mn0.5O3−δ | LSCM |
| BaCe0.7Zr0.1Y0.2O3−δ | BCZY71 | La0.7Sr0.3FeO3−δ | LSF73 |
| BaCe0.8Zr0.1Y0.1O3−δ | BCZY81 | La0.8Sr0.2FeO3−δ | LSF82 |
| BaCe0.4Zr0.4Y0.1Yb0.1O3−δ | BCZYYb4411 | La0.5Sr0.5MnO3−δ | LSM55 |
| BaCe0.7Zr0.1Y0.1Yb0.1O3−δ | BCZYYb7111 | La0.7Sr0.3MnO3−δ | LSM73 |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | BSCF | La0.8Sr0.2MnO3−δ | LSM82 |
| BaZrO3−δ | BZO | NdBa0.5Sr0.5Co1.5Fe0.5O5+δ | NBSCF |
| BaZr0.8Y0.2O3−δ | BZY20 | PrBaCo2O5+δ | PBCO |
| BaZr0.85Y0.15O3−δ | BZY15 | PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | PBSCF |
| BaZr0.9Y0.1O3−δ | BZY10 | Sm0.5Sr0.5FeO3−δ | SSF |
| Ce0.9Gd0.1O2−δ | 10GDC | Sm0.5Sr0.5CoO3−δ | SSC |
| Air Electrode | ASR (Ω·cm2) | MPD (W·cm−2) | Electrolyte | Thickness (µm) | Ref |
|---|---|---|---|---|---|
| Mixed proton–electron-conducting cathodes | |||||
| BaCe0.1Zr0.2Y0.1Fe0.6O3−δ | 0.21 (700 °C) | 0.240 (700 °C) | BCZYYb7111 | 30 | [33] |
| BaCe0.9-yPryGd0.1O3−δ | 0.47 (800 °C) | - | BCG20 | 510 | [21] |
| BaPr0.8In0.2O3−δ | 0.09 (700 °C) | 0.550 (700 °C) | BCZYYb7111 | 15 | [25] |
| BaZr0.2Fe0.6Y0.2O3−δ | 0.28 (700 °C) | 0.322 (700 °C) | BCZY71 | 20 | [32] |
| BaZr0.6Co0.4O3−δ | 0.19 (700 °C) | - | BCZY53 | 20 | [31] |
| Mixed oxide-ion–electron-conducting cathodes | |||||
| Ba0.5La0.5CoO3 | 1.4 (600 °C) | - | Ba(Zr0.5Ce0.4)8/9Y0.2O3−δ | 12 | [45] |
| Ba0.5Sr0.5Fe0.8Zn0.2O3−δ | 0.08 (700 °C) | 0.486 (700 °C) | BCZY71 | 15 | [62] |
| BaCo0.4Fe0.4Ce0.1Gd0.1O3−δ | 0.12 (650 °C) | 0.663 (700 °C) | BCZYYb7111 | 30 | [57] |
| BaCo0.4Fe0.4Ce0.1Y0.1O3−δ | 0.13 (650 °C) | 0.648 (700 °C) | BCZYYb7111 | 30 | [57] |
| BaCo0.4Fe0.4Zr0.2O3−δ | 1 (600 °C) | 0.225 (600 °C) | BCZYYb7111 | 60 | [56] |
| BSCF | 0.06 (700 °C) | 0.800 (700 °C) | BaZr0.4Ce0.45Y0.15O3−δ | 15.5 | [53] |
| Ca3Co4O9+δ | 0.17 (700 °C) | 0.290 (700 °C) | BCZY53 | 20 | [69] |
| Ca3-xLaxCo4O9+δ (x = 0, 0.3) | 2.2 (600 °C) | - | BCY10 | - | [68] |
| La0.6Ba0.4CoO3−δ | - | 0.180 (1000 °C) | BCS10 | 500 | [39] |
| LSC55 | 0.38 (700 °C) | 0.300 (700 °C) | BCZY71 | 500 | [40] |
| LSCF | - | 0.079 (700 °C) | BCZY27 | 20 | [47] |
| LSCF | - | 0.018 (600 °C) | BCZY62 | 500 | [48] |
| LSCF | 1.12 (700 °C) | 0.268 (700 °C) | BCZYYb7111 | 60 | [49] |
| LSCF: BaCO3 | 0.29 (700 °C) | 0.404 (700 °C) | BCZYYb7111 | 60 | [49] |
| LSCF | 0.26 (700 °C) | 0.753 (700 °C) | BaCe0.95Tb0.05O3−δ | 12–15 | [46] |
