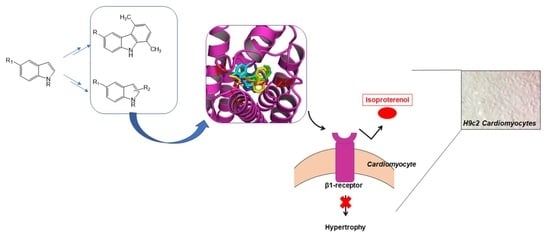
Carbazole and Simplified Derivatives: Novel Tools toward ?-Adrenergic Receptors Targeting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
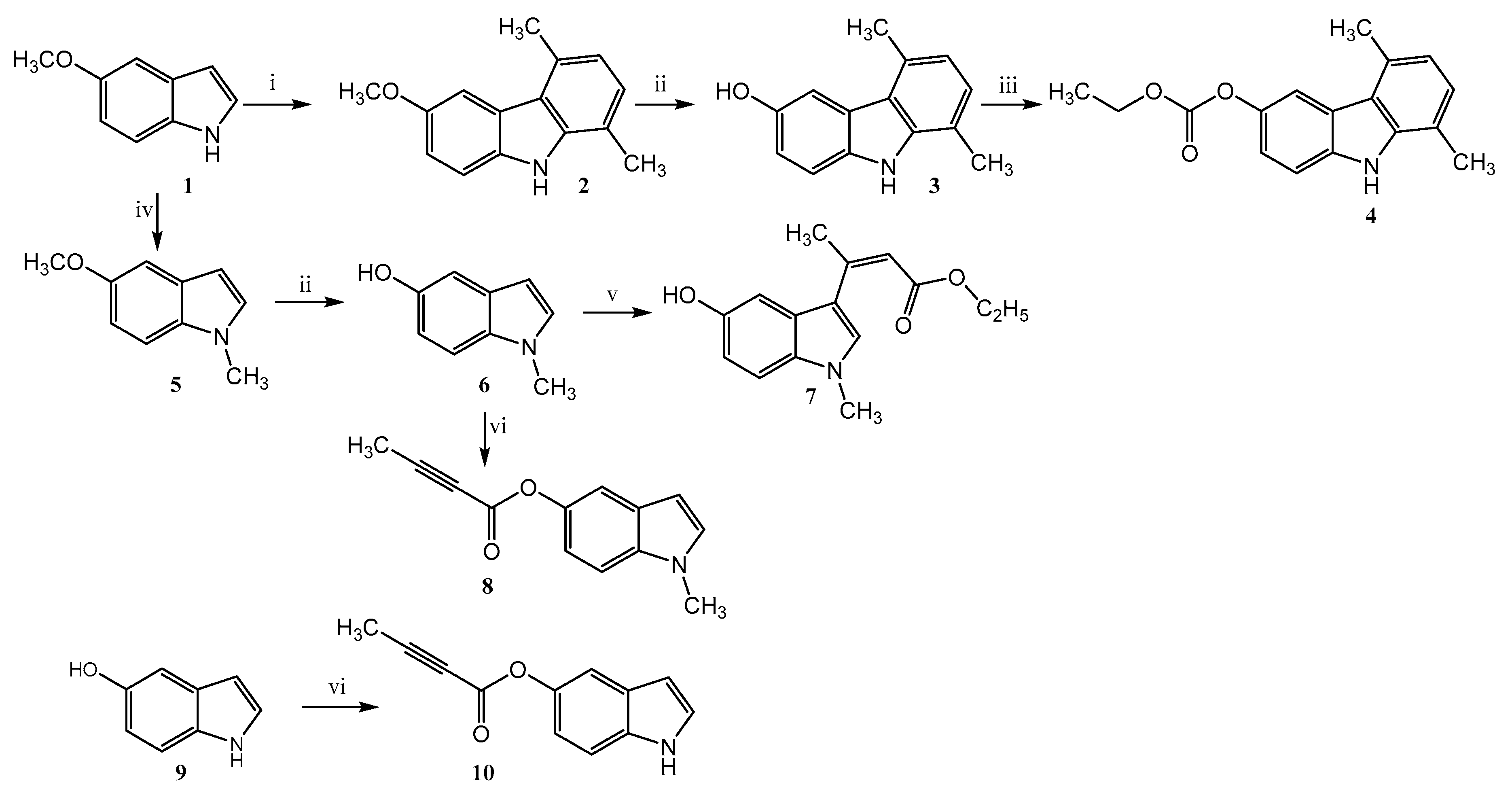
2.1.1. 1,4-Dimethyl-6-methoxy-carbazole (2)
2.1.2. 1,4-Dimethyl-6-hydroxy-carbazole (3)
2.1.3. 5,8-Dimethyl-9H-carbazol-3-yl Ethyl Carbonate (4)
2.1.4. 5-Methoxy-1-methylindole (5)
2.1.5. 5-Hydroxy-1-methylindole (6)
2.1.6. Ethyl 3-(5-hydroxy-1-methyl-1H-indol-3-yl)but-2-enoate (7)
2.1.7. 1-Methyl-1H-indol-5-yl-but-2-ynoate (8)
2.1.8. Indol-5-yl-but-2-ynoate (10)
2.2. Molecular Docking
2.3. Cell Culture
2.4. 3-(4,5-Dimethylthiazol-)2,5-diphenyl Tetrazolium Bromide (MTT) Assay
2.5. Morphological Analysis of H9c2 Cells
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. Molecular Docking
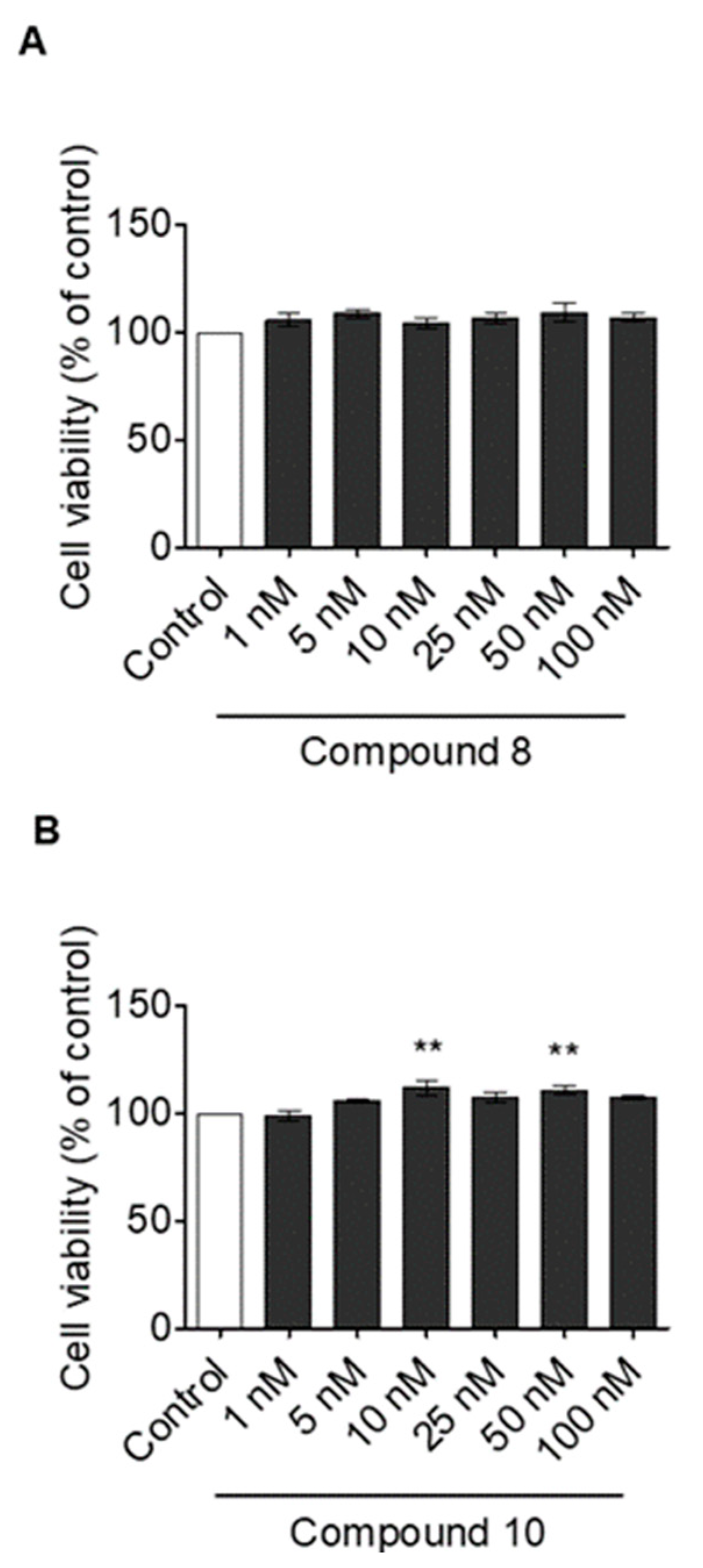
3.3. In Vitro Findings
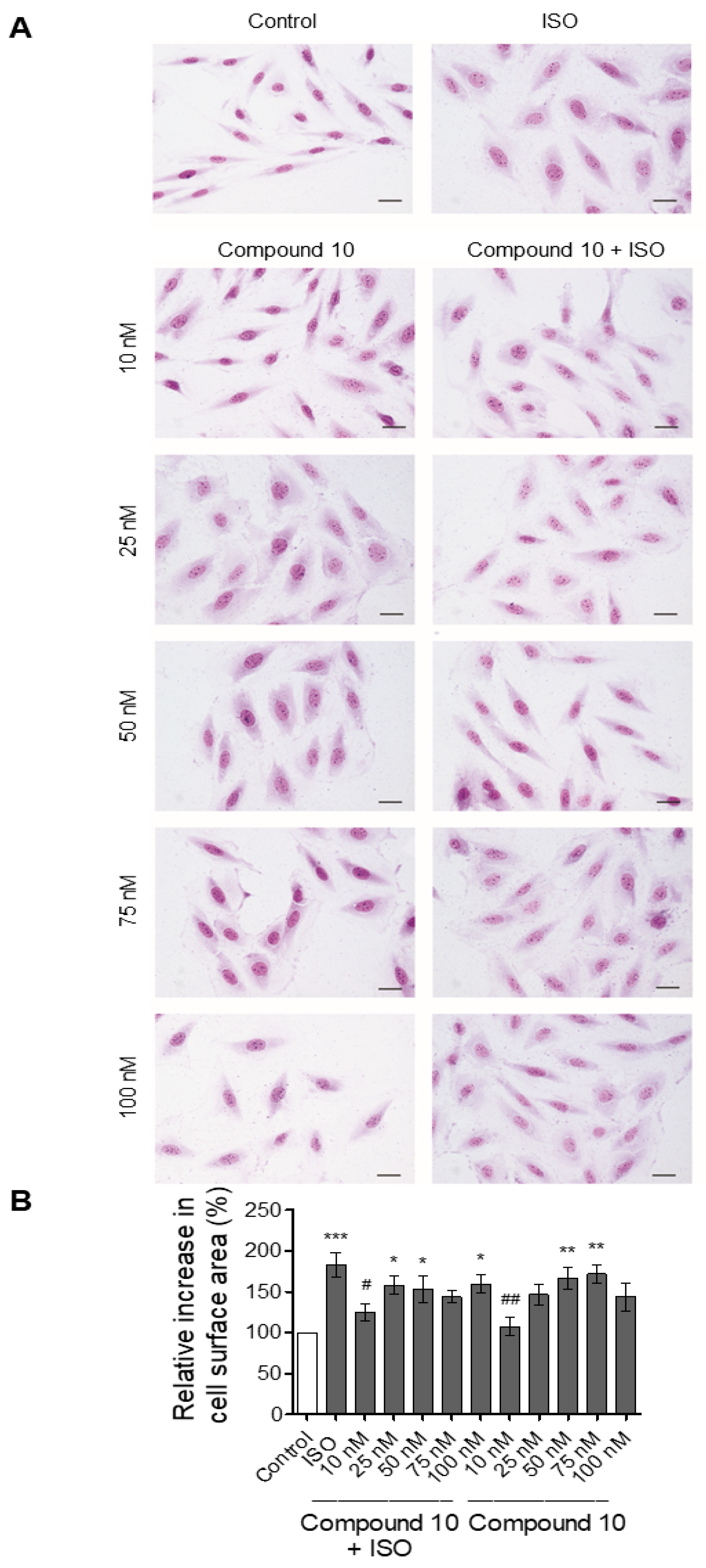
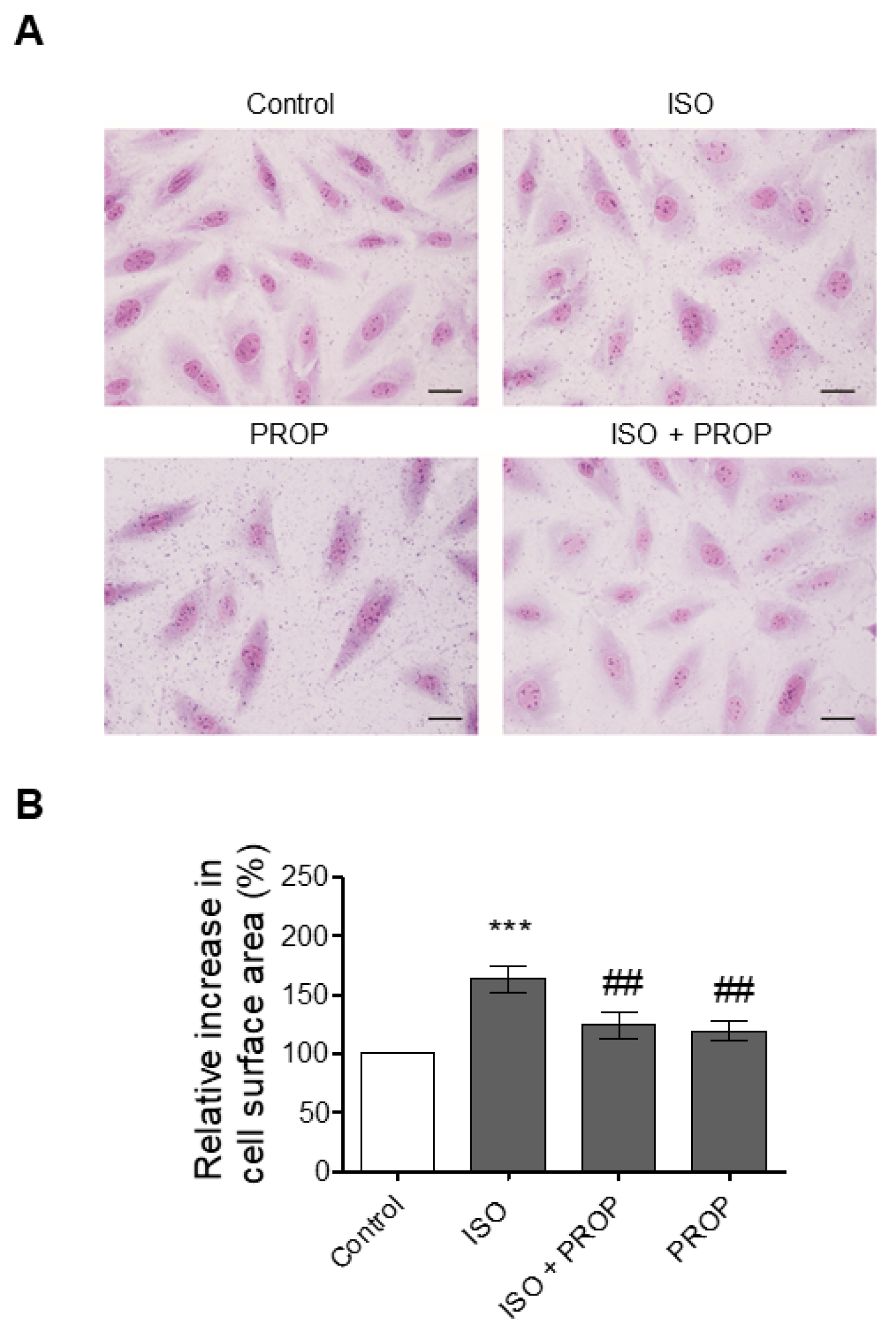
Effect of Compounds 8 and 10 on Cell Proliferation and ISO-Dependent Hypertrophy in H9c2 Cardiomyocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three generations of β-blockers: History, class differences and clinical applicability. Curr. Hypertens. Rev. 2018, 15, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and mechanisms of action. Rev. Española Cardiol. 2019, 72, 853–862. [Google Scholar] [CrossRef]
- Dale, H.H. On some physiological actions of ergot. J. Physiol. 1906, 34, 163–206. [Google Scholar] [CrossRef]
- Wang, J.; Gareri, C.; Rockman, H.A. G-protein-coupled receptors in heart disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef]
- Moran, N.C.; Perkins, M.E. Adrenergic blockade of the mammalian heart by a dichloro analogue of isoproterenol. J. Pharm. Exp. Ther. 1958, 124, 223–237. [Google Scholar]
