Mechanical and Gamma-Ray Interaction Studies of PbO–MoO3–Li2O–B2O3 Glass System for Shielding Applications in The Low Energy Region: A Theoretical Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Glass Samples
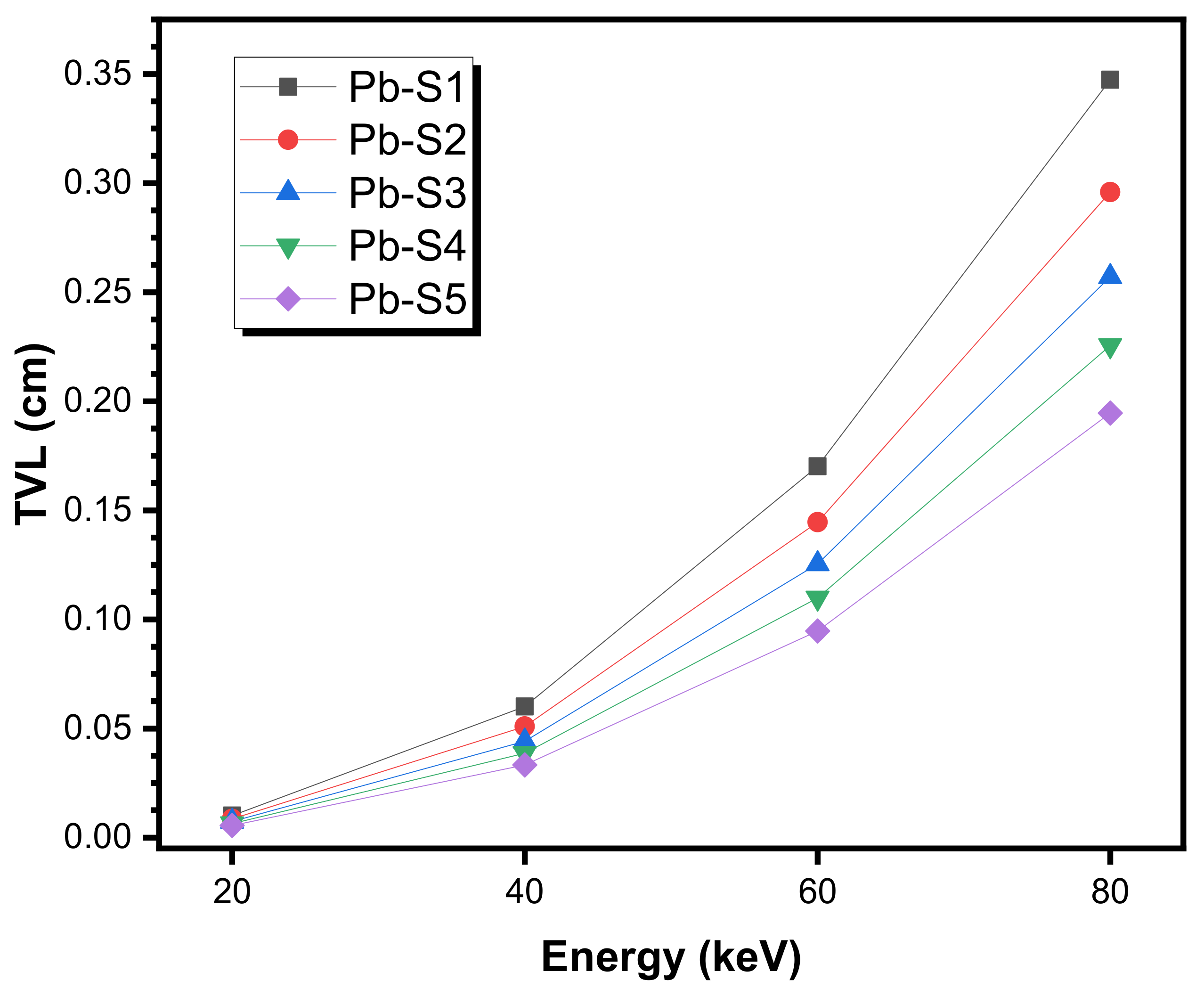
- Pb-S1: 30 PbO–5 MoO3–25 Li2O–40 B2O3 (density = 4.354 g/cm3)
- Pb-S2: 35 PbO–5 MoO3–20 Li2O–40 B2O3 (density = 4.838 g/cm3)
- Pb-S3: 40 PbO–5 MoO3–15 Li2O–40 B2O3 (density = 5.327 g/cm3)
- Pb-S4: 45 PbO–5 MoO3–10 Li2O–40 B2O3 (density = 5.853 g/cm3)
- Pb-S5: 50 PbO–5 MoO3–5 Li2O–40 B2O3 (density = 6.578 g/cm3)
2.2. Physical Properties
2.3. Mechanical Properties
2.4. Gamma Ray Shielding Properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tijani, S.A.; Al-Hadeethi, Y. The influence of TeO2 and Bi2O3 on the shielding ability of lead-free transparent bismuth tellurite glass at low gamma energy range. Ceram. Int. 2019, 45, 23572–23577. [Google Scholar] [CrossRef]
- Tijani, S.A.; Al-Hadeethi, Y. The use of isophthalic-bismuth polymer composites as radiation shielding barriers in nuclear medicine. Mater. Res. Express 2019, 6, 055323. [Google Scholar] [CrossRef]
- Kulali, F. Simulation Studies on Radiological Parameters for marble concrete. Emerg. Mater. Res. 2020, 9, 1341–1347. [Google Scholar] [CrossRef]
- Malidarrea, R.B.; Kulali, F.; Inal, A.; Oz, A. Monte Carlo simulation of the Waste Soda-Lime-Silica Glass system contained Sb2O3. Emerg. Mater. Res. 2020, 9, 1334–1340. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mhareb, M.H.A.; Alajerami, Y.S.M.; Mahmoud, K.A.; Imheidat, M.A.; Alshahri, F.; Alqahtani, M.; Al-Abdullah, T. Optical and radiation shielding features for a new series of borate glass samples. Optik 2021, 239, 166790. [Google Scholar] [CrossRef]