| Nd0.5Ba0.5Fe0.9Co0.1O3−δ | 0.18 (700 °C) | 0.390 (700 °C) | BCZY53 | 30 | [63] |
| SrCo0.9Nb0.1O3−δ | 0.09 (700 °C) | 0.348 (700 °C) | BCZY44 | 20 | [44] |
| SrCo0.9Sb0.1O3−δ | 0.14 (700 °C) | 0.259 (700 °C) | BCZY71 | 20 | [42] |
| SrFe0.95Nb0.05O3−δ | 0.23 (650 °C) | 0.538 (650 °C) | BCZY71 | 20 | [61] |
| SSC | 10 (700 °C) | 5.9·10−3 (700 °C) | BCZY62 | 500 | [43] |
| Y0.8Ca0.2BaCo4O7 | 0.12 (700 °C) | 0.472 (700 °C) | BCZY71 | 20 | [65] |
| Y0.8Ca0.2BaCo4O7 | - | 0.308 (725 °C) | BCZY53 | 30 | [66] |
| Triple protonic oxide ionic electron hole-conducting oxides | |||||
| Ba(Co0.4Fe0.4Zr0.1Y0.1)0.95O3−δ | 0.80 (550 °C) | 0.840 (650 °C) | Ba(Zr0.1Ce0.7Y0.1Yb0.1)0.95O3−δ | 12 | [119] |
| Ba(Zr0.1Ce0.7Y0.1Yb0.1)0.95O3−δ | 0.61 (550 °C) | 0.450 (550 °C) | BCZYYb7111 | 25 | [121] |
| Ba0.95Ca0.05Co0.4Fe0.4Zr0.1Y0.1O3−δ | 0.36 (700 °C) | 0.580 (700 °C) | BCZYYb4411 | 22 | [120] |
| Ba0.9Co0.4Fe0.4Zr0.1Y0.1O3−δ | 0.52 (500 °C) | 0.797 (650 °C) | BCZY71 | 40 | [118] |
| BaCo0.4Fe0.4Zr0.1Y0.1O3−δ | - | 0.455 (500 °C) | BCZYYb7111 | 20–30 | [115] |
| BaFe0.5Sn0.2Bi0.3O3−δ | 0.03 (700 °C) | 1.280 (700 °C) | BCZY71 | 12 | [125] |
| BaGd0.8La0.2Co2O6−δ | 0.05 (650 °C) | - | BCZY71 | - | [100] |
| BSCF | 0.18 (650 °C) | 0.622 (650 °C) | BCZYYb7111 | 21.3 | [106] |
| GBCO | 0.16 (700 °C) | 0.266 (700 °C) | BCZY71 | 10 | [94] |
| GdBaCuCoO5+x | 0.17 (700 °C) | 0.480 (700 °C) | BCZY71 | 20 | [107] |
| La1.2Sr0.8Ni0.6Fe0.4O4−δ | 0.08 (700 °C) | 0.781 (700 °C) | BCZY71 | 15 | [86] |
| La1.5Ca0.5NiO4−δ | 0.05 (700 °C) | 0.923 (700 °C) | BCZY71 | 15 | [87] |
| La3Ni1.6Fe0.4O7 | 0.15 (700 °C) | 0.398 (700 °C) | BCZY53 | 20 | [88] |
| LaBaCuCoO5-x | 0.15 (700 °C) | 0.432 (700 °C) | BCZY71 | 20 | [97] |
| LaBaCuFeO5+x | 0.27 (700 °C) | 0.327 (700 °C) | BCZY71 | 20 | [97] |
| LaNi0.6Fe0.4O3−δ | 0.13 (700 °C) | 0.550 (700 °C) | BCZY71 | 15 | [90] |
| LiNi0.8Co0.2O2 | 3.48 (650 °C) | 0.410 (650 °C) | BCZY71 | 24 | [92] |
| NBSCF | 0.08 (700 °C) | 1.370 (700 °C) | BCZYYb7111 | 14.7 | [98] |
| Nd2NiO4−δ | 4.8 (600 °C) | 0.060 (600 °C) | BCY10 | 25 | [79] |
| PBSCF | 0.13 (600 °C) | 0.500 (500 °C) | BCZYYb4411 | 15 | [101] |
| PBSCF | 0.30 (600 °C) | 0.650 (600°C) | BCZYYb6211 | 7.6 | [103] |
| PBSCF | - | 1.100 (650 °C) | BCZYYb4411 | 15 | [102] |
| Pr2BaNiMnO7−δ | 0.084 (700 °C) | 1.070 (700 °C) | BCZYYb7111 | 20 | [108] |
| Pr2NiO4−δ | 0.70 (650 °C) | 0.130 (650 °C) | BCY10 | 85 | [77] |