- Quirke, V. Putting theory into practice: James Black, receptor theory and the development of the beta-blockers at ICI, 1958–1978. Med. Hist. 2006, 50, 69–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfleger, J.; Gresham, K.; Koch, W.J. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat. Rev. Cardiol. 2019, 16, 612–622. [Google Scholar] [CrossRef]
- Giltrow, E.; Eccles, P.D.; Hutchinson, T.H.; Sumpter, J.P.; Rand-Weaver, M. Characterisation and expression of β1-, β2- and β3-adrenergic receptors in the fathead minnow (Pimephales promelas). Gen. Comp. Endocrinol. 2011, 173, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Leo, S.; Gattuso, A.; Mazza, R.; Filice, M.; Cerra, M.C.; Imbrogno, S. Cardiac influence of the β3-adrenoceptor in the goldfish (Carassius auratus): A protective role under hypoxia? J. Exp. Biol. 2019, 222, jeb211334. [Google Scholar] [CrossRef]
- Imbrogno, S.; Filice, M.; Cerra, M.C. Exploring cardiac plasticity in teleost: The role of humoral modulation. Gen. Comp. Endocrinol. 2019, 283, 113236. [Google Scholar] [CrossRef]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New insights in cardiac β-Adrenergic signaling during heart failure and aging. Front. Pharm. 2018, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.G.; Hill, S.J.; Summers, R.J. Evolution of β-blockers: From anti-anginal drugs to ligand-directed signalling. Trends Pharm. Sci. 2011, 32, 227–234. [Google Scholar] [CrossRef]
- Poirier, L.; Tobe, S.W. Contemporary use of β-blockers: Clinical relevance of subclassification. Can. J. Cardiol. 2014, 30, S9–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.W.; Kang, C.; Kim, Y.; Lee, M.G.; Kim, J.Y. Isoproterenol-induced hypertrophy of neonatal cardiac myocytes and H9c2 cell is dependent on TRPC3-regulated CaV1.2 expression. Cell Calcium 2020, 92, 102305. [Google Scholar] [CrossRef]
