Simple Thalidomide Analogs in Melanoma: Synthesis and Biological Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Biological Methods
2.2.1. Cell Culture
2.2.2. MTT Assay
2.2.3. Immunofluorescence Analysis
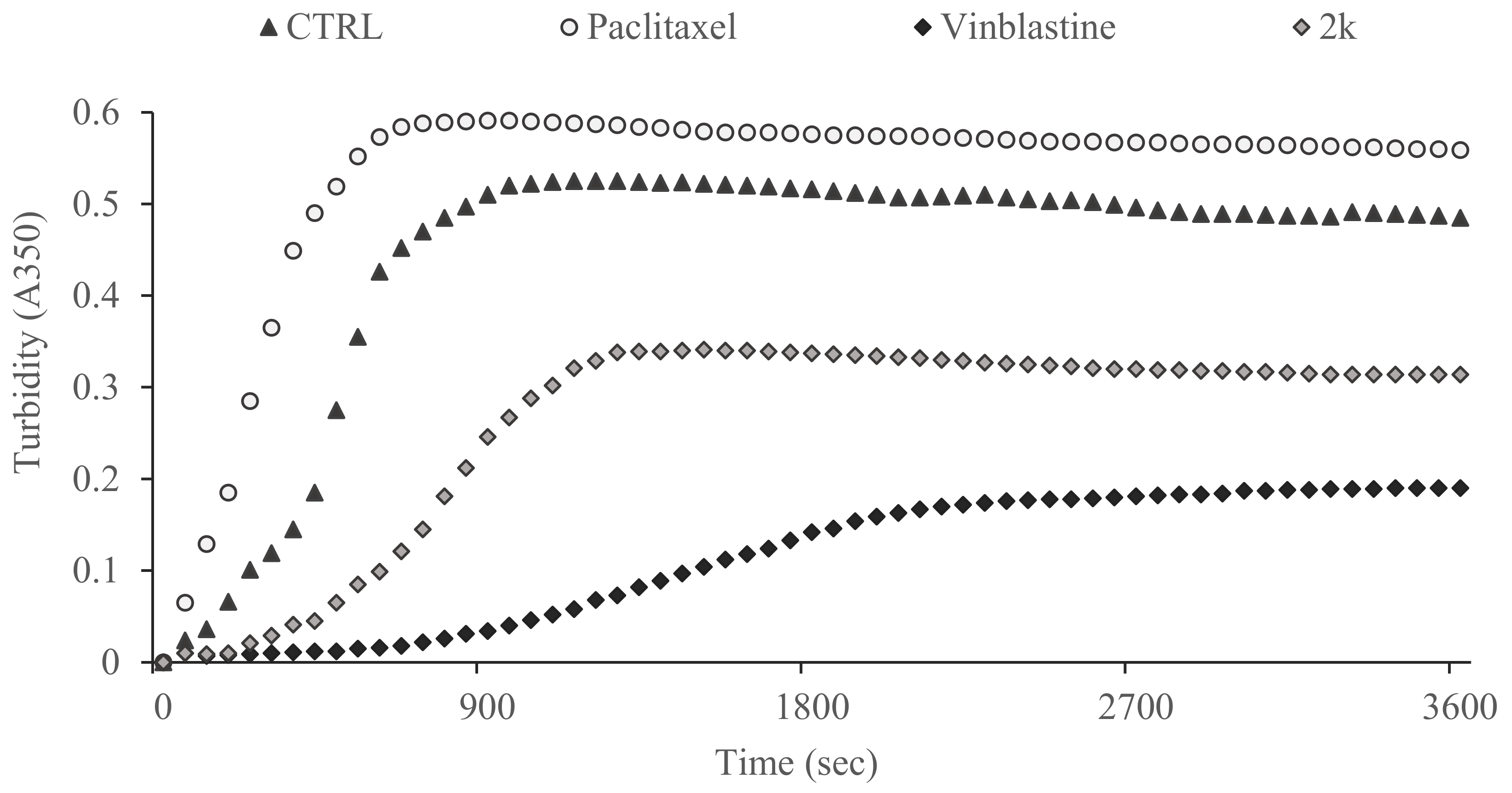
2.2.4. Tubulin Polymerization Assay
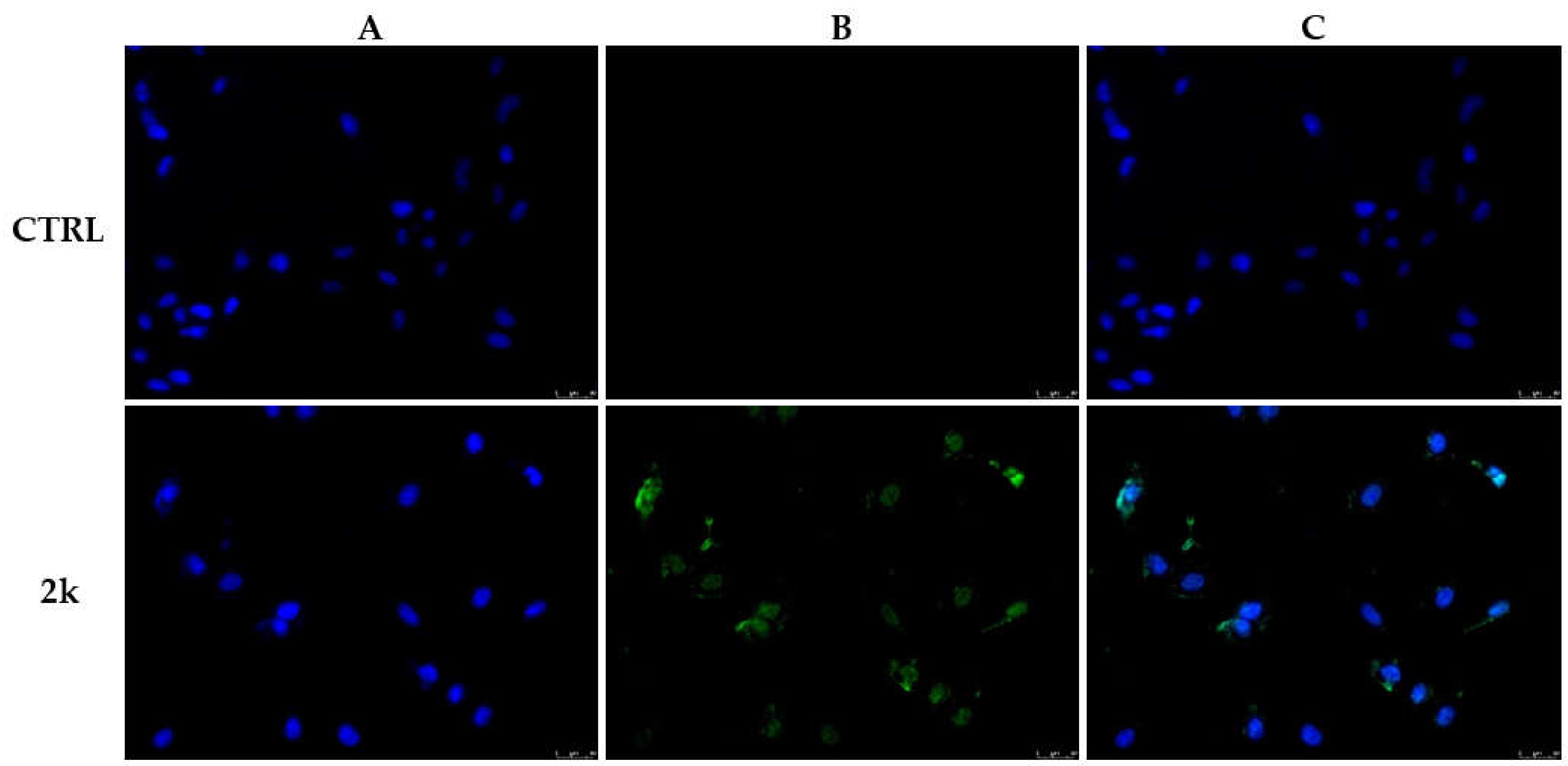
2.2.5. TUNEL Assay
2.2.6. Western Blotting Analysis
2.2.7. Docking Studies
3. Results and Discussion
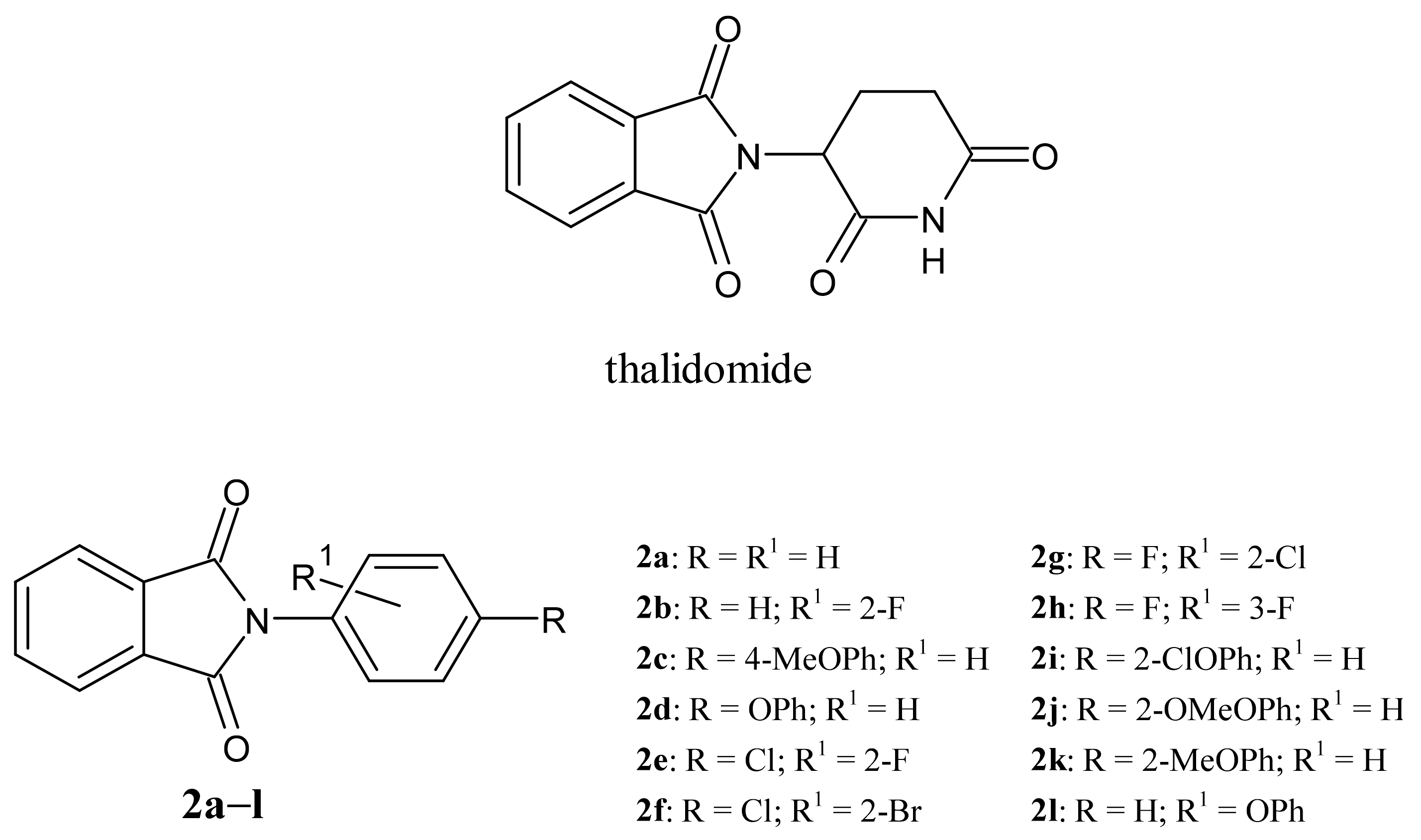
3.1. Chemistry
3.2. Biological Results
3.2.1. Cell Viability Assay
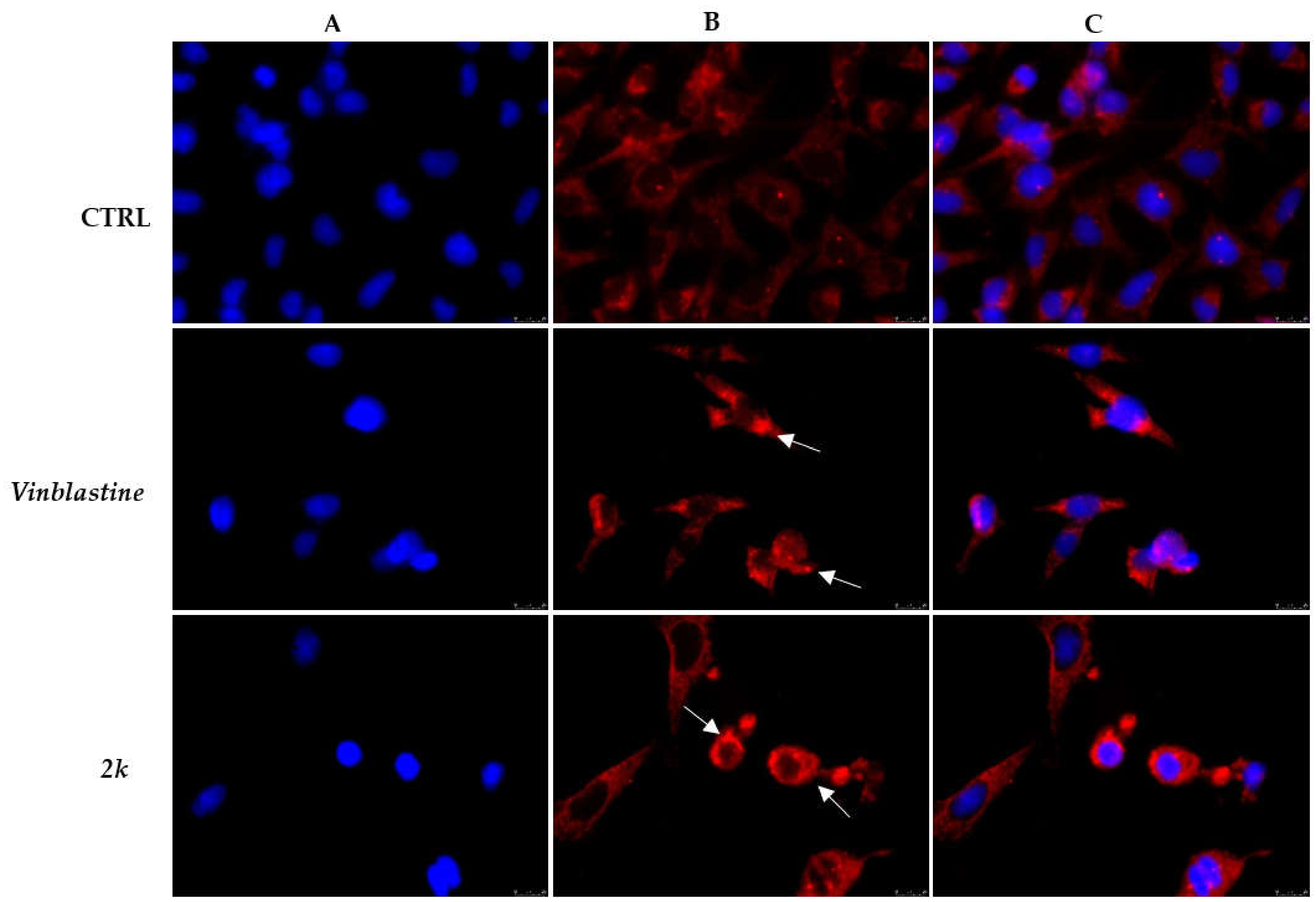
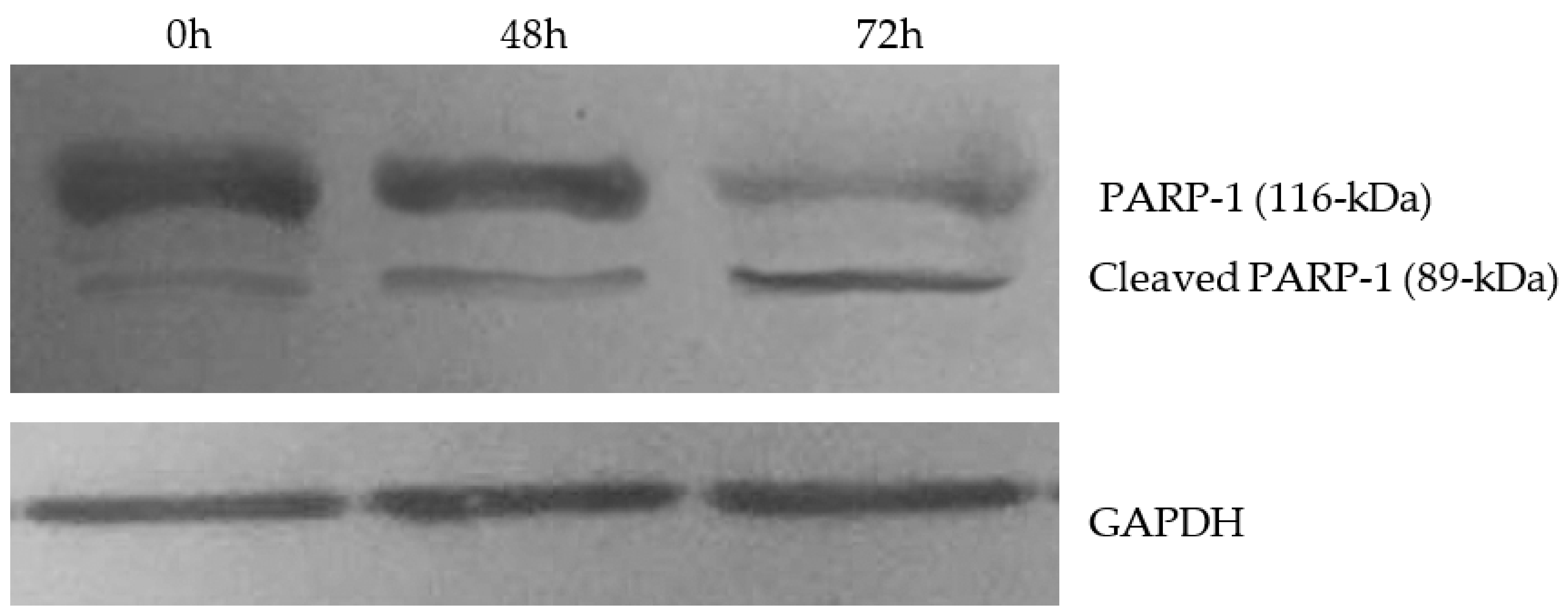
3.2.2. Effect of Compound 2k on Microtubule Dynamics and Cell Death by Apoptosis
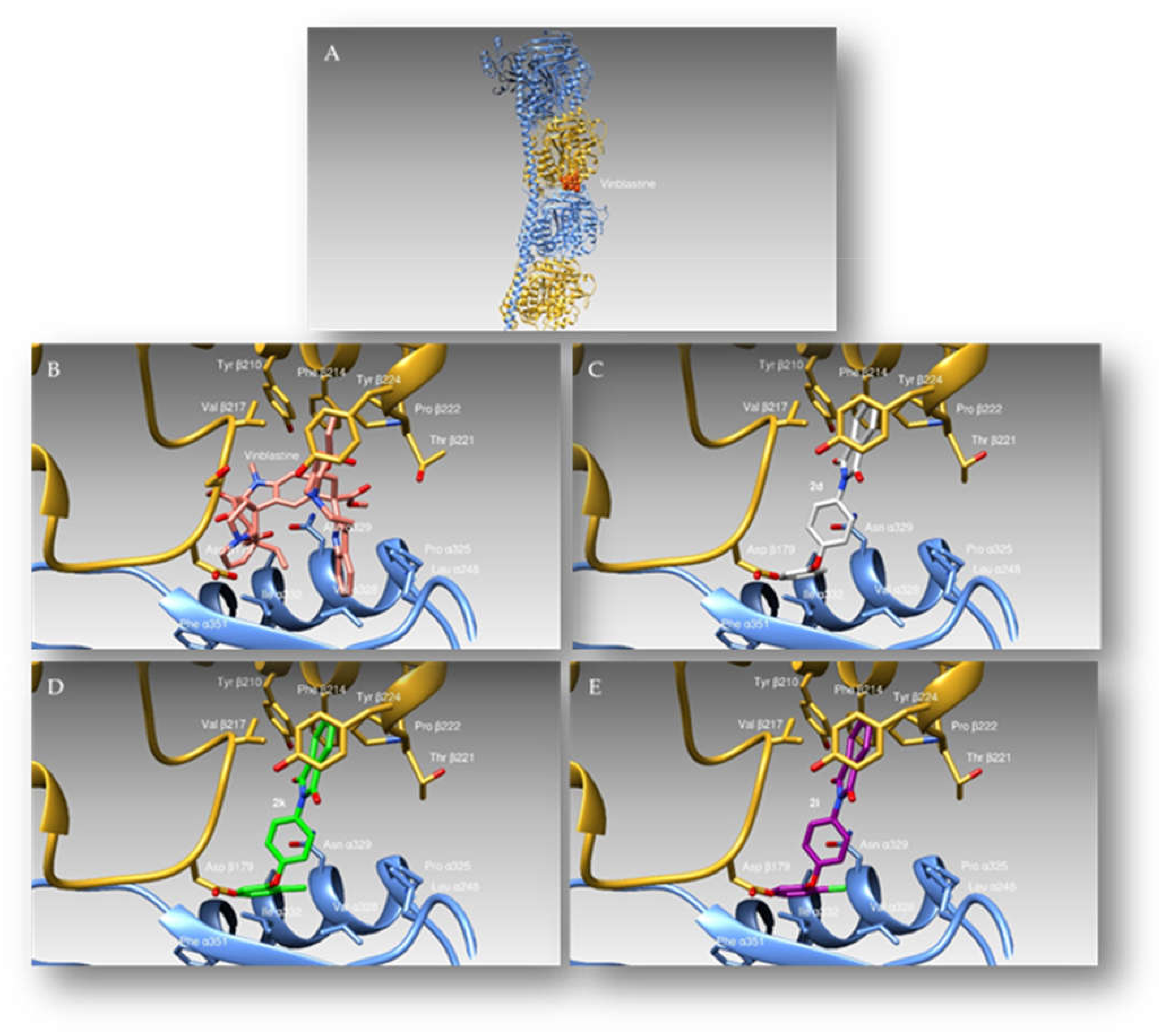
3.2.3. Docking Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Paolino, G.; Bekkenk, M.W.; Didona, D.; Eibenschutz, L.; Richetta, A.G.; Cantisani, C.; Viti, G.P.; Carbone, A.; Buccini, P.; De Simone, P.; et al. Is the prognosis and course of acral melanoma related to site-specific clinicopathological features? Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 842–848. [Google Scholar] [PubMed]
- Scali, E.; Mignogna, C.; Di Vito, A.; Presta, I.; Camastra, C.; Donato, G.; Bottoni, U. Inflammation and macrophage polarization in cutaneous melanoma: Histopathological and immunohistochemical study. Int. J. Immunopathol. Pharmacol. 2016, 29, 715–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Q.; Long, J.; Hu, L.; Chen, Z.; Li, Q.; Hu, G. Drug repurposing and rediscovery: Design, synthesis, and preliminary biological evaluation of 1-arylamino-3-aryloxypropan-2-ols as anti-melanoma agents. Bioorg. Med. Chem. 2020, 28, 115404. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, A.; Adriani, G.; Catalano, A.; Carocci, A.; Rao, L.; Lentini, G.; Cavalluzzi, M.M.; Franchini, C.; Vacca, A.; Corbo, F. A mini-review on thalidomide: Chemistry, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr. Med. Chem. 2017, 24, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, M.; Gu, Y.; Liu, Z.; Xu, S.; Cui, Y.; Sun, B. Thalidomide influences growth and vasculogenic mimicry channel formation in melanoma. J. Exp. Clin. Cancer Res. 2008, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rashid, A.; Kuppa, A.; Kunwar, A.; Panda, D. Thalidomide (5HPP-33) suppresses microtubule dynamics and depolymerizes the microtubule network by binding at the vinblastine binding site on tubulin. Biochemistry 2015, 54, 2149–2159. [Google Scholar] [CrossRef]
- Inatsuki, S.; Noguchi, T.; Miyachi, H.; Oda, S.; Iguchi, T.; Kizaki, M.; Hashimoto, Y.; Kobayashi, H. Tubulin-polymerization inhibitors derived from thalidomide. Bioorg. Med. Chem. Lett. 2005, 15, 321–325. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Rajkumar, S.V. Management of thalidomide toxicity. J. Support. Oncol. 2003, 1, 194–205. [Google Scholar]
- Pessoa, C.; Ferreira, P.M.P.; Lotufo, L.V.C.; de Moraes, M.O.; Cavalcanti, S.M.; Coêlho, L.C.D.; Hernandes, M.Z.; Leite, A.C.L.; De Simone, C.A.; Costa, V.M.; et al. Discovery of phthalimides as immunomodulatory and antitumor drug prototypes. ChemMedChem Chem. Enabling Drug Discov. 