- Akkurt, I.; Tekin, H.O. Radiological parameters for bismuth oxide glasses using Phy-X/PSD software. Emerg. Mater. Res. 2020, 9, 1020–1027. [Google Scholar] [CrossRef]
- Akkurt, I.; Basyigit, C.; Kilincarslan, S.; Mavi, B.; Akkurt, A. Radiation shielding of concretes containing different aggregates. Cem. Concr. Compos. 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Akkurt, I.; Akyıldırım, H.; Mavi, B.; Kilincarslan, S.; Basyigit, C. Photon attenuation coefficients of concrete includes barite in different rate. Ann. Nucl. Energy 2010, 37, 910–914. [Google Scholar] [CrossRef]
- Kurtulus, R.; Kavas, T.; Akkurt, I.; Gunoglu, K. An experimental study and WinXCom calculations on X-ray photon characteristics of Bi2O3 and Sb2O3 added waste soda-lime-silica glass. Ceram. Int. 2020, 46, 21120–21127. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Olarinoye, O.I.; Elsafi, M. Assessment of gamma-radiation attenuation characteristics of Bi2O3–B2O3–SiO2–Na2O glasses using Geant4 simulation code. Eur. Phys. J. Plus 2021, 136, 535. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Kumar, A.; Mahmoud, K.A.; El-Mallawany, R.; El-Agawany, F.I.; Susoy, G.; Tekin, H.O. SnO-reinforced silicate glasses and utilization in gamma radiation shielding applications. Emerg. Mater. Res. 2020, 9, 1000–1008. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.I. Radiation shielding characterizations and investigation of TeO2–WO3–Bi2O3 and TeO2–WO3–PbO glasses. Appl. Phys. A 2021, 127, 190. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Lacomme, E.; AlShammari, M.M.; Dwaikat, N.; Alajerami, Y.S.M.; Alqahtani, M.; El-bashir, B.O.; Mhareb, M.H.A. Development of a novel MoO3-doped borate glass network for gamma-ray shielding applications. Eur. Phys. J. Plus 2021, 136, 108. [Google Scholar] [CrossRef]