- Hescheler, J.; Meyer, R.; Plant, S.; Krautwurst, D.; Rosenthal, W.; Schultz, G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ. Res. 1991, 69, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Angelone, T.; Caruso, A.; Rochais, C.; Caputo, A.M.; Cerra, M.C.; Dallemagne, P.; Filice, E.; Genest, D.; Pasqua, T.; Puoci, F.; et al. Indenopyrazole oxime ethers: Synthesis and β1-adrenergic blocking activity. Eur. J. Med. Chem. 2015, 92, 672–681. [Google Scholar] [CrossRef]
- Grande, F.; Parisi, O.I.; Mordocco, R.A.; Rocca, C.; Puoci, F.; Scrivano, L.; Quintieri, A.M.; Cantafio, P.; Ferla, S.; Brancale, A.; et al. Quercetin derivatives as novel antihypertensive agents: Synthesis and physiological characterization. Eur. J. Pharm. Sci. 2016, 82, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Di Nunno, L.; Franchini, C.; Scilimati, A.; Sinicropi, M.S.; Tortorella, P. Chemical and chemoenzymatic routes to 1-(benzothiazol-2-ylsulfanyl)-3-chloropropan-2-ol, a precursor of drugs with potential β-blocker activity. Tetrahedron Asymmetry 2000, 11, 1571–1583. [Google Scholar] [CrossRef]
- Go, J.G.; Santiago, L.D.; Miranda, A.C.; Jara, R.D. Effect of Beta-blockers on Hypertension and Heart Failure with Reduced Ejection Fraction: A Systematic Review of Randomized Controlled Trials. Hypertens. J. 2019, 5. [Google Scholar] [CrossRef]
- Bennett, M.; Chang, C.L.; Tatley, M.; Savage, R.; Hancox, R.J. The safety of cardio-selective beta1-blockers in asthma: Literature review and search of global pharmacovigilance safety reports. ERJ Open Res. 2021, 7, 00801–02020. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Yeung, S.; Thakkar, A.; Huang, K.M.; Liu, M.M.; Kanassatega, R.-S.; Parsa, C.; Orlando, R.; Jackson, E.K.; Andresen, B.T.; et al. Prevention of skin carcinogenesis by the β-blocker carvedilol. Prev. Cancer Prev. Res. 2015, 8, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Kaindl, J.; Clark, M.J.; Hübner, H.; Hirata, K.; Sunahara, R.K.; Gmeiner, P.; Kobilka, B.K.; Liu, X. Binding pathway determines norepinephrine selectivity for the human β1AR over β2AR. Cell Res. 2021, 31, 569–579. [Google Scholar] [CrossRef]