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Da Costa, P.M.; da Costa, M.P.; Carvalho, A.A.; Cavalcanti, S.M.T.; de Oliveira Cardoso, M.V.; de Oliveira Filho, G.B.; Viana, D.D.A.; Fechine-Jamacaru, F.V.; Leite, A.L.; De Moraes, M.O.; et al. Improvement of in vivo anticancer and antiangiogenic potential of thalidomide derivatives. Chem. Biol. Interact. 2015, 239, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Saturnino, C.; Caruso, A.; Longo, P.; Capasso, A.; Pingitore, A.; Cristina Caroleo, M.; Cione, E.; Perri, M.; Nicolo, F.; Mollica Nardo, V.; et al. Crystallographic study and biological evaluation of 1, 4-dimethyl-N-alkylcarbazoles. Curr. Top. Med. Chem. 2015, 15, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Caruso, A.; Occhiuzzi, M.A.; Iacopetta, D.; Barbarossa, A.; Rizzuti, B.; Dallemagne, P.; Rault, S.; El-Kashef, H.; Saturnino, C.; et al. Benzothienoquinazolinones as new multi-target scaffolds: Dual inhibition of human Topoisomerase I and tubulin polymerization. Eur. J. Med. Chem. 2019, 181, 111583. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muià, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceramella, J.; Mariconda, A.; Rosano, C.; Iacopetta, D.; Caruso, A.; Longo, P.; Sinicropi, M.S.; Saturnino, C. α–ω Alkenyl-bis-S-Guanidine Thiourea Dihydrobromide Affects HeLa Cell Growth Hampering Tubulin Polymerization. ChemMedChem 2020, 15, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Lappano, R.; Mariconda, A.; Ceramella, J.; Sinicropi, M.S.; Saturnino, C.; Talia, M.; Cirillo, F.; Martinelli, F.; Puoci, F.; et al. Newly Synthesized Imino-Derivatives Analogues of Resveratrol Exert Inhibitory Effects in Breast Tumor Cells. Int. J. Mol. Sci. 2020, 21, 7797. [Google Scholar] [CrossRef]