- Tekin, H.O.; Issa, S.A.M.; Mahmoud, K.A.; El-Agawany, F.I.; Rammah, Y.S.; Susoy, G.; Al-Buriahi, M.S.; Abuzaid, M.M.; Akkurt, I. Nuclear radiation shielding competences of Barium (Ba) reinforced borosilicate glasses. Emerg. Mater. Res. 2020, 9, 1131–1144. [Google Scholar] [CrossRef]
- Alorfi, H.S.; Hussein, M.A.; Tijani, S.A. The use of rocks in lieu of bricks and concrete as radiation shielding barriers at low gamma and nuclear medicine energies. Constr. Build. Mater. 2020, 251, 118908. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Rashid, M.A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Yasmin, S.; Rozaila, Z.S.; Khandaker, M.U.; Barua, B.S.; Chowdhury, F.U.Z.; Rashid, M.A.; Bradley, D.A. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Radiat. Eff. Defects Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mohammed, F.Q.; Mahmoud, K.A.; Lacomme, E.; Kaky, K.M.; Khandaker, M.U.; Faruque, M.R.I. Evaluation of radiation shielding features of Co and Ni-based superalloys using MCNP-5 code: Potential use in nuclear safety. Appl. Sci. 2020, 10, 7680. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Kothan, S.; Kedkaew, C.; Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 2020, 172, 108791. [Google Scholar] [CrossRef]