- Wong, G.W.K.; Boyda, H.N.; Wright, J.M. Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension. Cochrane Database Syst. Rev. 2014, 12, 2017. [Google Scholar] [CrossRef]
- Willette, R.N.; Mitchell, M.P.; Ohlstein, E.H.; Lukas, M.A.; Ruffolo, R.R. Evaluation of intrinsic sympathomimetic activity of bucindolol and carvedilol in rat heart. Pharmacology 1998, 56, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Sophie Voisin-Chiret, A.; Lancelot, J.-C.; Sinicropi, S.; Garofalo, A.; Rault, S. Novel and efficient synthesis of 5,8-dimethyl-9h-carbazol-3-ol via a hydroxydeboronation reaction. Heterocycles 2007, 71, 2203–2210. [Google Scholar] [CrossRef]
- Saturnino, C.; Grande, F.; Aquaro, S.; Caruso, A.; Iacopetta, D.; Bonomo, M.; Longo, P.; Schols, D.; Sinicropi, M. Chloro-1,4-dimethyl-9H-carbazole Derivatives Displaying Anti-HIV Activity. Molecules 2018, 23, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizza, P.; Pellegrino, M.; Caruso, A.; Iacopetta, D.; Sinicropi, M.S.; Rault, S.; Lancelot, J.C.; El-Kashef, H.; Lesnard, A.; Rochais, C.; et al. 3-(Dipropylamino)-5-hydroxybenzofuro[2,3-f]quinazolin-1(2H)-one (DPA-HBFQ-1) plays an inhibitory role on breast cancer cell growth and progression. Eur. J. Med. Chem. 2016, 107, 275–287. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muià, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Caruso, A.; Lancelot, J.C.; El-Kashef, H.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Rault, S. A rapid and versatile synthesis of novel pyrimido[5,4-b]carbazoles. Tetrahedron 2009, 65, 10400–10405. [Google Scholar] [CrossRef]
- Saturnino, C.; Caruso, A.; Longo, P.; Capasso, A.; Pingitore, A.; Caroleo, M.; Cione, E.; Perri, M.; Nicolo, F.; Nardo, V.; et al. Crystallographic Study and Biological Evaluation of 1,4-dimethyl-N-alkylcarbazoles. Curr. Top. Med. Chem. 2015, 15, 973–979. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Grande, F.; Rizzuti, B.; Occhiuzzi, M.A.; Ioele, G.; Casacchia, T.; Gelmini, F.; Guzzi, R.; Garofalo, A.; Statti, G. Identification by molecular docking of homoisoflavones from leopoldia comosa as ligands of estrogen receptors. Molecules 2018, 23, 894. [Google Scholar] [CrossRef] [Green Version]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A pilot study on the nutraceutical properties of the Citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J. Funct. Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Rocca, C.; Grande, F.; Granieri, M.C.; Colombo, B.; De Bartolo, A.; Giordano, F.; Rago, V.; Amodio, N.; Tota, B.; Cerra, M.C.; et al. The chromogranin A1-373 fragment reveals how a single change in the protein sequence exerts strong cardioregulatory effects by engaging neuropilin-1. Acta Physiol. 2020, 231, e13570. [Google Scholar]
- Rocca, C.; Femminò, S.; Aquila, G.; Granieri, M.C.; De Francesco, E.M.; Pasqua, T.; Rigiracciolo, D.C.; Fortini, F.; Cerra, M.C.; Maggiolini, M.; et al. Notch1 mediates preconditioning protection induced by gper in normotensive and hypertensive female rat hearts. Front. Physiol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Nettore, I.C.; Rocca, C.; Mancino, G.; Albano, L.; Amelio, D.; Grande, F.; Puoci, F.; Pasqua, T.; Desiderio, S.; Mazza, R.; et al. Quercetin and its derivative Q2 modulate chromatin dynamics in adipogenesis and Q2 prevents obesity and metabolic disorders in rats. J. Nutr. Biochem. 2019, 69, 151–162. [Google Scholar] [CrossRef]
- Rocca, C.; De Bartolo, A.; Grande, F.; Rizzuti, B.; Pasqua, T.; Giordano, F.; Granieri, M.C.; Occhiuzzi, M.A.; Garofalo, A.; Amodio, N.; et al. Cateslytin abrogates lipopolysaccharide-induced cardiomyocyte injury by reducing inflammation and oxidative stress through toll like receptor 4 interaction. Int. Immunopharmacol. 2021, 94, 107487. [Google Scholar] [CrossRef]
