Polymeric Coatings for AR-Glass Fibers in Cement-Based Matrices: Effect of Nanoclay on the Fiber-Matrix Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fibers
2.2. Coating Preparation and Characterization
- First, 2.5 g of nano-clay particles were slowly added to 50 mL distilled water in a 100 mL round flask under vigorous stirring. The mixture was stirred for about 5 min and then was placed in an ultrasonic bath at 40 °C for 1 h.
- The mixture was then refluxed for 3 h at 80 °C until a homogeneous dispersion was obtained. Once the dispersion was cooled down, it was filtered with a 14–19 μm filter in order to remove possible agglomerated particles. In order to exclude the presence of agglomerates, the particles size distribution of the nanoclay in the aqueous dispersion was analyzed with Malvern Zetasizer Nano ZS Particle and ZetaPotential Analyzer. The distribution obtained was found equal to that reported in the technical sheet for CloisiteNa, which indicates that the nanoclays were well dispersed;
- The nanoclay dispersion was stirred for 15 min with the SB latex and distilled water. The proportion between nanoclay dispersion (MMTD), SB latex (SB), and distilled water (HO) was calculated as follows: first, the amount of SB latex and MMTD dispersion were determined according to Equations (1) and (2), and then the obtained dispersion was diluted with distilled water in order to obtain a final solid content of 20% (Equation (3)).
- x is the polymer solid fraction in the SB-MMT coating;
- SBMMT is the desired amount of SB-MMT coating expressed in gram;
- scSBC is the SB latex solid content determined according to DIN EN ISO 3251;
- scMMTD is the solid content of the MMTD dispersion determined according to DIN EN ISO 3251.
2.3. Cementitious Matrix
- CEM II/B-LL 42.5 R—165 kg/m;
- CEM II/B-LL 32.5 R—83 kg/m;
- Hydrated lime—110 kg/m;