- Kaewjang, S.; Maghanemi, U.; Kothan, S.; Kim, H.J.; Limkitjaroenporn, P.; Kaewkhao, J. New gadolinium based glasses for gamma-rays shielding materials. Nucl. Eng. Des. 2014, 280, 21–26. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Tashlykov, O.L.; Khandaker, M.U.; Faruque, M.R.I. Enhancement of the Shielding Capability of Soda–Lime Glasses with Sb2O3 Dopant: A Potential Material for Radiation Safety in Nuclear Installations. Appl. Sci. 2021, 11, 326. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Khattari, Z.Y.; Kumar, A.; Al-Jundi, J.; Dong, M.G.; Al Zaatreh, M.Y. Radiation shielding parameters of BaO–Nb2O5–P2O5 glass system using MCNP5 code and XCOM software. Mater. Res. Express 2018, 5, 115203. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.P.; Elmahroug, Y.; Kara, U.; Tekin, H.O.; Sayyed, M.I. Gamma ray shielding studies on 26.66 B2O3–16GeO2–4Bi2O3–(53.33–x) PbO–xPbF2 glass system using MCNPX, Geant4 and XCOM. Mater. Res. Express 2018, 5, 095203. [Google Scholar] [CrossRef]
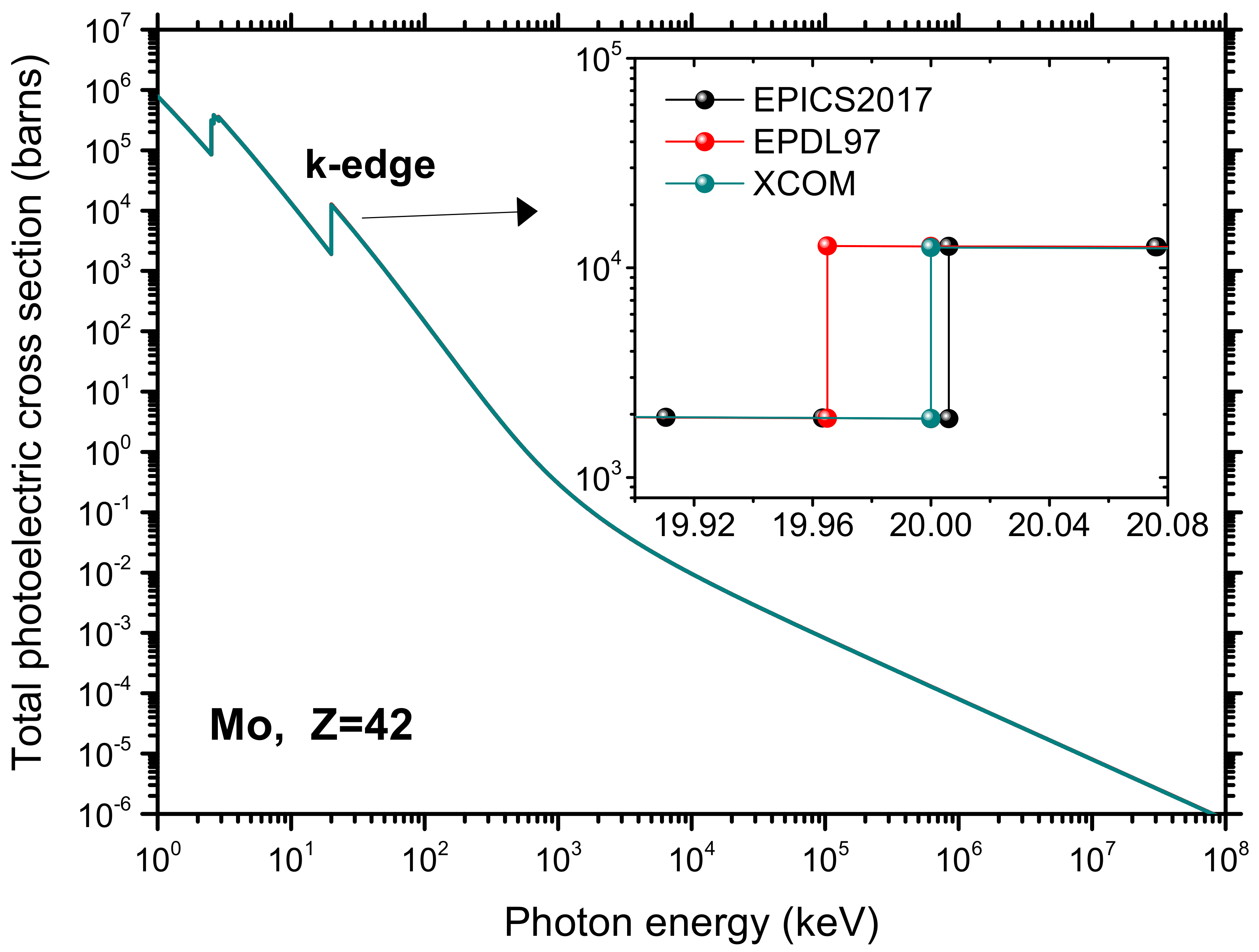
- Cullen, D.E. Nuclear Data Preparation. In Handbook of Nuclear Engineering; Springer: Boston, MA, USA, 2010; pp. 279–425. [Google Scholar]
- Cullen, D.E. A Survey of Photon Cross Section Data for Use in EPICS2017; IAEA-NDS-225; IAEA-NDS: Vienna, Austria, 2018. [Google Scholar]
- Cullen, D.E. EPICS2017: April 2019 Status Report; IAEA-NDS-228; IAEA-NDS: Vienna, Austria, 2019. [Google Scholar]
- Brown, D.A.; Chadwick, M.B.; Capote, R.; Kahler, A.C.; Trkov, A.; Herman, M.W.; Sonzogni, A.A.; Danon, Y.; Carlson, A.D.; Dunn, M.; et al. ENDF/B-VIII.0: The 8th Major Release of the Nuclear Reaction Data Library with CIELO-project Cross Sections, New Standards and Thermal Scattering Data. Nucl. Data Sheets 2018, 148, 1–142. [Google Scholar] [CrossRef]