- Cai, L.; Fan, G.; Wang, F.; Liu, S.; Li, T.; Cong, X.; Chun, J.; Chen, X. Protective role for LPA3 in cardiac hypertrophy induced by myocardial infarction but not by isoproterenol. Front. Physiol. 2017, 8, 356. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Caruso, A.; Conforti, F.; Marrelli, M.; El Kashef, H.; Lancelot, J.; Rault, S.; Statti, G.A.; Menichini, F. Synthesis, inhibition of NO production and antiproliferative activities of some indole derivatives. J. Enzyme Inhib. Med. Chem. 2009, 24, 1148–1153. [Google Scholar] [CrossRef]
- Cranwell, P.A.; Saxton, J.E. A synthesis of ellipticine. J. Chem. Soc. 1962, 683, 3482–3487. [Google Scholar] [CrossRef]
- Letois, B.; Lancelot, J.; Rault, S.; Robba, M.; Tabka, T.; Gauduchon, P.; Bertreux, E.; Le Talaer, J. Étude de la cytotoxicitéin vitro de dérivés du carbazole III. 3-Amino et 3-nitro-1,4-diméthyl-9H-carbazoles diversement substitués en position. Eur. J. Med. Chem. 1990, 25, 775–784. [Google Scholar]
- Yadav, M.R.; Grande, F.; Chouhan, B.S.; Naik, P.P.; Giridhar, R.; Garofalo, A.; Neamati, N. Cytotoxic potential of novel 6,7-dimethoxyquinazolines. Eur. J. Med. Chem. 2012, 48, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Chimento, A.; El-Kashef, H.; Lancelot, J.-C.; Panno, A.; Pezzi, V.; Saturnino, C.; Sinicropi, M.S.; Sirianni, R.; Rault, S. Antiproliferative activity of some 1,4-dimethylcarbazoles on cells that express estrogen receptors: Part I. J. Enzyme Inhib. Med. Chem. 2012, 27, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Dalic, A.; Fang, L.; Kiriazis, H.; Ritchie, R.H.; Sim, K.; Gao, X.M.; Drummond, G.; Sarwar, M.; Zhang, Y.Y.; et al. Myocardial oxidative stress contributes to transgenic β 2- adrenoceptor activation-induced cardiomyopathy and heart failure. Br. J. Pharm. 2011, 162, 1012–1028. [Google Scholar] [CrossRef] [Green Version]
- Morisco, C.; Zebrowski, D.C.; Vatner, D.E.; Vatner, S.F.; Sadoshima, J. β-adrenergic cardiac hypertrophy is mediated primarily by the β1-subtype in the rat heart. J. Mol. Cell. Cardiol. 2001, 33, 561–573. [Google Scholar] [CrossRef]