- Calcium carbonate 600—206 kg/m;
- Calcium carbonate 400—715 kg/m;
- Vac/VeVa/E—21 kg/m.
2.4. Single Fiber Pull-Out Test
2.5. Hydration Products
3. Results and Discussion
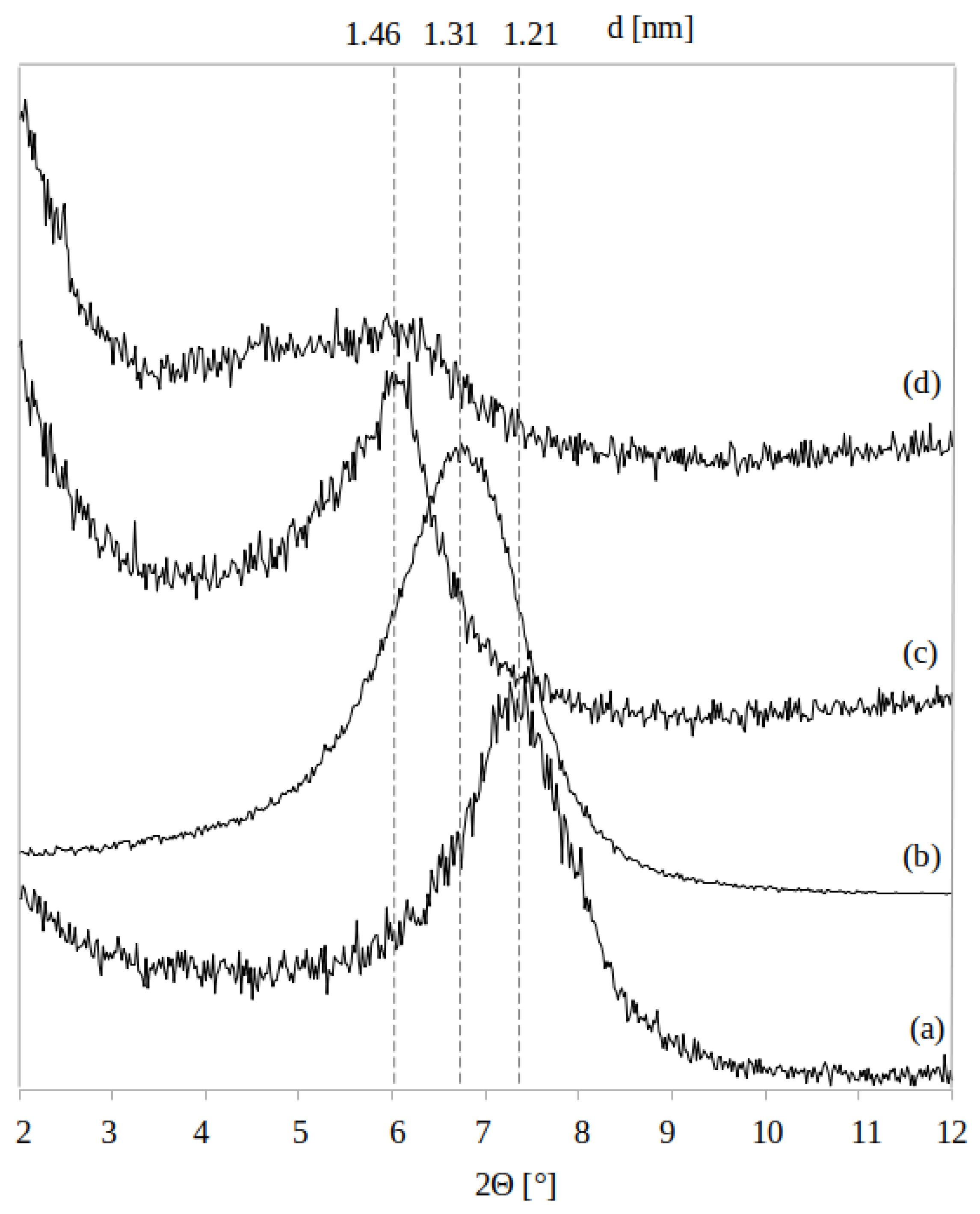
3.1. Coatings Nanostructure
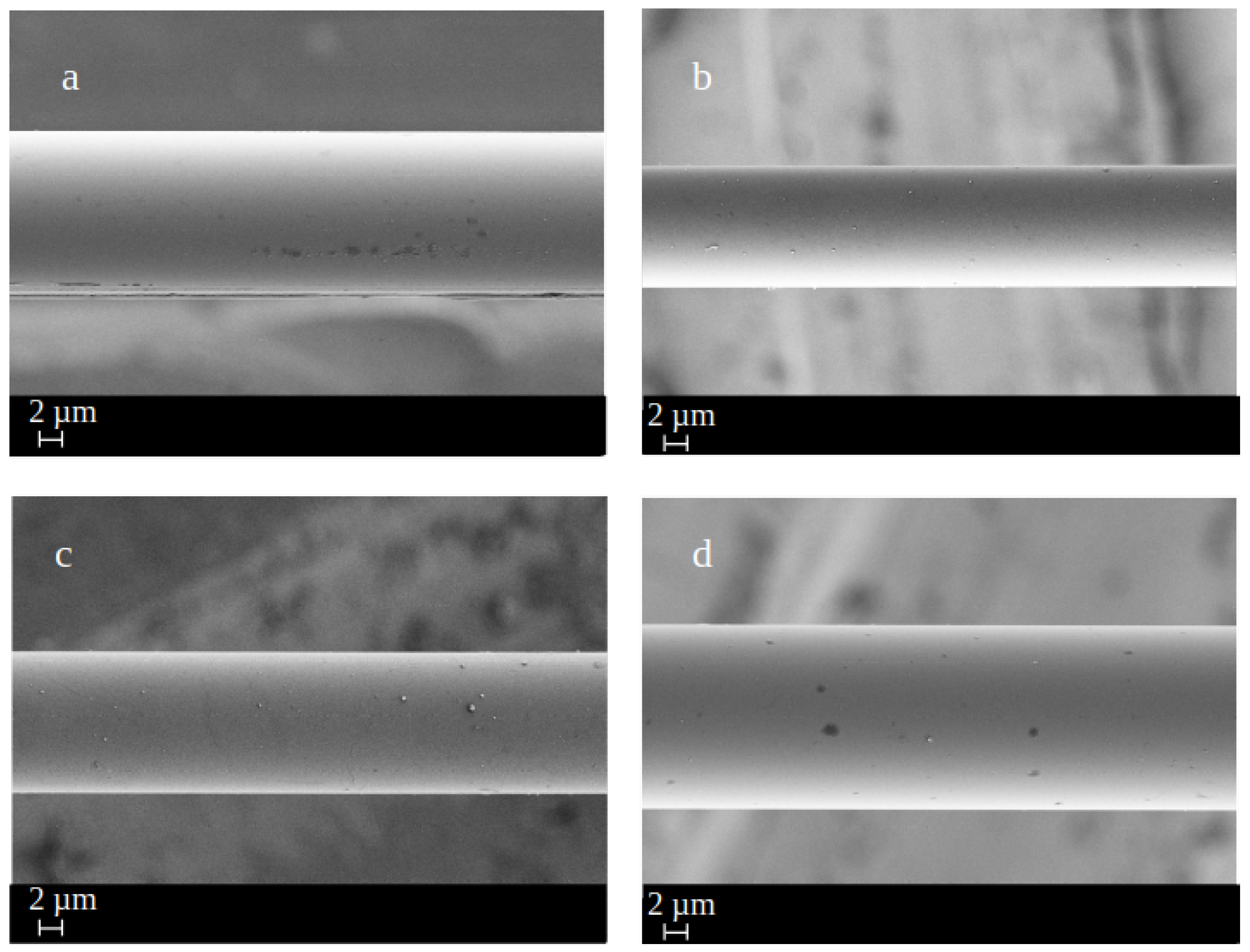
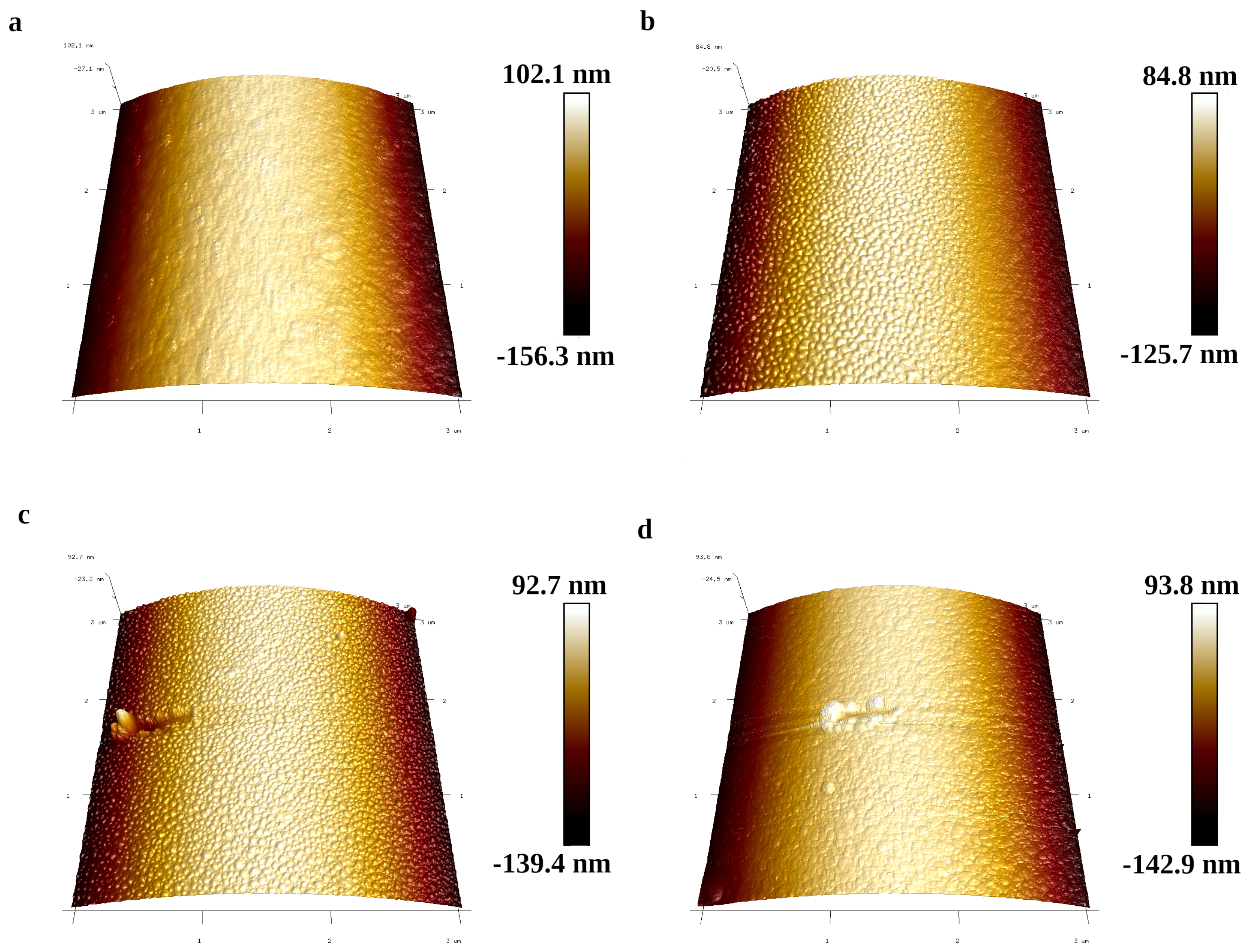
3.2. Fiber Surface Morphology
3.3. Single Fiber Tensile Strength
3.4. Formation of Hydration Products
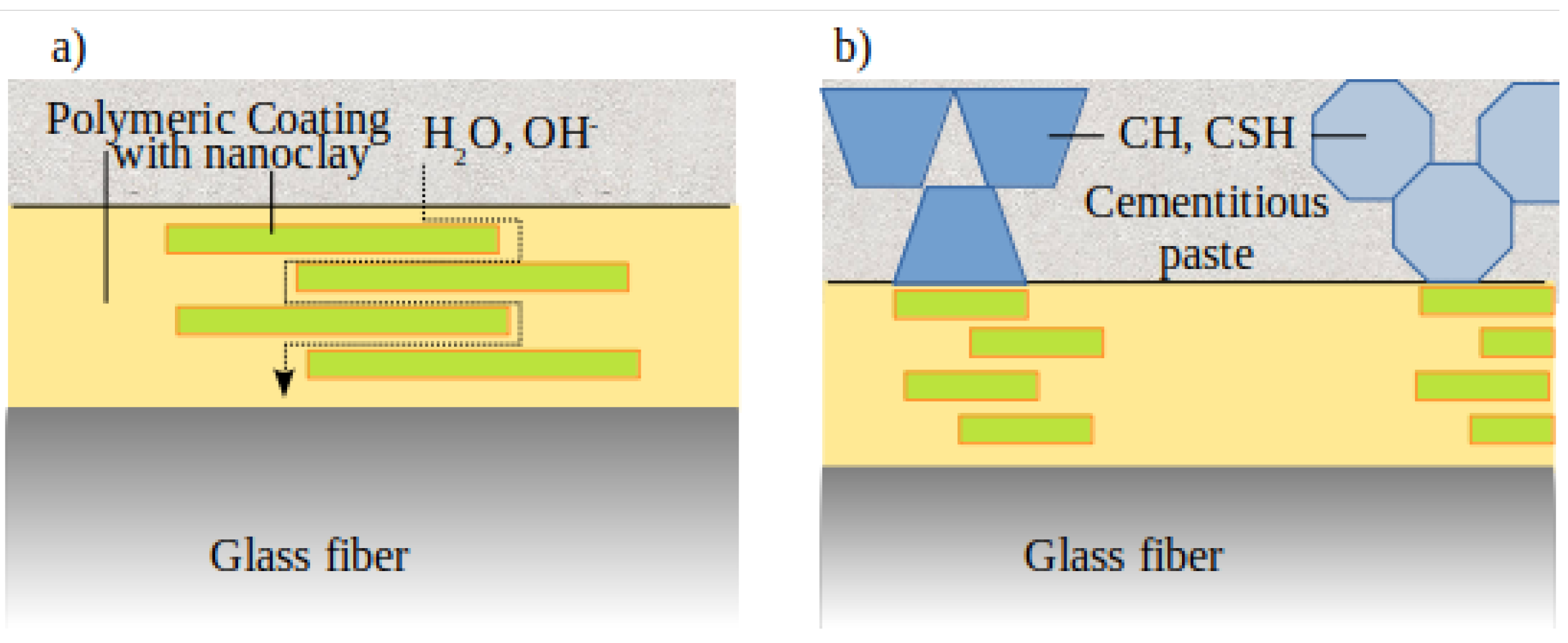
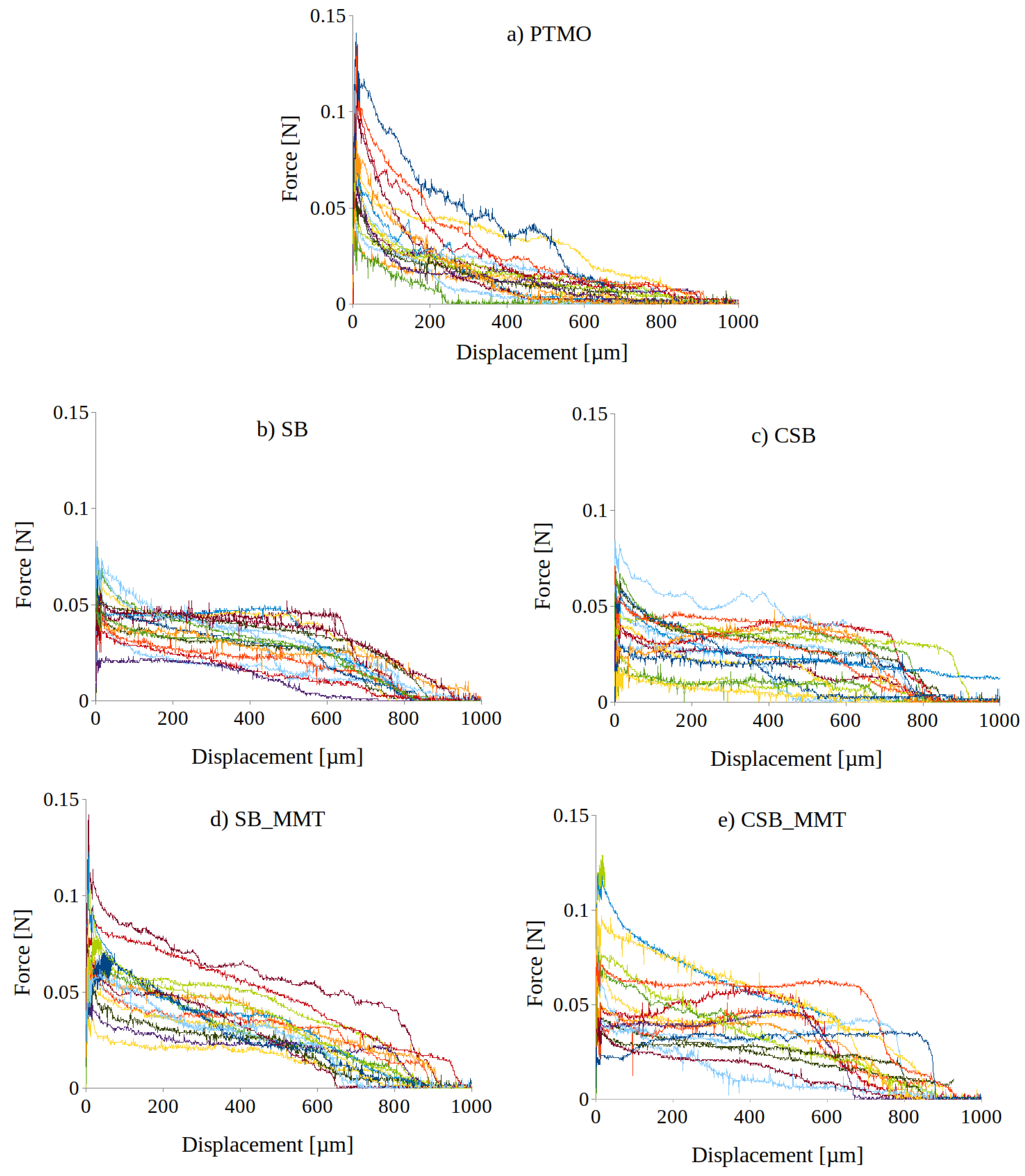
3.5. Fiber Matrix Interaction
4. Conclusions
- The dispersion quality of nanoclay particles in the coating can be affected by further ingredients, as it was observed in this work for the cross linking agent.
- The single fiber tensile strength is mainly depending on the silane treatment; no positive effect was arising from the nanoclay containing polymer coatings.
- The silane-treated fibers (PTMO) display higher IFSS but lower W. This indicates that the PTMO fibers have a high interfacial adhesion due to the chemical bonding with the matrix. However, the resulting force drop after reaching F indicates only minor fiber-matrix interaction during further pull-out; hence, brittle failure occurs.
- The application of a coating increases the friction between the fiber and the matrix resulting in higher pull-out work.
- For both SB and CSB coatings, the addition of MMT particles produced an increase of the IFSS and the pull-out work. This can be attributed to a better interaction with the cementitious matrix and to the ability of nanoclay particles to work as nucleating species for the formation of hydration products.
- The increase of the polymer roughness did not lead to an increase of the pull-out load. This suggests that the modification of the chemical characteristics have a major impact on the interaction with the cementitious matrix.