- Iacopetta, D.; Carocci, A.; Sinicropi, M.S.; Catalano, A.; Lentini, G.; Ceramella, J.; Curcio, R.; Caroleo, M.C. Old drug scaffold, new activity: Thalidomide-correlated compounds exert different effects on breast cancer cell growth and progression. ChemMedChem 2017, 12, 381–389. [Google Scholar] [CrossRef]
- Aliabadi, A.; Gholamine, B.; Karimi, T. Synthesis and antiseizure evaluation of isoindoline-1,3-dione derivatives in mice. Med. Chem. Res. 2014, 23, 2736–2743. [Google Scholar] [CrossRef]
- Assis, S.P.O.; Araújo, T.G.; Sena, V.L.; Catanho, M.T.J.; Ramos, M.N.; Srivastava, R.M.; Lima, V.L. Synthesis, hypolipidemic, and anti-inflammatory activities of arylphthalimides. Med. Chem. Res. 2014, 23, 708–716. [Google Scholar] [CrossRef]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muià, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and β-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Saturnino, C.; Malzert-Fréon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the way to fight cancer paved with gold? Metal-based carbene complexes with multiple and fascinating biological features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar]
- Rechoum, Y.; Rovito, D.; Iacopetta, D.; Barone, I.; Andò, S.; Weigel, N.L.; O’Malley, B.W.; Brown, P.H.; Fuqua, S.A.W. AR collaborates with ERα in aromatase inhibitor-resistant breast cancer. Breast Cancer Res. Treat. 2014, 147, 473–485. [Google Scholar] [CrossRef]