- Cullen, D.E.; Hubbell, J.H.; Kissel, L. EPDL97: The Evaluated Photo Data Library ′97 Version; USDOE: Washington, DC, USA, 1997.
- Hila, F.C.; Amorsolo, A.V.; Javier-Hila, A.M.V.; Guillermo, N.R.D. A simple spreadsheet program for calculating mass attenuation coefficients and shielding parameters based on EPICS2017 and EPDL97 photoatomic libraries. Radiat. Phys. Chem. 2020, 177, 109122. [Google Scholar] [CrossRef]
- Hila, F.C.; Asuncion-Astronomo, A.; Dingle, C.A.M.; Jecong, J.F.M.; Javier-Hila, A.M.V.; Gili, M.B.Z.; Balderas, C.V.; Lopez, G.E.P.; Guillermo, N.R.D.; Amorsolo, A.V., Jr. EpiXS: A Windows-based program for photon attenuation, dosimetry and shielding based on EPICS2017. Radiat. Phys. Chem. 2021, 182, 109331. [Google Scholar] [CrossRef]
- Ali, A.M.; Sayyed, M.I.; Rashad, M.; Kumar, A.; Kaur, R.; Aşkın, A.; Algarni, H. Gamma ray shielding behavior of Li2O doped PbO–MoO3–B2O3 glass system. Appl. Phys. A 2019, 125, 671. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, R.; Sayyed, M.I.; Rashad, M.; Singh, M.; Ali, A.M. Physical, structural, optical and gamma ray shielding behavior of (20 + x) PbO–10 BaO–10 Na2O–10 MgO–(50 − x) B2O3 glasses. Phys. B Condens. Matter 2019, 552, 110–118. [Google Scholar] [CrossRef]
- Hager, I.Z.; El-Mallawany, R. Preparation and structural studies in the (70 − x)TeO2–20WO3–10Li2O–xLn2O3 glasses. J. Mater. Sci. 2010, 45, 897–905. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non-Cryst. Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Direct calculations of Young modulus of glass. J. Non-Cryst. Solids 1973, 12, 35–45. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Almuqurin, A.H.; Sayyed, M.I.; Kumar, A.; El-bashir, B.O.; Akkurt, I. Optical, mechanical properties and gamma ray shielding behavior of TeO2-Bi2O3-PbO-MgO-B2O3 glasses using FLUKA simulation code. Opt. Mat. 2021, 113, 110900. [Google Scholar] [CrossRef]