- According to the results presented, the main benefit obtained from the dispersion of nanoclay particles in the coating consists in providing a better interaction based on chemical bonding in combination with improved mechanical interlocking. However, the potential, in terms of durability and mechanical performances, of engineering the fiber-matrix interface with a nanostructured coating are much greater. Further studies are certainly needed to better explore the applications of this technology. Particularly, future works should focus on the improvement of the coating in order to obtain a better dispersion of the nanoclay particles in the polymer. For this purpose, different protocols for the preparation of the coating can be considered. In addition, the possibility to use other polymers and nanoclay particles can be examined. Moreover, the optimal amount of nanoclay dispersed in the coating should be defined. Finally, the results obtained on a microscale should be confirmed by investigation conducted on a macroscale.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FRCM | Fabric Reinforced Cementitious Matrix |
| TRC | Textile Reinforced Concrete |
| AR-Glass | Alkali Resistant Glass |
| AFM | Atomic Force Microscopy |
| SEM | Scanning Electron Microscope |
| SEM-EDX | Scanning Electron Microscope—Energy-Dispersive X-ray |
| IFSS | Interfacial Shear Strength |
| SFPO | Single Fiber Pull-out |
| MMT | Montmorillonite |
| XRD | X-ray Diffraction |
| IPF | Institut für Polymerforschung |
| SB | Styrene-Butadiene |
| RH | Relative Humidity |
| PLS | Polymer Layered Silicate |
| VAc/VeVa/E | Vinyl acetate/vinyl esther of versatic acid/ethylene |
| Diffraction angle | |
| wave length of the X-ray radiation | |
| d | layered silicate interlayer |
| R | average roughness |
| R | square roughness |
| R | maximum roughness |
| F | maximum pull-out force |
| W | total pull-out work |
| fiber diameter | |
| embedded fiber length |
References
- Bentur, A.; Mindess, S. Fiber Reinfroced Cementitious Composites, 2nd ed.; Taylor & Francis: Abingdon, UK, 2007. [Google Scholar]
- Koutas, L.N.; Tetta, Z.; Bournas, D.A.; Triantafillou, T.C. Strengthening of Concrete Structures with Textile Reinforced Mortars: State-of-the-Art Review. J. Compos. Constr. 2019, 23, 1–20. [Google Scholar] [CrossRef]
- Nanni, A. A New Tool for Concrete and Masonry Repair. Concr. Int. 2012, 34, 43–49. [Google Scholar]
- Parisi, F.; Menna, C.; Prota, A. 10-Fabric-Reinforced Cementitious Matrix (FRCM) composites: Mechanical behavior and application to masonry walls. In Failure Analysis in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2019; pp. 199–227. [Google Scholar] [CrossRef]
- Kouris, L.A.S.; Triantafillou, T.C. State-of-the-Art on Strengthening of Masonry Structures with Textile Reinforced Mortar (TRM). Constr. Build. Mater. 2018, 188, 1221–1233. [Google Scholar] [CrossRef]
- Institute, A.C. (Ed.) Guide to Design and Construction of Externally Bonded Fabric-Reinforced Cementitious Matrix (FRCM) Systems for Repair and Strengthening Concrete and Masonry Structures; Number 549.4R-13 in ACI; American Concrete Institute: Farmington Hills, MI, USA, 2013. [Google Scholar]
- Bompadre, F.; Donnini, J. Surface Modification of Glass Textile for the Reinforcement of a Cement-Based Composite: A Review. Appl. Sci. 2021, 11, 2028. [Google Scholar] [CrossRef]
- Messori, M.; Nobili, A.; Signorini, C.; Sola, A. Mechanical Performance of Epoxy Coated AR-Glass Fabric Textile Reinforced Mortar: Influence of Coating Thickness and Formulation. Compos. Part Eng. 2018, 149, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Mäder, E.; Plonka, R.; Schiekel, M.; Hempel, R. Coatings on Alkali-Resistant Glass Fibres for the Improvement of Concrete. J. Ind. Text. 2004, 33, 191–207. [Google Scholar] [CrossRef]
- Donnini, J.; Corinaldesi, V. Mechanical Characterization of Different FRCM Systems for Structural Reinforcement. Constr. Build. Mater. 2017, 145, 565–575. [Google Scholar] [CrossRef]
- Reinhardt, H.W.; Krüger, M.; Bentur, A.; Brameshuber, W.; Banholzer, B.; Curbach, M.; Jesse, F.; Mambasher, B.; Peled, A.; Schorn, H. Composite Materials—6.1 Bond. In Textile Reinforced Concrete-State-of-the-Art Report of RILEM TC 201-TRC; Brameshuber, W., Ed.; RILEM Publications SARL: Paris, France, 2006. [Google Scholar]
- Signorini, C.; Nobili, A.; Sola, A.; Messori, M. Designing Epoxy Viscosity for Optimal Mechanical Performance of Coated Glass Textile Reinforced Mortar (GTRM) Composites. Constr. Build. Mater. 2020, 233, 117325. [Google Scholar] [CrossRef]
- Raupach, M.; Orlowsky, J.; Büttner, T.; Dilthey, U.; Schleser, M. Epoxy-Impregnated Textiles in Concrete. Load Bearing Capacity and Durability. In Proceedings of the ICTRC’2006—1st International RILEM Conference on Textile Reinforced Concret, Aachen, Germany, 6 September 2006; Hegger, J.W.B., Will, N., Eds.; RILEM Publications SARL: Paris, France, 2006; pp. 77–88. [Google Scholar]