- Dangel, V.; Giray, J.; Ratge, D.; Wisser, H. Regulation of β-adrenoceptor density and mRNA levels in the rat heart cell-line H9c. Biochem. J. 1996, 317, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Lohse, M.J.; Engelhardt, S.; Eschenhagen, T. What is the role of β-adrenergic signaling in heart failure? Circ. Res. 2003, 93, 896–906. [Google Scholar] [CrossRef]
- Vidal, M.; Wieland, T.; Lohse, M.J.; Lorenz, K. β-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathway. Cardiovasc. Res. 2012, 96, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Leitz, M.R.; Oberdorf-Maass, S.; Lohse, M.J.; Klotz, K.N. Comparative pharmacology of human β-adrenergic receptor subtypes-characterization of stably transfected receptors in CHO cells. Naunyn. Schmiedebergs. Arch. Pharm. 2004, 369, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Del Carmine, R.; Ambrosio, C.; Sbraccia, M.; Cotecchia, S.; Ijzerman, A.P.; Costa, T. Mutations inducing divergent shifts of constitutive activity reveal different modes of binding among catecholamine analogues to the β2-adrenergic receptor. Br. J. Pharm. 2002, 135, 1715–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, J.N. Titration of β-blockers in heart failure: How to maximize benefit while minimizing adverse events. Postgrad Med. 2003, 113, 63–70. [Google Scholar] [CrossRef]
- Schmitt, B.; Li, T.; Kutty, S.; Khasheei, A.; Schmitt, K.R.L.; Anderson, R.H.; Lunkenheimer, P.P.; Berger, F.; Kühne, T.; Peters, B. Call for papers impact of sympathoexcitation on cardiovascular function in humans effects of incremental beta-blocker dosing on myocardial mechanics of the human left ventricle: MRI 3D-tagging insight into pharmacodynamics supports theory of inner antagonism. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H45–H52. [Google Scholar]