- Waight, A.B.; Bargsten, K.; Doronina, S.; Steinmetz, M.O.; Sussman, D.; Prota, A.E. Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLoS ONE 2016, 11, e0160890. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Sanner, M.F.; Duncan, B.S.; Carrillo, C.J.; Olson, A.J. Integrating computation and visualization for biomolecular analysis: An example using python and AVS. Pac. Symp. Biocomput. 1999, 8, 401–412. [Google Scholar]
- Cesarini, S.; Spallarossa, A.; Ranise, A.; Schenone, S.; Rosano, C.; La Colla, P.; Sanna, G.; Busonera, B.; Loddo, R. N-acylated and N,N’-diacylated imidazolidine-2-thione derivatives and N,N’-diacylated tetrahydropyrimidine-2(1H)-thione analogues: Synthesis and antiproliferative activity. Eur. J. Med. Chem. 2009, 44, 1106–1118. [Google Scholar] [CrossRef]
- Viale, M.; Cordazzo, C.; de Totero, D.; Budriesi, R.; Rosano, C.; Leoni, A.; Ioan, P.; Aiello, C.; Croce, M.; Andreani, A.; et al. Inhibition of MDR1 activity and induction of apoptosis by analogues of nifedipine and diltiazem: An in vitro analysis. Investig. New Drugs 2011, 29, 98–109. [Google Scholar] [CrossRef]
- Rosano, C.; Lappano, R.; Santolla, M.F.; Ponassi, M.; Donadini, A.; Maggiolini, M. Recent advances in the rationale design of GPER ligands. Curr. Med. Chem. 2012, 19, 6199–6206. [Google Scholar] [CrossRef]