- Gowda, V.C.V. Physical, thermal, infrared and optical properties of Nd3+ doped lithium–lead-germanate glasses. Phys. B 2015, 456, 298–305. [Google Scholar] [CrossRef]
- Saddeek, Y.B. Structural and acoustical studies of lead sodium borate glasses. J. Alloys Compd. 2009, 467, 14–21. [Google Scholar] [CrossRef]
- Sidek, H.A.A.; El-Mallawany, R.; Matori, K.A.; Halimah, M.K. Effect of PbO on the elastic behavior of ZnO–P2O5 glass systems. Results Phys. 2016, 6, 449–455. [Google Scholar] [CrossRef]
- Saunders, G.A.; Brennan, T.; Acet, M.; Cankurtaran, M.; Senin, H.B.; Sidek, H.A.A.; Federico, M. Elastic and non-linear acoustic properties and thermal expansion of cerium metaphosphate glasses. J. Non-Cryst. Solids 2001, 282, 291–305. [Google Scholar] [CrossRef]
- Alazoumi, S.H.; Sidek, H.A.A.; Halimah, M.K.; Matori, K.A.; Zaid, M.H.M.; Abdulbaset, A.A. Synthesis and elastic properties of ternary ZnO-PbO-TeO glasses. Chalcogenide Lett. 2017, 14, 303–320. [Google Scholar]
- Issa, S.A.M.; Kumar, A.; Sayyed, M.I.; Dong, M.G.; Elmahroug, Y. Mechanical and gamma-ray shielding properties of TeO2-ZnO-NiO glasses. Mater. Chem. Phys. 2018, 212, 12–20. [Google Scholar] [CrossRef]
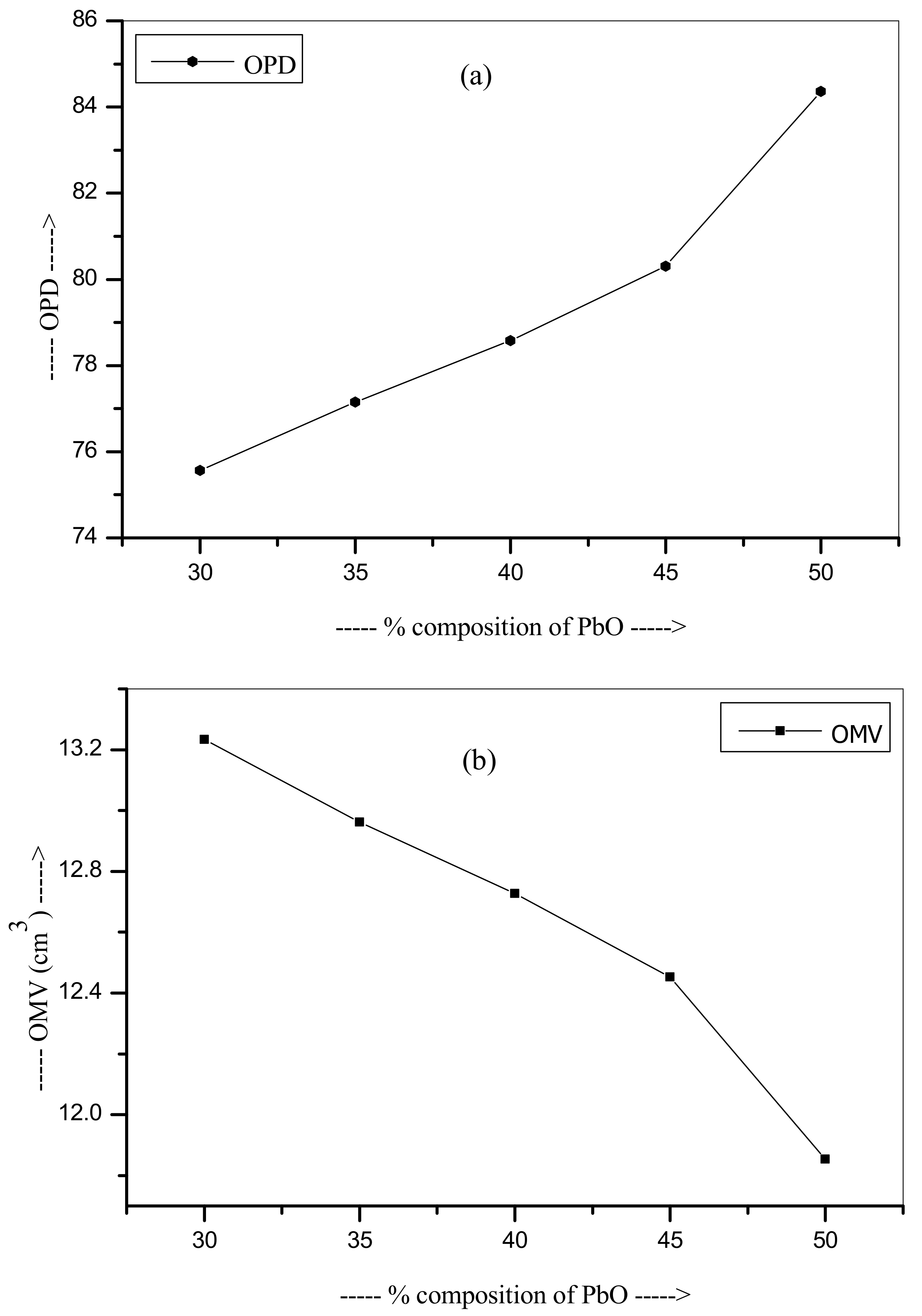
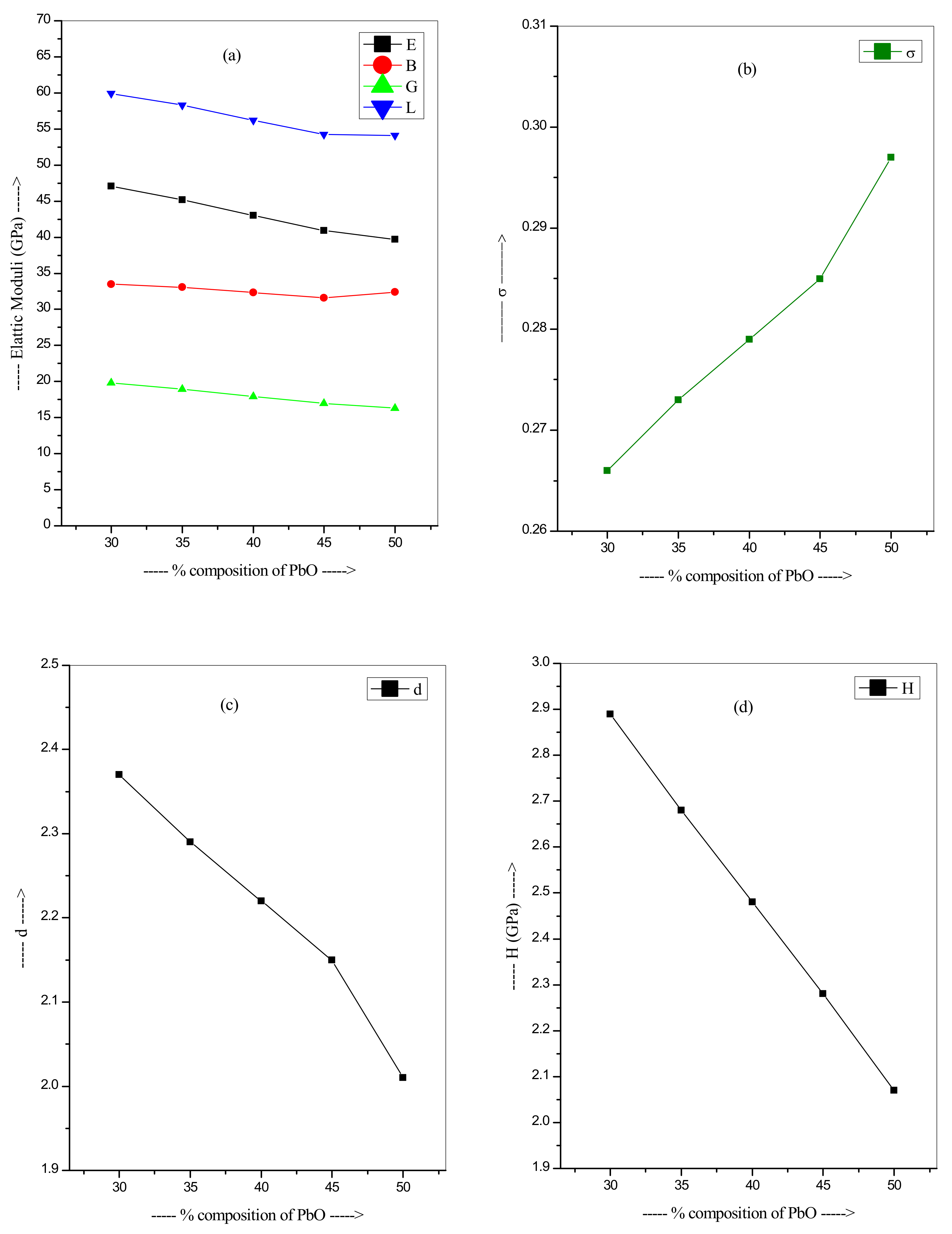



| Glass Code | Mole % of Oxides Present | Density (g/cm3) | Vm (cm3/mol) | OPD (mol/L) | OMV (cm3/mol) | |||
|---|---|---|---|---|---|---|---|---|
| PbO | MoO3 | Li2O | B2O3 | |||||
| Pb-S1 | 30 | 5 | 25 | 40 | 4.354 | 25.144 | 75.563 | 13.234 |