- Donnini, J.; Corinaldesi, V.; Nanni, A. Mechanical Properties of FRCM Using Carbon Fabrics with Different Coating Treatments. Compos. Part Eng. 2016, 88. [Google Scholar] [CrossRef]
- Purnell, P.; Bentur, A.; Raupach, M.; Hempel, R.; Orlowsky, J.; Schiekel, M. 6.3 Durability. In Textile Reinforced Concrete-State-of-the-Art Report of RILEM TC 201-TRC; RILEM Publications SARL: Paris, France, 2006; pp. 187–209. [Google Scholar]
- Scheffler, C. Zur Beurteilung von AR-Glasfasern in Alkalischer Umgebung. Ph.D. Thesis, Technischen Universität Dresden, Dresden, Germany, 2009. [Google Scholar]
- Scheffler, C.; Gao, S.L.; Plonka, R.; Mäder, E.; Hempel, S.; Butler, M.; Mechtcherine, V. Interphase Modification of Alkali-Resistant Glass Fibres and Carbon Fibres for Textile Reinforced Concrete I: Fibre Properties and Durability. Compos. Sci. Technol. 2009, 69, 531–538. [Google Scholar] [CrossRef]
- Büttner, T.; Raupach, M. Polymer Impregnation of Textile Reinforced Concrete to Enhance Durability—Laboratory Testing and Long-Term Modelling. Restor. Build. Monum. 2012, 18, 185–194. [Google Scholar] [CrossRef]
- Gao, S.L.; Mäder, E.; Plonka, R. Coatings for Glass Fibers in a Cementitious Matrix. Acta Mater. 2004, 52, 4745–4755. [Google Scholar] [CrossRef]
- Raoof, S.M.; Koutas, L.N.; Bournas, D.A. Bond between Textile-Reinforced Mortar (TRM) and Concrete Substrates: Experimental Investigation. Compos. Part Eng. 2016, 98, 350–361. [Google Scholar] [CrossRef]
- Signorini, C.; Sola, A.; Nobili, A.; Siligardi, C. Lime-Cement Textile Reinforced Mortar (TRM) with Modified Interphase. J. Appl. Biomater. Funct. Mater. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Nadiv, R.; Peled, A.; Mechtcherine, V.; Hempel, S.; Schroefl, C. Micro- and Nanoparticle Mineral Coating for Enhanced Properties of Carbon Multifilament Yarn Cement-Based Composites. Compos. Part Eng. 2017, 111, 179–189. [Google Scholar] [CrossRef]
- Lu, M.; Xiao, H.; Liu, M.; Li, X.; Li, H.; Sun, L. Improved Interfacial Strength of SiO2 Coated Carbon Fiber in Cement Matrix. Cem. Concr. Compos. 2018, 91, 21–28. [Google Scholar] [CrossRef]
- Signorini, C.; Nobili, A.; González, E.I.C.; Siligardi, C. Silica Coating for Interphase Bond Enhancement of Carbon and AR-Glass Textile Reinforced Mortar (TRM). Compos. Part Eng. 2018, 141, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Homoro, O.; Michel, M.; Baranger, T.N. Improvement of the Mechanical Properties of a Glass Multifilament Yarn Reinforced Ettringitic Matrix Using an Innovative Pre-Impregnation Process. Eur. J. Environ. Civ. Eng. 2020, 1–16. [Google Scholar] [CrossRef]
- Shim, E. 10-Bonding requirements in coating and laminating of textiles. In Joining Textiles; Jones, I., Stylios, G., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2013; pp. 309–351. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B. 14—Application technologies for coating, lamination and finishing of technical textiles. In Advances in the Dyeing and Finishing of Technical Textiles; Gulrajani, M., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2013; pp. 355–411. [Google Scholar] [CrossRef]
- Zamir, M.; Sripada, R.; Peled, A. Hybrid Fillers in Carbon-Fabric-Reinforced Cement-Based Composites. Cem. Concr. Compos. 2019, 98, 113–124. [Google Scholar] [CrossRef]
- Hempel, S.; Butler, M.; Kratz, M.; Scheffler, C.; Plonka, R. Improvement of Bond Behaviour and Durability of AR-Glassfibre-Reinforced Concrete by Polymer-Fibre Coatings. In Proceedings of the 15th Congress of the Glassfibre Reinforced Concrete Association Internationa, Prague, Czech Republic, 20–23 April 2008; pp. 225–233. [Google Scholar]
- Scheffler, C.; Gao, S.L.; Plonka, R.; Mäder, E.; Hempel, S.; Butler, M.; Mechtcherine, V. Interphase Modification of Alkali-Resistant Glass Fibres and Carbon Fibres for Textile Reinforced Concrete II: Water Adsorption and Composite Interphases. Compos. Sci. Technol. 2009, 69, 905–912. [Google Scholar] [CrossRef]
- Rallini, M.; Kenny, J. Nanofillers in Polymers. In Modification of Polymer Properties; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–86. [Google Scholar] [CrossRef]
- Hu, H.; Onyebueke, L.; Abatan, A. Characterizing and Modeling Mechanical Properties of Nanocomposites-Review and Evaluation. J. Miner. Mater. Charact. Eng. 2010, 9, 275–319. [Google Scholar] [CrossRef]
- Gao, F. (Ed.) Advances in Polymer Nanocomposites Types and Applications; Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Conradi, M. Nanosilica-Reinforced Polymer Composites. Mater. Technol. 2013, 47, 285–293. [Google Scholar]