- Saturnino, C.; Iacopetta, D.; Sinicropi, M.S.; Rosano, C.; Caruso, A.; Caporale, A.; Marra, N.; Marengo, B.; Pronzato, M.A.; Parisi, O.I.; et al. N-alkyl carbazole derivatives as new tools for Alzheimer’s disease: Preliminary studies. Molecules 2014, 19, 9307–9317. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera- A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Zhang, D.; Kanakkanthara, A. Beyond the paclitaxel and Vinca alkaloids: Next generation of plant-derived microtubule-targeting agents with potential anticancer activity. Cancers 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Yele, V.; Pindiprolu, S.K.S.; Sana, S.; Ramamurty, D.S.V.N.M.; Madasi, J.R.; Vadlamani, S. Synthesis and preclinical evaluation of indole triazole conjugates as microtubule targeting agents that are effective against MCF-7 breast cancer cell lines. Anti-Cancer Agents Med. Chem. 2021, 21, 1047–1055. [Google Scholar] [CrossRef]
- Karahalil, B.; Yardım-Akaydin, S.; Nacak Baytas, S. An overview of microtubule targeting agents for cancer therapy. Arch. Hyg. Rada Toksikol. 2019, 70, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Kaur, G.; Gill, R.K.; Soni, R.; Bariwal, J. Recent developments in tubulin polymerization inhibitors: An overview. Eur. J. Med. Chem. 2014, 87, 89–124. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D.; Zhu, X.; Yu, L.; Mao, M.; Liu, Y. Anticancer evaluation of a novel dithiocarbamate hybrid as the tubulin polymerization inhibitor. Investig. New Drugs 2020, 38, 525–532. [Google Scholar] [CrossRef]
- Donthiboina, K.; Anchi, P.; Ramya, P.S.; Karri, S.; Srinivasulu, G.; Godugu, C.; Shankaraiah, N.; Kamal, A. Synthesis of substituted biphenyl methylene indolinones as apoptosis inducers and tubulin polymerization inhibitors. Bioorg. Chem. 2019, 86, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Barbarossa, A.; Carocci, A.; Fazio, A.; La Torre, C.; Caruso, A.; Ponassi, M.; Rosano, C.; et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorg. Chem. 2020, 105, 104440. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Almahli, H.; Hadchity, E.; Jaballah, M.Y.; Daher, R.; Ghabbour, H.A.; Kabil, M.M.; Al-Shakliah, N.S.; Eldehna, W.M. Development of novel synthesized phthalazinone-based PARP-1 inhibitors with apoptosis inducing mechanism in lung cancer. Bioorg. Chem. 2018, 77, 443–456. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell. Commun. Signal 2010, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappano, R.; Rosano, C.; Pisano, A.; Santolla, M.F.; De Francesco, E.M.; De Marco, P.; Dolce, V.; Ponassi, M.; Felli, L.; Cafeo, G.; et al. Calixpyrrole derivative acts as an antagonist to GPER, a G-protein coupled receptor: Mechanisms and models. Dis. Model. Mech. 2015, 8, 1237–1246. [Google Scholar] [CrossRef] [Green Version]
- Sinicropi, M.S.; Lappano, R.; Caruso, A.; Santolla, M.F.; Pisano, A.; Rosano, C.; Capasso, A.; Panno, A.; Lancelot, J.C.; Rault, S.; et al. (6-bromo-1,4-dimethyl-9H-carbazol-3-yl-methylene)-hydrazine (carbhydraz) acts as a GPER agonist in breast cancer cells. Curr. Top. Med. Chem. 2015, 15, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Stec-Martyna, E.; Ponassi, M.; Miele, M.; Parodi, S.; Felli, L.; Rosano, C. Structural comparison of the interaction of tubulin with various ligands affecting microtubule dynamics. Curr. Cancer Drug Targets 2012, 12, 658–666. [Google Scholar] [CrossRef]

