Spherical Polydopamine-Modified Carbon-Felt Cathode with an Active Indole Structure for Efficient Hydrogen Peroxide Electroproduction
Abstract
1. Introduction
2. Experiment
2.1. Materials and Reagents
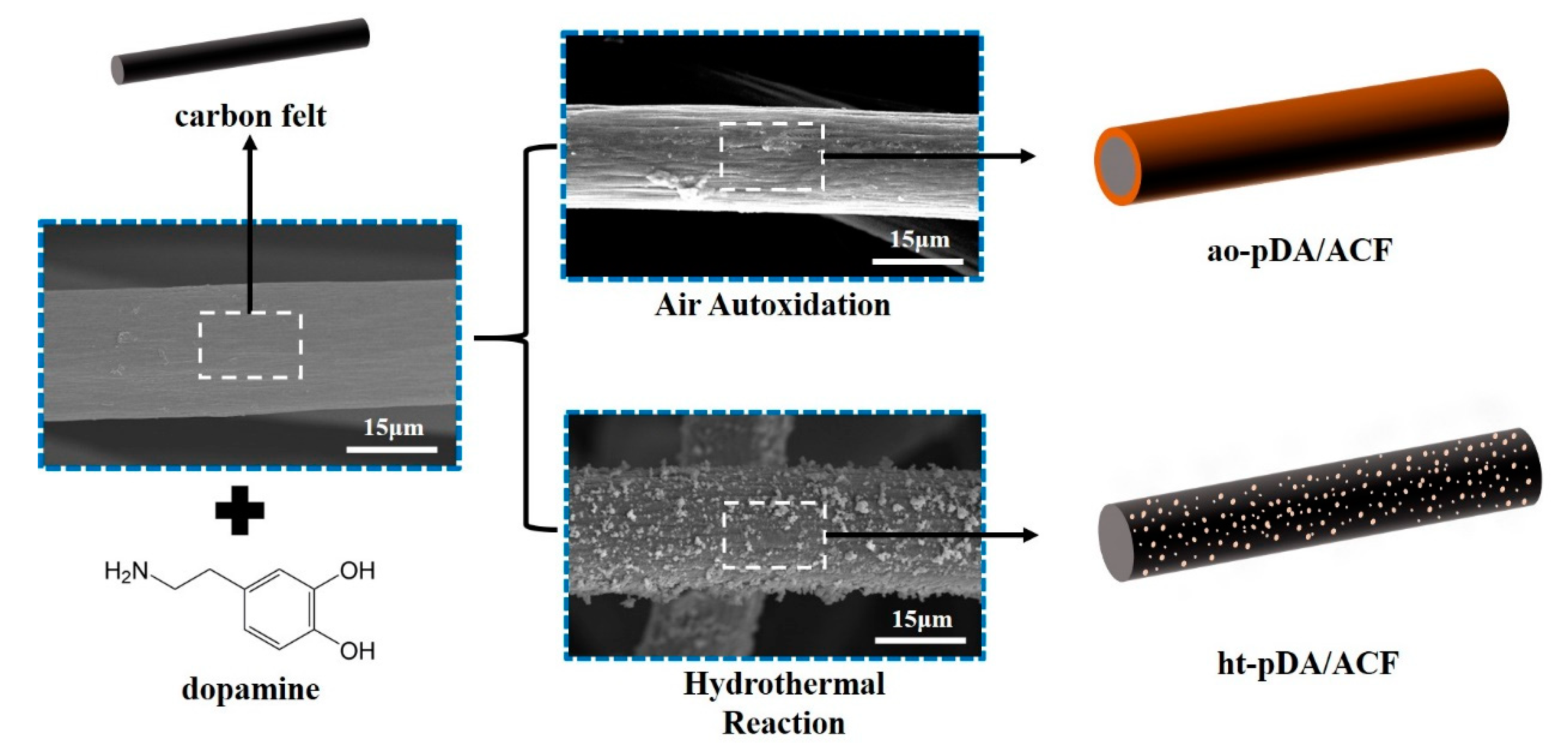
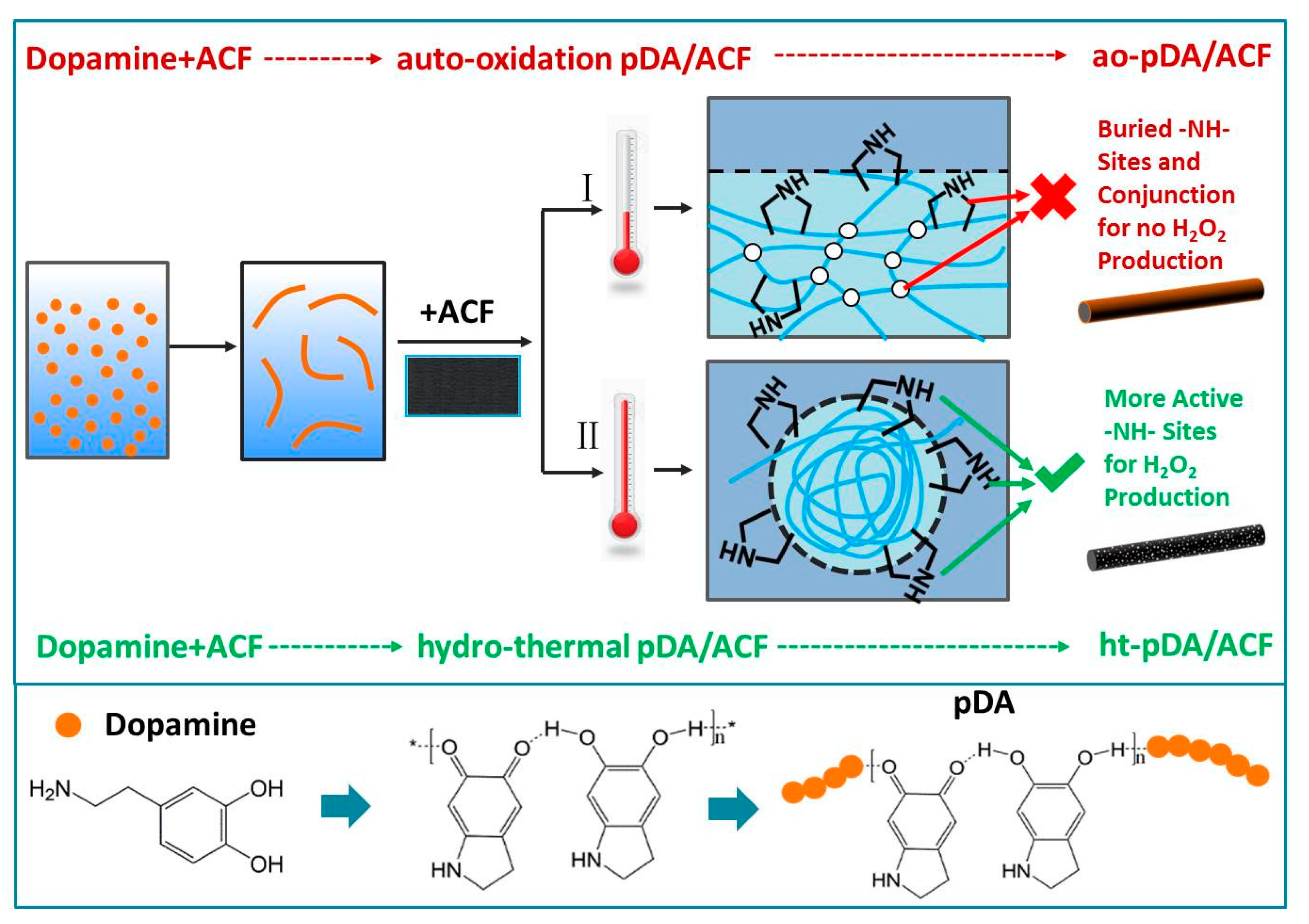
2.2. Preparation of ht-pDA/ACF and ao-pDA/ACF
2.3. Electrocatalytic ORR for H2O2 Synthesis at the ht-pDA/ACF Cathode
2.4. Characterization
3. Results and Discussion
3.1. Cyclic Voltammetry Test
3.2. H2O2 Production
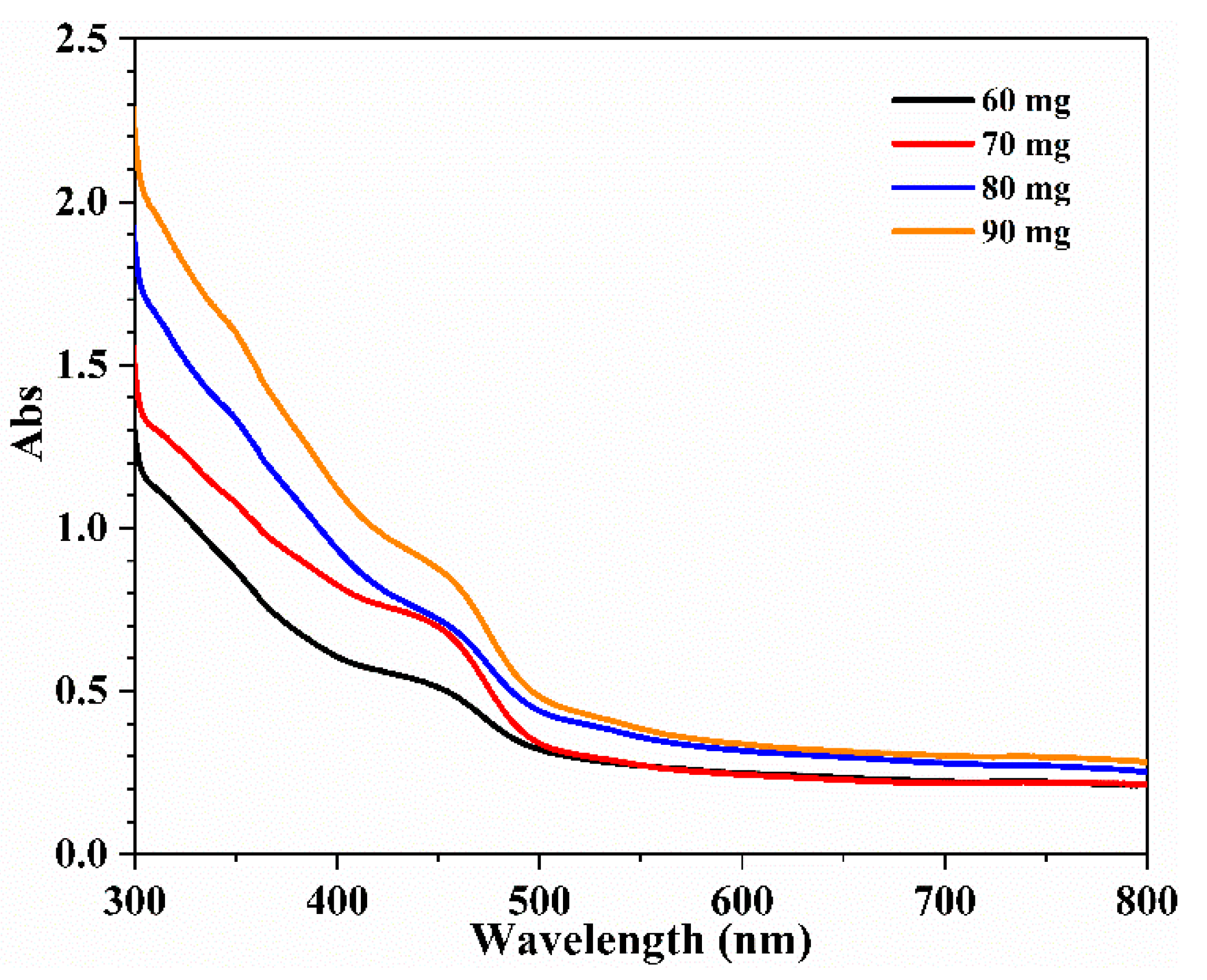
3.3. UV-Visible Spectra
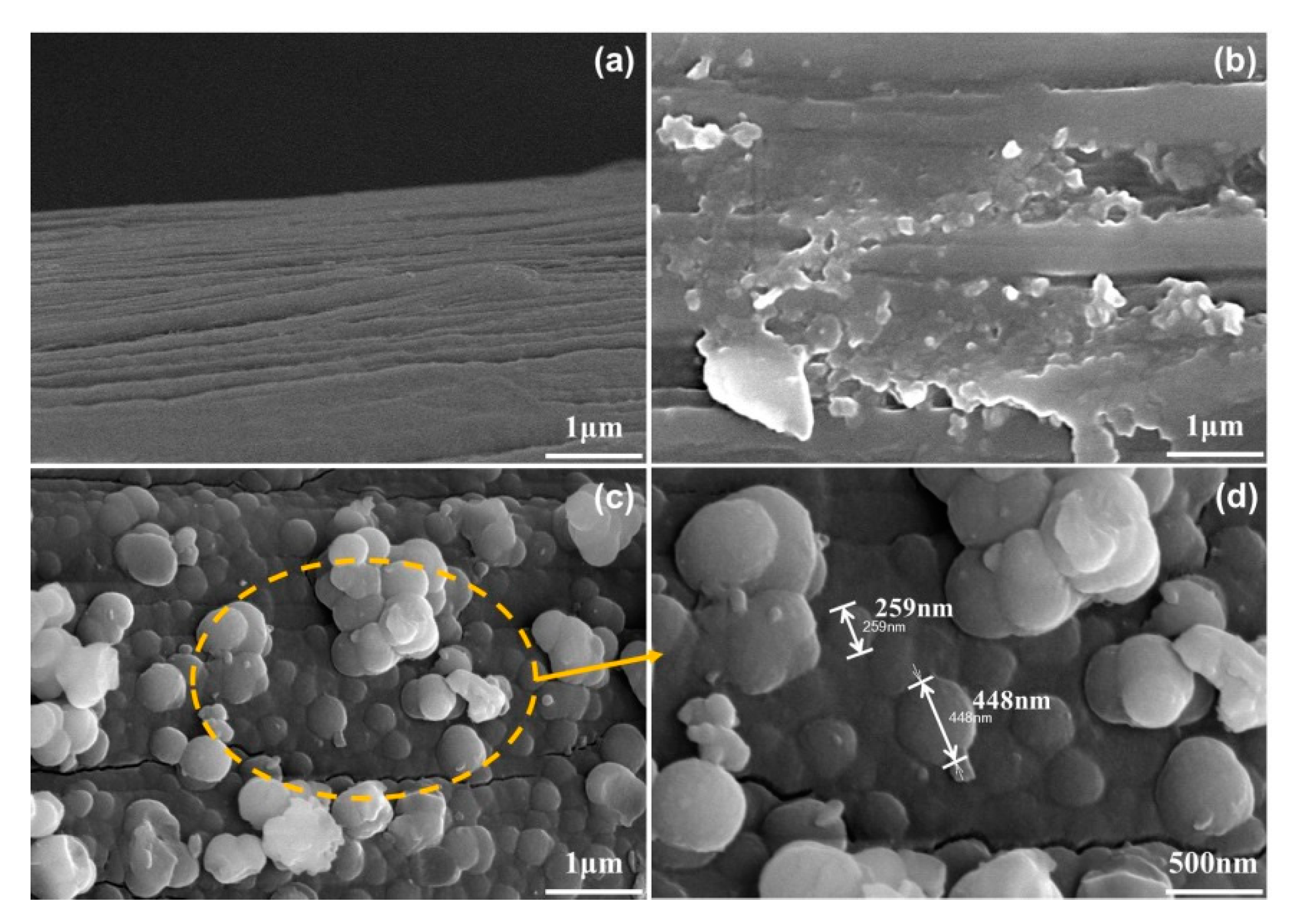
3.4. SEM
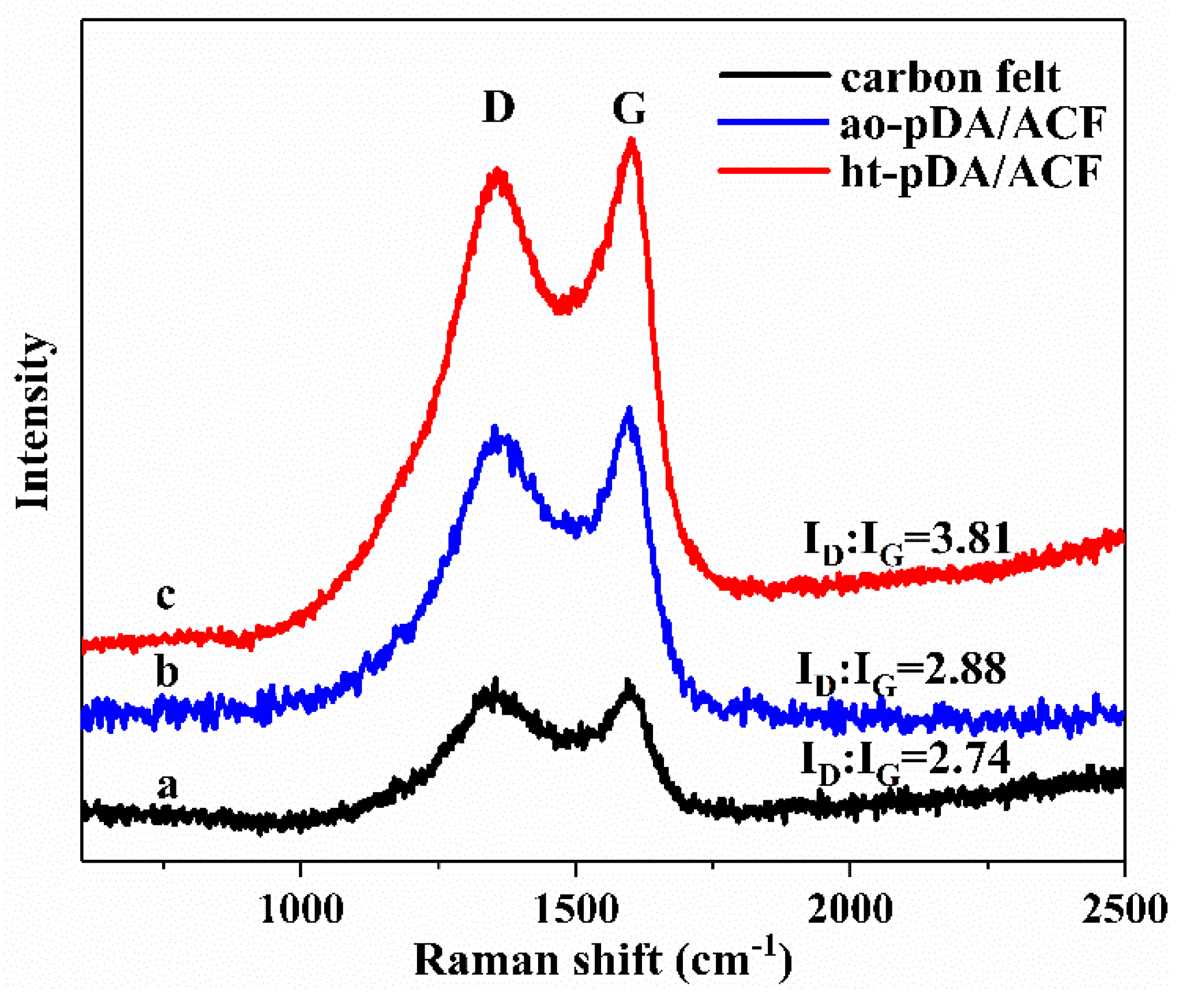
3.5. Raman Spectra
3.6. FT-IR Spectra
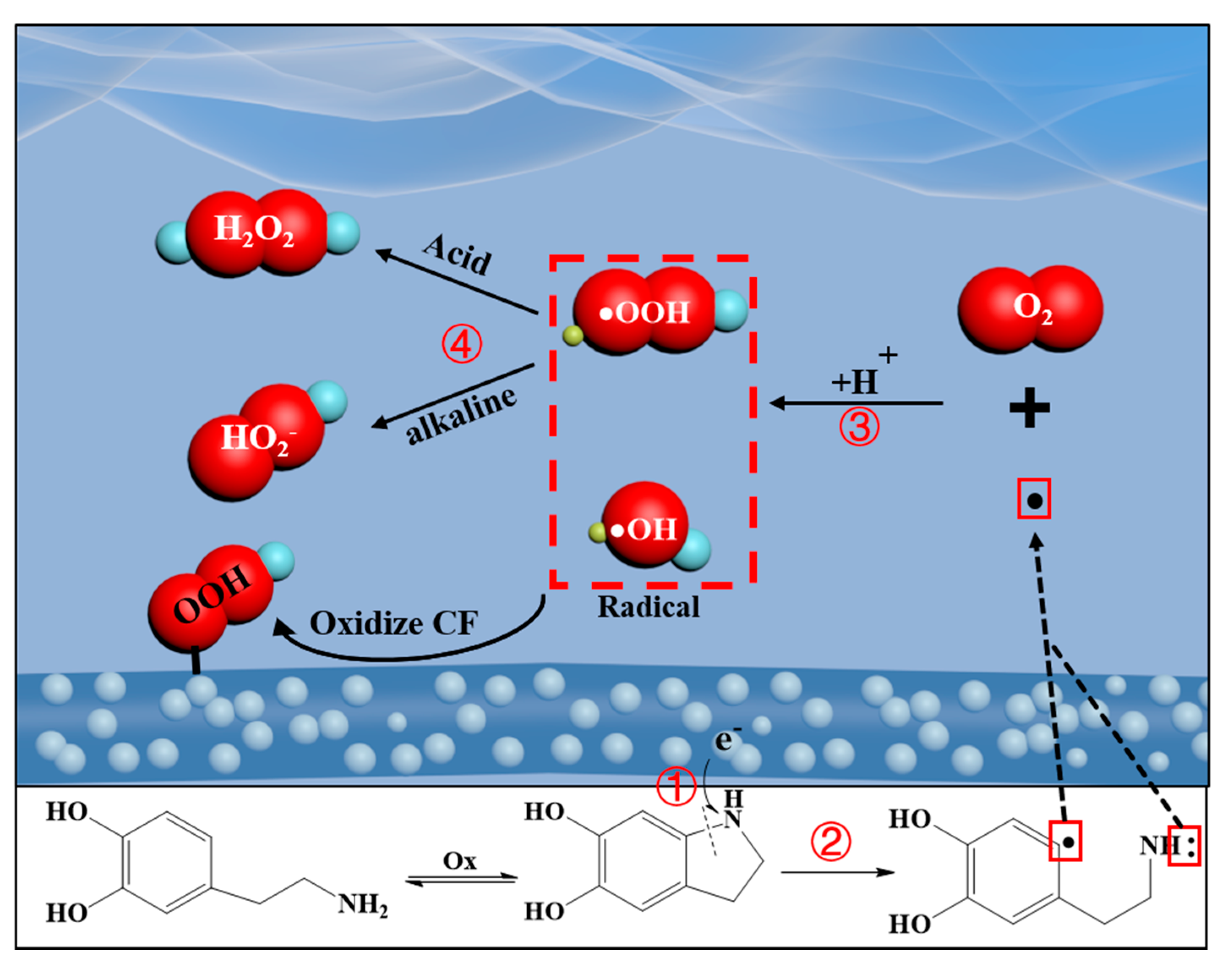
3.7. Mechanistic Analysis of ht-pDA/ACF Formation and the Production of H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, L.; Li, G.; Guo, H. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: Noble-metal catalytic method, fuel-cell method and plasma method. Catal. Sci. Technol. 2016, 6, 1593–1610. [Google Scholar] [CrossRef]
- Flaherty, D.W. Direct Synthesis of H2O2 from H2 and O2 on Pd Catalysts: Current Understanding, Outstanding Questions, and Research Needs. ACS Catal. 2018, 8, 1520–1527. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A.N. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review. Chemosphere 2019, 225, 588–607. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Li, B.; Zhao, C.; Liu, J.; Zhang, Q. Electrosynthesis of Hydrogen Peroxide Synergistically Catalyzed by Atomic Co–N x –C Sites and Oxygen Functional Groups in Noble-Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, e1808173. [Google Scholar] [CrossRef] [PubMed]
- Jirkovský, J.S.; Panas, I.; Ahlberg, E.; Halasa, M.; Romani, S.; Schiffrin, D.J. Single Atom Hot-Spots at Au–Pd Nanoalloys for Electrocatalytic H2O2 Production. J. Am. Chem. Soc. 2011, 133, 19432–19441. [Google Scholar] [CrossRef] [PubMed]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef]
- Verdaguer-Casadevall, A.; Deiana, D.; Karamad, M.; Siahrostami, S.; Malacrida, P.; Hansen, T.W.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Trends in the Electrochemical Synthesis of H2O2: Enhancing Activity and Selectivity by Electrocatalytic Site Engineering. Nano Lett. 2014, 14, 1603–1608. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Cai, J.; Liang, L.; Ren, G.; Jiang, L. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. J. Mater. Chem. A 2017, 5, 8070–8080. [Google Scholar] [CrossRef]