| Pb-S2 | 35 | 5 | 20 | 40 | 4.838 | 24.626 | 77.151 | 12.962 |
| Pb-S3 | 40 | 5 | 15 | 40 | 5.327 | 24.180 | 78.575 | 12.727 |
| Pb-S4 | 45 | 5 | 10 | 40 | 5.853 | 23.658 | 80.307 | 12.452 |
| Pb-S5 | 50 | 5 | 5 | 40 | 6.578 | 22.520 | 84.366 | 11.853 |
| Glass Code | Mechanical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| nb (cm−3) | nc | E (GPa) | B (GPa) | G (GPa) | L (GPa) | σ | d | H (GPa) | |
| Pb-S1 | 1.030 × 1023 | 1.939 | 47.06 | 33.51 | 19.82 | 59.94 | 0.266 | 2.37 | 2.89 |
| Pb-S2 | 1.076 × 1023 | 2.000 | 45.17 | 33.07 | 18.93 | 58.30 | 0.273 | 2.29 | 2.68 |
| Pb-S3 | 1.121 × 1023 | 2.064 | 43.03 | 32.30 | 17.94 | 56.23 | 0.279 | 2.22 | 2.48 |
| Pb-S4 | 1.171 × 1023 | 2.133 | 40.89 | 31.59 | 16.96 | 54.21 | 0.285 | 2.15 | 2.28 |
| Pb-S5 | 1.257 × 1023 | 2.207 | 39.67 | 32.41 | 16.29 | 54.14 | 0.297 | 2.01 | 2.07 |
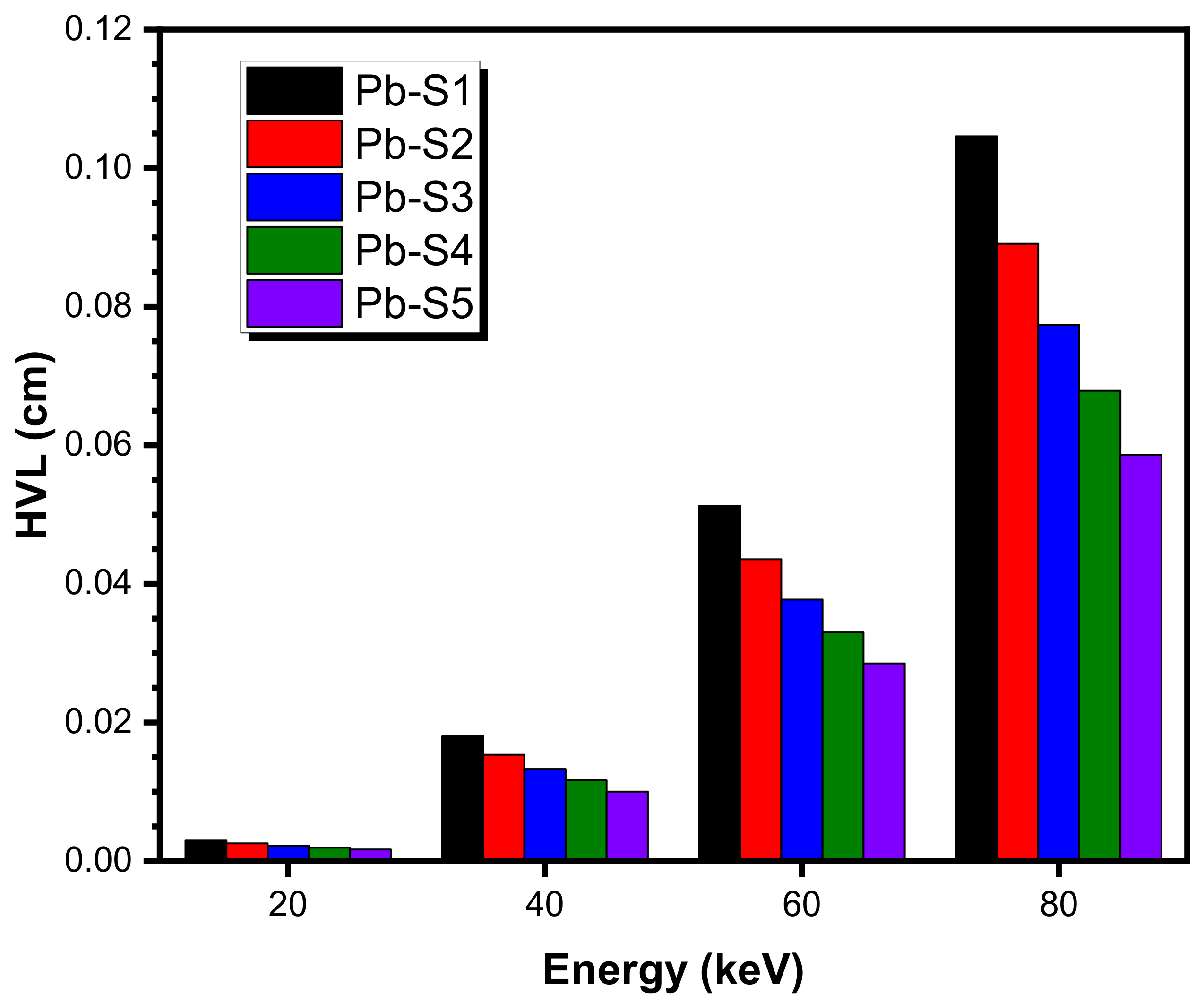
| Energy (keV) | Pb-S1 | Pb-S2 | Pb-S3 | ||||||
| EPICS2017, ENDF/B-VIII (EpiXS) | EPDL97, ENDF/B-VI.8 (EpiXS) | Phy-X/PSD | EPICS2017, ENDF/B-VIII (EpiXS) | EPDL97, ENDF/B-VI.8 (EpiXS) | Phy-X/PSD | EPICS2017, ENDF/B-VIII (EpiXS) | EPDL97, ENDF/B-VI.8 (EpiXS) | Phy-X/PSD | |
| 20 | 49.703 | 52.619 | 52.799 | 53.155 | 55.827 | 56.025 | 56.089 | 58.554 | 58.767 |
| 40 | 8.808 | 8.791 | 8.811 | 9.340 | 9.323 | 9.344 | 9.792 | 9.774 | 9.797 |
| 60 | 3.085 | 3.084 | 3.107 | 3.267 | 3.266 | 3.291 | 3.421 | 3.421 | 3.447 |
| 80 | 1.486 | 1.494 | 1.522 | 1.570 | 1.578 | 1.608 | 1.641 | 1.650 | 1.682 |
| Energy (keV) | Pb-S4 | Pb-S5 | |||||||
| EPICS2017, ENDF/B-VIII (EpiXS) | EPDL97, ENDF/B-VI.8 (EpiXS) | Phy-X/PSD | EPICS2017, ENDF/B-VIII (EpiXS) | EPDL97, ENDF/B-VI.8 (EpiXS) | Phy-X/PSD | ||||