- Pavlidou, S.; Papaspyrides, C.D. A Review on Polymer-Layered Silicate Nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Coleman, J.N.; Cadek, M.; Blake, R.; Nicolosi, V.; Ryan, K.P.; Belton, C.; Fonseca, A.; Nagy, J.B.; Gun’ko, Y.K.; Blau, W.J. High Performance Nanotube-Reinforced Plastics: Understanding the Mechanism of Strength Increase. Adv. Funct. Mater. 2004, 14, 791–798. [Google Scholar] [CrossRef]
- Rahman, R. (Ed.) Silica and Clay Dispersed Polymer Nanocomposites Preparation, Properties and Applications; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Savic, I.; Stojiljkovic, S.; Gajic, D. Industrial Application of Clays and Clay Minerals. In Clays and Clay Minerals Geological Origin, Mechanical Properties and Industrial Application; Wesely, L.R., Ed.; Earth Science in the 21st Century; Nova Publishers: New York, NY, USA, 2014; Chapter 15; pp. 379–402. [Google Scholar]
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q.M.; Paul, D.R. Clay-Based Polymer Nanocomposites: Research and Commercial Development. J. Nanosci. Nanotechnol. 2005, 5, 1574–1592. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of Metakaolin on the Properties of Mortar and Concrete: A Review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Brameshub, W.; Brockmann, T.; Wastiels, J.; Curbach, M.; Meyer, C.; Vilkner, G.; Mobasher, B.; Peled, A.; Reinhardt, H.; Krüger, M. Concrete/Matrix. In Textile Reinforced Concrete-State-of-the-Art Report of RILEM TC 201-TRC; RILEM Publications SARL: Paris, France, 2006; pp. 29–56. [Google Scholar]
- Mechtcherine, V.; Schneider, K.; Brameshuber, W. Mineral-Based Matrices for Textile-Reinforced Concrete. In Textile Fibre Composites in Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–43. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in Concrete—A Review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Krøyer, H.; Lindgreen, H.; Jakobsen, H.J.; Skibsted, J. Hydration of Portland Cement in the Presence of Clay Minerals Studied by 29Si and 27Al MAS NMR Spectroscopy. Adv. Cem. Res. 2003, 15, 103–112. [Google Scholar] [CrossRef]
- Lindgreen, H.; Geiker, M.; Krøyer, H.; Springer, N.; Skibsted, J. Microstructure Engineering of Portland Cement Pastes and Mortars through Addition of Ultrafine Layer Silicates. Cem. Concr. Compos. 2008, 30, 686–699. [Google Scholar] [CrossRef]
- Gao, S.L.; Mäder, E.; Plonka, R. Nanostructured Coatings of Glass Fibers: Improvement of Alkali Resistance and Mechanical Properties. Acta Mater. 2007. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-Layered Silicate Nanocomposites: Preparation, Properties and Uses of a New Class of Materials. Mater. Sci. Eng. Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Khan, S.U. Fatigue Damage Behaviors of Carbon Fiber-Reinforced Epoxy Composites Containing Nanoclay. Compos. Sci. Technol. 2010, 70, 2077–2085. [Google Scholar] [CrossRef]
- Dai, G.; Mishnaevsky, L., Jr. Fatigue of Multiscale Composites with Secondary Nanoplatelet Reinforcement: 3D Computational Analysis. Compos. Sci. Technol. 2014, 91, 71–81. [Google Scholar] [CrossRef]
- Mishnaevsky, L., Jr. Nanostructured Interfaces for Enhancing Mechanical Properties of Composites: Computational Micromechanical Studies. Compos. Part Eng. 2015, 68, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Isitman, N.A.; Aykol, M.; Kaynak, C. Nanoclay Assisted Strengthening of the Fiber/Matrix Interface in Functionally Filled Polyamide 6 Composites. Compos. Struct. 2010, 92, 2181–2186. [Google Scholar] [CrossRef]
- Khan, S.U. Quasi-Static and Impact Fracture Behaviors of CFRPs with Nanoclay-Filled Epoxy Matrix. Compos. Part Appl. Sci. Manuf. 2011, 42, 253–264. [Google Scholar] [CrossRef]
- Mishnaevsky, L., Jr. Micromechanical Analysis of Nanocomposites Using 3D Voxel Based Material Model. Compos. Sci. Technol. 2012, 72, 1167–1177. [Google Scholar] [CrossRef]
- Mishnaevsky, L., Jr.; Dai, G. Hybrid and Hierarchical Nanoreinforced Polymer Composites: Computational Modelling of Structure Properties Relationships. Compos. Struct. 2014, 117, 156–168. [Google Scholar] [CrossRef]
- Donnini, J.; Bompadre, F.; Corinaldesi, V. Tensile Behavior of a Glass FRCM System after Different Environmental Exposures. Processes 2020, 8, 1274. [Google Scholar] [CrossRef]
- Zhandarov, S.; Mäder, E.; Scheffler, C.; Kalinka, G.; Poitzsch, C.; Fliescher, S. Investigation of Interfacial Strength Parameters in Polymer Matrix Composites: Compatibility and Reproducibility. Adv. Ind. Eng. Polym. Res. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Mäder, E.; Grundke, K.; Jacobasch, H.J.; Wachinger, G. Surface, Interphase and Composite Property Relations in Fibre-Reinforced Polymers. Composites 1994, 25, 739–744. [Google Scholar] [CrossRef]
- Scheffler, C.; Zhandarov, S.; Mäder, E. Alkali Resistant Glass Fiber Reinforced Concrete: Pull-out Investigation of Interphase Behavior under Quasi-Static and High Rate Loading. Cem. Concr. Compos. 2017, 84, 19–27. [Google Scholar] [CrossRef]