| Pr2NiO4−δ | 0.06 (650 °C) | 0.820 (650 °C) | BaCe0.55Zr0.3Y0.15O3−δ | 5 | [82] |
| (PrBa0.8Ca0.2)0.95Co2O6−δ | 0.14 (600 °C) | 0.540 (600 °C) | BCZYYb6211 | 20 | [105] |
| PrNi0.5Co0.5O3−δ | 0.05 (500 °C) | 0.611 (600 °C) | BCZYYb4411 | 10 | [114] |
| SmBa0.5Sr0.5Co2O5+δ | 0.08 (700 °C) | 0.533 (700 °C) | BCZY71 | 15 | [97] |
| SmBaCo2O5+x | 0.15 (700 °C) | 0.382 (700 °C) | BCZY71 | 25 | [95] |
| Sr2Fe1.5Mo0.4Zr0.1O6−δ | 0.17 (700 °C) | 0.790 (700 °C) | BCZYYb7111 | 20 | [159] |
| Sr2Sc0.1Nb0.1Co1.5Fe0.3O6−δ | 0.14 (650 °C) | 0.840 (650 °C) | BCZYYb7111 | 18.5 | [106] |
| Composite cathodes | |||||
| Ba0.4K0.1Sr0.5Co0.8Fe0.2O3−δ: BCZY71 | 0.05 (700 °C) | 1.275 (750 °C) | BCZY71 | 10 | [152] |
| Ba0.95Ca0.05Fe0.85Sn0.05Y0.1O3−δ: SDC | 0.05 (700 °C) | 0.950 (700 °C) | BCZY71 | 17 | [171] |
| Ba0.95La0.05Fe0.8Zn0.2O3−δ: BCZY71 | 0.08 (750 °C) | 0.330 (750 °C) | BCZYYb7111 | 42 | [197] |
| Ba0.9Co0.4Fe0.4Zr0.1Y0.1O3−δ: BCZY71 | 0.15 (700 °C) | 0.540 (700 °C) | BCZY71 | 25 | [196] |
| Ba4Sr2Sm2Co4O15: BaCe0.5Pr0.3Y0.2O3−δ | 0.20 (600 °C) | 0.197 (600 °C) | BCY10 | 60 | [139] |
| BaCe0.4Fe0.4Co0.2O3−δ | 0.075 (700 °C) | 0.335 (700 °C) | BCZYYb7111 | 70 | [169] |
| BaCo0.4Fe0.4Zr0.1Y0.1O3−δ: BCZYYb7111 | 0.25 (600 °C) | 0.660 (600 °C) | BCZYYb7111 | 10–20 | [153] |
| BSCF: BCY10 | 0.53 (600 °C) | 0.293 (700 °C) | BCY10 | 15 | [149] |
| BSCF: BCZY71 | 0.10 (700 °C) | 0.420 (700 °C) | BCZY71 | 20 | [148] |
| BSCF: BCZYYb7111 | 0.27 (600 °C) | 0.420 (600 °C) | BCZYYb7111 | 4 | [150] |
| Ca0.3Y0.7Fe0.5Co0.5O3−δ: BCZY71 | 0.07 (750 °C) | 0.798 (750 °C) | BCZY71 | 150 | [146] |
| La0.35Pr0.15Sr0.5FeO3−δ: Pr | 0.06 (700 °C) | 1.080 (700 °C) | BCZY71 | 18 | [166] |
| La0.8Ba0.2CoO3−δ: BaZr0.6Co0.4O3−δ | 1.54 (600 °C) | - | BZY10 | - | [140] |
| La0.6Ba0.4CoO3−δ: BaZr0.8Co0.2O3−δ | 1.76 (600 °C) | - | BZY10 | - | [140] |
| La0.5Sr1.5MnO4−δ: LSM55 | 0.08 (700 °C) | 0.940 (700 °C) | BCZY71 | 12 | [179] |
| La0.62Ba0.38CoO3−δ: BaZr0.68Y0.07Co0.25O3−δ | 0.21 (600 °C) | - | BZY10 | - | [141] |
| La0.6Ba0.4CoO3−δ: BCZYYb7111 | 0.02 (700 °C) | 1.000 (700 °C) | BCZYYb7111 | 10 | [147] |
| La2NiO4+δ: LaNi0.6Fe0.4O3−δ | 0.09 (700 °C) | 0.590 (700 °C) | BCZY71 | 20 | [164] |
| La2NiO4+δ: BCZY71 | 6.7 (700 °C) | 0.032 (700 °C) | BCZY71 | 25 | [183] |
| La2NiO4+δ: LaNi0.6Fe0.4O3−δ | 0.03 (700 °C) | 0.969 (700 °C) | BCZY71 | - | [187] |