- Contreras, E.; Dominguez, D.; Tiznado, H.; Guerrero-Sanchez, J.; Takeuchi, N.; Alonso-Nunez, G.; Contreras, O.E.; Oropeza-Guzmán, M.T.; Romo-Herrera, J.M. N-Doped carbon nanotubes enriched with graphitic nitrogen in a buckypaper configuration as efficient 3D electrodes for oxygen reduction to H2O2. Nanoscale 2019, 11, 2829–2839. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, L.; Wu, P.; Xu, R.; He, J.; Jiang, W. Improved H2O2 photogeneration by KOH-doped g-C3N4 under visible light irradiation due to synergistic effect of N defects and K modification. Appl. Surf. Sci. 2020, 527, 146584. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Zheng, K.; Li, F.; Ma, G.; Yao, H.-C.; Wang, X.; Chen, Y. KOH-treated reduced graphene oxide: 100% selectivity for H2O2 electroproduction. Carbon 2019, 153, 6–11. [Google Scholar] [CrossRef]
- Xu, Y.; Mo, Y.; Tian, J.; Wang, P.; Yu, H.; Yu, J. The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites. Appl. Catal. B Environ. 2016, 181, 810–817. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, X.; Fan, X.; Wang, H.; Chen, S. High-Yield Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction by Hierarchically Porous Carbon. Angew. Chem. Int. Ed. 2015, 54, 6837–6841. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous Nitrogen-Doped Carbon for the Electrocatalytic Synthesis of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef] [PubMed]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-N x /C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, Y.; Lei, W.-X.; Wan, L.; Ji, J.; Xu, Z. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Ma, B.; Hung, C.-T.; Li, W.; Zhao, D. Spherical Mesoporous Materials from Single to Multilevel Architectures. Accounts Chem. Res. 2019, 52, 2928–2938. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.; Chen, Z.; Duan, Y.; Lai, Y.; Fang, Q.; Wang, F.; Li, S. Performance of boron-doped graphene aerogel modified gas diffusion electrode for in-situ metal-free electrochemical advanced oxidation of Bisphenol A. Appl. Catal. B Environ. 2019, 255, 117784. [Google Scholar] [CrossRef]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon Dots Prepared by Hydrothermal Treatment of Dopamine as an Effective Fluorescent Sensing Platform for the Label-Free Detection of Iron(III) Ions and Dopamine. Chem. A Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Jin, S.; Zhou, Y.; Ji, Q.; Lan, H.; Liu, H.; Qu, J. Graphitic N in nitrogen-Doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction. Carbon 2020, 163, 154–161. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. BiVO4/CeO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3718–3723. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Cai, W.; Zhang, S.; Cui, S. Revealing the formation mechanism of insoluble polydopamine by using a simplified model system. Polym. Chem. 2017, 8, 860–864. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]



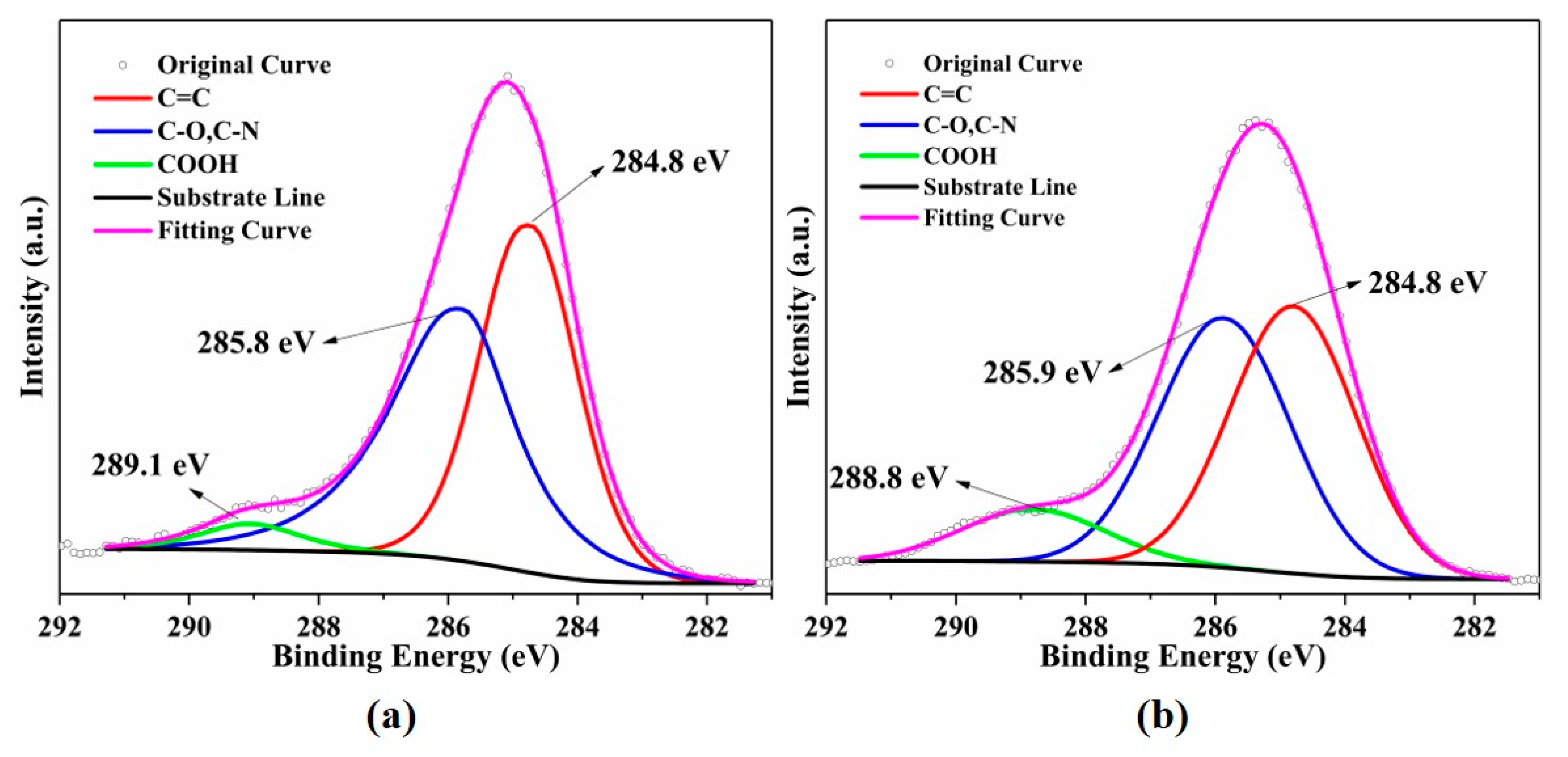

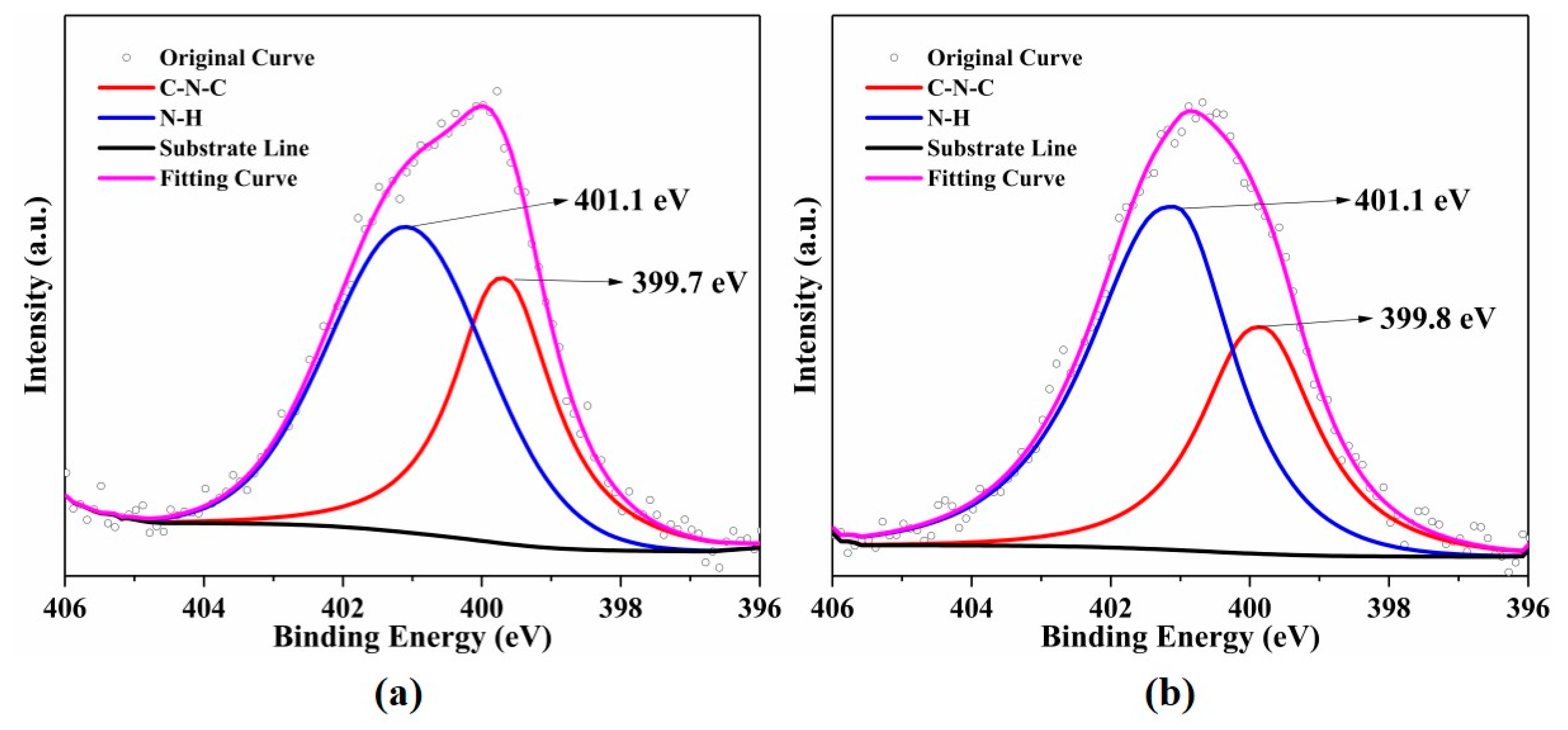

| As-Prepared (%) | After (%) | |||
|---|---|---|---|---|
| Element (%) | C 1s | 76.71 | 76.58 | |
| N 1s | 17.03 | 20.07 | ||
| O 1s | 6.25 | 2.70 | ||
| Group (%) | C 1s | C=C | 47.57 | 45.34 |
| C-N, C-O | 48.64 | 44.48 | ||
| -COOH | 3.79 | 10.18 | ||
| N 1s | C-N-C | 38.95 | 35.97 | |
| N-H | 61.05 | 64.03 | ||
| O 1s | O-C | 70.22 | 53.82 | |
| O=C | 29.78 | 46.18 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yuan, S.; Wang, H.; Zhu, Y.; Fu, D.; Li, Z. Spherical Polydopamine-Modified Carbon-Felt Cathode with an Active Indole Structure for Efficient Hydrogen Peroxide Electroproduction. Appl. Sci. 2021, 11, 5371. https://doi.org/10.3390/app11125371
Chen L, Yuan S, Wang H, Zhu Y, Fu D, Li Z. Spherical Polydopamine-Modified Carbon-Felt Cathode with an Active Indole Structure for Efficient Hydrogen Peroxide Electroproduction. Applied Sciences. 2021; 11(12):5371. https://doi.org/10.3390/app11125371
Chicago/Turabian StyleChen, Lei, Sicheng Yuan, Huaiyuan Wang, Yanji Zhu, Dengyu Fu, and Zhenggui Li. 2021. "Spherical Polydopamine-Modified Carbon-Felt Cathode with an Active Indole Structure for Efficient Hydrogen Peroxide Electroproduction" Applied Sciences 11, no. 12: 5371. https://doi.org/10.3390/app11125371
APA StyleChen, L., Yuan, S., Wang, H., Zhu, Y., Fu, D., & Li, Z. (2021). Spherical Polydopamine-Modified Carbon-Felt Cathode with an Active Indole Structure for Efficient Hydrogen Peroxide Electroproduction. Applied Sciences, 11(12), 5371. https://doi.org/10.3390/app11125371