- Gislason, G.H.; Rasmussen, J.N.; Abildstrøm, S.Z.; Gadsbøll, N.; Buch, P.; Friberg, J.; Rasmussen, S.; Køber, L.; Stender, S.; Madsen, M.; et al. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur. Heart J. 2006, 27, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimmitt, S.B.; Stampfer, H.G. Low drug doses may improve outcomes in chronic disease. Med. J. Aust. 2009, 191, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Dimmitt, S.B.; Stampfer, H.G.; Warren, J.B. β-adrenoceptor blockers valuable but higher doses not necessary. Br. J. Clin. Pharm. 2014, 78, 1076–1079. [Google Scholar] [CrossRef] [Green Version]


| Compound | Binding Energy (kal/mol) |
|---|---|
| 4 | −8.0 |
| 7 | −7.7 |
| 8 | −7.5 |
| 10 | −7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, F.; De Bartolo, A.; Occhiuzzi, M.A.; Caruso, A.; Rocca, C.; Pasqua, T.; Carocci, A.; Rago, V.; Angelone, T.; Sinicropi, M.S. Carbazole and Simplified Derivatives: Novel Tools toward ?-Adrenergic Receptors Targeting. Appl. Sci. 2021, 11, 5486. https://doi.org/10.3390/app11125486
Grande F, De Bartolo A, Occhiuzzi MA, Caruso A, Rocca C, Pasqua T, Carocci A, Rago V, Angelone T, Sinicropi MS. Carbazole and Simplified Derivatives: Novel Tools toward ?-Adrenergic Receptors Targeting. Applied Sciences. 2021; 11(12):5486. https://doi.org/10.3390/app11125486
Chicago/Turabian StyleGrande, Fedora, Anna De Bartolo, Maria Antonietta Occhiuzzi, Anna Caruso, Carmine Rocca, Teresa Pasqua, Alessia Carocci, Vittoria Rago, Tommaso Angelone, and Maria Stefania Sinicropi. 2021. "Carbazole and Simplified Derivatives: Novel Tools toward ?-Adrenergic Receptors Targeting" Applied Sciences 11, no. 12: 5486. https://doi.org/10.3390/app11125486
APA StyleGrande, F., De Bartolo, A., Occhiuzzi, M. A., Caruso, A., Rocca, C., Pasqua, T., Carocci, A., Rago, V., Angelone, T., & Sinicropi, M. S. (2021). Carbazole and Simplified Derivatives: Novel Tools toward ?-Adrenergic Receptors Targeting. Applied Sciences, 11(12), 5486. https://doi.org/10.3390/app11125486