| LaCoO3: BCZY27 | 0.11 (600 °C) | - | BCZY27 | - | [138] |
| LSCF: BaCe0.5Zr0.35Y0.15O3−δ | 0.18 (700 °C) | 0.537 (700 °C) | BaCe0.5Zr0.35Y0.15O3−δ | 8 | [145] |
| LSCF: BCYb10 | 0.14 (700 °C) | 0.050 (700 °C) | BCY20 | 15–20 | [143] |
| LSCF: BCY10 | 0.49 (600 °C) | - | BZY20 | - | [144] |
| LSF73: 20SDC | 0.074 (650 °C) | 0.542 (650 °C) | BCZY71 | 13 | [163] |
| LSF73: BCZY71 | 0.13 (700 °C) | 0.450 (700 °C) | BCZY71 | 15 | [162] |
| LSF82: Ba(Ce0.51Zr0.30Y0.15Zn0.04)O3−δ | 0.09 (700 °C) | 0.425 (700 °C) | Ba(Ce0.51Zr0.30Y0.15Zn0.04)O3−δ | 70 | [156] |
| LSM82: La0.995Ca0.005NbO4 | 25 (750 °C) | - | La0.995Ca0.005NbO4 | - | [174] |
| LSM82: La5.5WO12−δ | 8 (750 °C) | - | La5.5WO12-δ | - | [175] |
| LSM82: BCY15 | 0.11 (750 °C) | 0.394 (750 °C) | BCY15 | 15 | [178] |
| LSM82: La5.5WO11.25−δ: 20SDC | 1.4 (750 °C) | - | La5.5WO11.25−δ | - | [176] |
| LSM82: La28-xW4+xO54+3x/2 | 40 (650 °C) | - | La28-xW4+xO54+3x/2 | - | [177] |
| LSM82: 50LDC | 0.69 (800 °C) | 0.065 (800 °C) | La0.99Ca0.01NbO4 | 20 | [173] |
| LSM82: BCZY71: Pr | 0.33 (700 °C) | BCZY71 | - | [180] | |
| Nd(Ba0.75Ca0.25)Co1.5Fe0.4Ni0.1O5+δ: 10GDC | 0.091 (650 °C) | 0.880 (650 °C) | BCZYYb7111 | 20 | [191] |
| Nd0.5Ba0.5Fe0.9Ni0.1O3−δ: BCZY71 | 0.15 (700 °C) | 0.490 (700 °C) | BCZY71 | 40 | [151] |
| Nd0.5Ba0.5Fe0.9Ni0.1O3−δ: BCZY71 | 0.15 (700 °C) | 0.490 (700 °C) | BCZY71 | 40 | [168] |
| Nd1.95NiO4−δ: BCZYYb7111 | 0.43 (750 °C) | 0.154 (750 °C) | BCZYYb7111 | 60 | [182] |
| NdBa0.5Sr0.5Co1.5Fe0.5O5−δ: Ce0.9Nd0.1O2−δ | 0.18 (650 °C) | 0.719 (650 °C) | BCZYYb7111 | 20 | [190] |
| PBCO: BCZY71: 10GDC | 0.05 (700 °C) | 1.020 (700 °C) | BCZY71 | 12 | [199] |
| PBCO: BCZYYb7111 | 0.08 (750 °C) | 0.490 (750 °C) | BCZYYb7111 | 30 | [193] |
| Pr0.58Sr0.4Fe0.8Co0.2O3−δ: BCYb10 | 0.50 (700 °C) | - | BCYb10 | 20 | [167] |
| Pr0.6Sr0.4Cu0.2Fe0.8O3−δ: 20SDC | 0.14 (650 °C) | 0.456 (650 °C) | BCZY53 | 20 | [165] |
| Pr0.6Sr0.4Cu0.2Fe0.8O3−δ: 20SDC | - | 0.556 (650 °C) | BCZY71 | 14 | [165] |
| (Pr0.9La0.1)2(Ni0.74Cu0.21Nb0.05)O4+δ: BCZY71 | 0.13 (700 °C) | 0.770 (700 °C) | BCZY71 | 12 | [184] |
| Pr1.7Ca0.3NiO4+δ: BaCe0.8Gd0.19Cu0.01O3−δ | 0.26 (700 °C) | 0.170 (700 °C) | BaCe0.8Gd0.19Cu0.01O3−δ | 25 | [188] |
| Pr1.9Ba0.1NiO4−δ:BaCe0.5Zr0.3Dy0.2O3−δ | 0.43 (700 °C) | 0.470 (700 °C) | BaCe0.5Zr0.3Dy0.2O3−δ | 30 | [185] |
| Pr2NiO4: BCZY62 | 0.31 (700 °C) | 0.977 (700 °C) | BCZY62 | 20 | [186] |