- Mayer, H. Introductory Solid State Physics, 2nd ed.; Taylor & Francis: Abingdon, UK, 1997. [Google Scholar]
- Fukushima, Y. X-ray Diffraction Study of Aqueous Montmorillonite Emulsions. Clays Clay Miner. 1984, 32, 320–326. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mizukami, F.; Miyamoto, A. Effect of Exchangeable Cation and Hydration Layer on the Swelling Property of 2:1 Dioctahedral Smectite Clay—A Periodic Density Functional Study. Stud. Surf. Sci. Catal. 2005, 156, 335–342. [Google Scholar] [CrossRef]
- Asgari, M. Silane Functionalization of Sodium Montmorillonite Nanoclay The Effect of Dispersing Media on Intercalation and Chemical Grafting. Appl. Clay Sci. 2018, 153, 228–238. [Google Scholar] [CrossRef]
- Slaný, M.; Ľuboš, J.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, T.K.; Wang, Y.C.; Alamani, B.G.; Lin, J.J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New Polylactide/Layered Silicate Nanocomposites: Role of Organoclays. Chem. Mater. 2002, 14, 4654–4661. [Google Scholar] [CrossRef]
- Keledi, G.; Hári, J.; Pukánszky, B. Polymer nanocomposites: Structure, interaction, and functionality. Nanoscale 2012, 4, 1919–1938. [Google Scholar] [CrossRef]
- Wójcik-Bania, M. Influence of the addition of organo-montmorillonite nanofiller on cross-linking of polysiloxanes—FTIR studies. Spectrochim. Acta Part Mol. Biomol. 2021, 252, 119491. [Google Scholar] [CrossRef] [PubMed]
- Román, F.; Montserrat, S.; Hutchinson, J.M. On the Effect of Montmorillonite in the Curing Reaction of Epoxy Nanocomposites. J. Therm. Anal. Calorim. 2007, 87, 113–118. [Google Scholar] [CrossRef]
- Bensadoun, F.; Kchit, N.; Billotte, C.; Trochu, F.; Ruiz, E. A Comparative Study of Dispersion Techniques for Nanocomposite Made with Nanoclays and an Unsaturated Polyester Resin. J. Nanomater. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Thomason, J.L. Glass Fibre Sizing: A Review. Compos. Part Appl. Sci. Manuf. 2019, 127. [Google Scholar] [CrossRef]
- Zinck, P.; Mäder, E.; Gerard, J.F. Role of Silane Coupling Agent and Polymeric Film Former for Tailoring Glass Fiber Sizings from Tensile Strength Measurements. J. Mater. Sci. 2001, 36, 5245–5252. [Google Scholar] [CrossRef]
- Zhandarov, S.; Mäder, E. Characterization of Fiber/Matrix Interface Strength: Applicability of Different Tests, Approaches and Parameters. Compos. Sci. Technol. 2005, 65, 149–160. [Google Scholar] [CrossRef]





| Sample Name | Sizing (1st Treatment Step) | Coating (2nd Treatment Step) |
|---|---|---|
| PTMO | 1% N-propyltrimethoxysilane (PTMO) in distilled water | none |
| SB | 1% 3-Aminopropyltriethoxysilane (AMEO) in distilled water | self-crosslinking styrene-butadiene copolymer (SB) |
| CSB | 1% AMEO in distilled water | SB + 7% crosslinking agent (C) |
| SB-MMT | 1% AMEO in distilled water | SB + 5% montmorillonite (MMT) |
| CSB-MMT | 1% AMEO in distilled water | CSB + 5% MMT |
| Fiber Type | R [nm] | R [nm] | R [nm] |
|---|---|---|---|
| SB | 2.27 ± 0.29 | 3.33 ± 0.4 | 38.88 ± 32.34 |
| SB-MMT | 11.90 ± 1.17 | 16.91 ± 1.61 | 160 ± 19.65 |
| CSB | 2.46 ± 1.03 | 3.66 ± 1.91 | 46.05 ± 30.96 |
| CSB-MMT | 1.92 ± 0.5 | 3.70 ± 1.22 | 107.20 ± 26.93 |
| Sample | Young’s Modulus [MPa] | Tensile Strength [MPa] | Strain at Break [%] |
|---|---|---|---|
| unsized | 76.14 ± 2.5 | 1018.48 ± 379.23 | 1.51 ± 0.58 |
| PTMO | 76.16 ± 1.00 | 1534.47 ± 433.88 | 2.35 ± 0.71 |
| SB-MMT | 76.34 ± 0.85 | 1520.79 ± 392.86 | 2.29 ± 0.62 |
| CSB | 77.06 ± 0.89 | 1578.88 ± 371.82 | 2.35 ± 0.58 |
| Sample Name | IFSS [MPa] | W [mN*mm] | [mm] |
|---|---|---|---|
| PTMO | 4.2 ± 2.4 | 15.2 ± 7.6 | 663 ± 245 |
| SB | 1.9 ± 0.3 | 25.3 ± 5.6 | 894 ± 163 |
| CSB | 1.5 ± 0.5 | 20.3 ± 7.5 | 847 ± 106 |
| SB-MMT | 2.2 ± 0.5 | 28.8 ± 10.1 | 782 ± 117 |
| CSB-MMT | 1.9 ± 0.5 | 34.4 ± 11.3 | 883 ± 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bompadre, F.; Scheffler, C.; Utech, T.; Donnini, J. Polymeric Coatings for AR-Glass Fibers in Cement-Based Matrices: Effect of Nanoclay on the Fiber-Matrix Interaction. Appl. Sci. 2021, 11, 5484. https://doi.org/10.3390/app11125484
Bompadre F, Scheffler C, Utech T, Donnini J. Polymeric Coatings for AR-Glass Fibers in Cement-Based Matrices: Effect of Nanoclay on the Fiber-Matrix Interaction. Applied Sciences. 2021; 11(12):5484. https://doi.org/10.3390/app11125484
Chicago/Turabian StyleBompadre, Francesca, Christina Scheffler, Toni Utech, and Jacopo Donnini. 2021. "Polymeric Coatings for AR-Glass Fibers in Cement-Based Matrices: Effect of Nanoclay on the Fiber-Matrix Interaction" Applied Sciences 11, no. 12: 5484. https://doi.org/10.3390/app11125484
APA StyleBompadre, F., Scheffler, C., Utech, T., & Donnini, J. (2021). Polymeric Coatings for AR-Glass Fibers in Cement-Based Matrices: Effect of Nanoclay on the Fiber-Matrix Interaction. Applied Sciences, 11(12), 5484. https://doi.org/10.3390/app11125484