| 20 | 58.613 | 60.900 | 61.126 | 60.808 | 62.939 | 63.177 | |||
| 40 | 10.182 | 10.163 | 10.187 | 10.520 | 10.501 | 10.526 | |||
| 60 | 3.554 | 3.554 | 3.581 | 3.670 | 3.669 | 3.698 | |||
| 80 | 1.702 | 1.711 | 1.744 | 1.755 | 1.765 | 1.799 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqrin, A.H.; Sayyed, M.I.; Albarzan, B.; Javier-Hila, A.M.V.; Alwadai, N.; Kumar, A. Mechanical and Gamma-Ray Interaction Studies of PbO–MoO3–Li2O–B2O3 Glass System for Shielding Applications in The Low Energy Region: A Theoretical Approach. Appl. Sci. 2021, 11, 5538. https://doi.org/10.3390/app11125538
Almuqrin AH, Sayyed MI, Albarzan B, Javier-Hila AMV, Alwadai N, Kumar A. Mechanical and Gamma-Ray Interaction Studies of PbO–MoO3–Li2O–B2O3 Glass System for Shielding Applications in The Low Energy Region: A Theoretical Approach. Applied Sciences. 2021; 11(12):5538. https://doi.org/10.3390/app11125538
Chicago/Turabian StyleAlmuqrin, Aljawhara H., M. I. Sayyed, Badriah Albarzan, Abigaile Mia V. Javier-Hila, Norah Alwadai, and Ashok Kumar. 2021. "Mechanical and Gamma-Ray Interaction Studies of PbO–MoO3–Li2O–B2O3 Glass System for Shielding Applications in The Low Energy Region: A Theoretical Approach" Applied Sciences 11, no. 12: 5538. https://doi.org/10.3390/app11125538
APA StyleAlmuqrin, A. H., Sayyed, M. I., Albarzan, B., Javier-Hila, A. M. V., Alwadai, N., & Kumar, A. (2021). Mechanical and Gamma-Ray Interaction Studies of PbO–MoO3–Li2O–B2O3 Glass System for Shielding Applications in The Low Energy Region: A Theoretical Approach. Applied Sciences, 11(12), 5538. https://doi.org/10.3390/app11125538