| PrBa0.9Ca0.1Co1.85Zn0.15O5+δ: BCZYYb7111 | 0.04 (750 °C) | 0.870 (750 °C) | BCZYYb7111 | 17 | [195] |
| PrBaCo1.75Ta0.25O5+δ: BCZYYb7111 | 0.05 (700 °C) | 0.755 (700 °C) | BCZYYb7111 | 10 | [194] |
| Sr2Fe1.5Mo0.5O6−δ: BZY20 | 0.48 (600 °C) | - | BZY20 | 16 | [160] |
| (SrBa)0.6Pr0.4(CuTi)0.2Fe0.8O3−δ: 20SDC | 0.07 (750 °C) | 0.925 (750 °C) | BCZY71 | 13 | [172] |
| SrCo0.7Fe0.2Zr0.1O3−δ: BCZY71 | - | 0.129 (700 °C) | BCZY71 | - | [142] |
| SrCo0.8Fe0.15Zr0.05O3−δ: BCZYYb7111 | 0.07 (700 °C) | 0.712 (700 °C) | BCZYYb7111 | 38 | [132] |
| SrEu2Fe1.8Co0.2O7−δ: BCZY71 | 0.13 (700 °C) | 0.560 (700 °C) | BCZY71 | 15 | [189] |
| SSC: BaCe0.5Zr0.35Y0.15O3−δ | 0.18 (700 °C) | 0.642 (700 °C) | BaCe0.5Zr0.35Y0.15O3−δ | 10 | [131] |
| SSC: BaCe0.5Zr0.3Y0.16Zn0.04O3−δ | 0.15 (700 °C) | 0.528 (700 °C) | BaCe0.5Zr0.3Y0.16Zn0.04O3−δ | 20 | [128] |
| SSC: BCS20 | 0.21 (700 °C) | 0.240 (700 °C) | BCS20 | 70 | [127] |
| SSC: BZY20 | 0.3 (600 °C) | 0.300 (600 °C) | BCZY71 | 20 | [130] |
| SSC: BCZY81 | 0.10 (700 °C) | 0.529 (700 °C) | BCZY81 | 9 | [129] |
| SSC: BZY20 | 0.30 (600 °C) | 0.300 (600 °C) | BCZY71 | 20 | [130] |
| SSC: BZY20 | 0.08 (600 °C) | 0.600 (600 °C) | BZY20 | 15 | [132] |
| SSC: SmBaCo2O5+δ | 0.02 (750 °C) | 1.570 (750 °C) | BCZYYb7111 | 30 | [137] |
| SSF: BCZY71 | 0.10 (700 °C) | 0.341 (700 °C) | BCZY71 | 20 | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mather, G.C.; Muñoz-Gil, D.; Zamudio-García, J.; Porras-Vázquez, J.M.; Marrero-López, D.; Pérez-Coll, D. Perspectives on Cathodes for Protonic Ceramic Fuel Cells. Appl. Sci. 2021, 11, 5363. https://doi.org/10.3390/app11125363
Mather GC, Muñoz-Gil D, Zamudio-García J, Porras-Vázquez JM, Marrero-López D, Pérez-Coll D. Perspectives on Cathodes for Protonic Ceramic Fuel Cells. Applied Sciences. 2021; 11(12):5363. https://doi.org/10.3390/app11125363
Chicago/Turabian StyleMather, Glenn C., Daniel Muñoz-Gil, Javier Zamudio-García, José M. Porras-Vázquez, David Marrero-López, and Domingo Pérez-Coll. 2021. "Perspectives on Cathodes for Protonic Ceramic Fuel Cells" Applied Sciences 11, no. 12: 5363. https://doi.org/10.3390/app11125363
APA StyleMather, G. C., Muñoz-Gil, D., Zamudio-García, J., Porras-Vázquez, J. M., Marrero-López, D., & Pérez-Coll, D. (2021). Perspectives on Cathodes for Protonic Ceramic Fuel Cells. Applied Sciences, 11(12), 5363. https://doi.org/10.3390/app11125363