| IC50 (µM) | ||||||
|---|---|---|---|---|---|---|
| Compound | A2058 | Sk-Mel28 | MDA-MB-231 | MCF-7 | HeLa | Hek-293 |
| 2a | >200 | >200 | >200 | >200 | >200 | >200 |
| 2b | >200 | >200 | >200 | >200 | >200 | >200 |
| 2c | >200 | >200 | >200 | >200 | >200 | 65.63 ± 0.8 |
| 2d | 124.7 ± 0.9 | >200 | 76.5 ± 1.2 | 177.0 ± 0.7 | 42.1 ± 1.1 | >200 |
| 2e | >200 | >200 | >200 | >200 | >200 | >200 |
| 2f | >200 | >200 | >200 | >200 | >200 | >200 |
| 2g | >200 | >200 | >200 | >200 | >200 | >200 |
| 2h | >200 | >200 | >200 | >200 | >200 | >200 |
| 2i | 23.26 ± 1.1 | >200 | 25.34 ± 1.0 | 31.2 ± 1.2 | 54.5 ± 0.8 | 149.2 ± 0.9 |
| 2j | >200 | >200 | >200 | >200 | >200 | >200 |
| 2k | 15.37 ± 0.7 | >200 | 20.99 ± 0.8 | 22.72 ± 0.9 | >200 | >200 |
| 2l | >200 | >200 | >200 | >200 | >200 | >200 |
| thalidomide | >200 | >200 | >200 | >200 | >200 | >200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarossa, A.; Catalano, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosano, C.; Franchini, C.; Sinicropi, M.S. Simple Thalidomide Analogs in Melanoma: Synthesis and Biological Activity. Appl. Sci. 2021, 11, 5823. https://doi.org/10.3390/app11135823
Barbarossa A, Catalano A, Ceramella J, Carocci A, Iacopetta D, Rosano C, Franchini C, Sinicropi MS. Simple Thalidomide Analogs in Melanoma: Synthesis and Biological Activity. Applied Sciences. 2021; 11(13):5823. https://doi.org/10.3390/app11135823
Chicago/Turabian StyleBarbarossa, Alexia, Alessia Catalano, Jessica Ceramella, Alessia Carocci, Domenico Iacopetta, Camillo Rosano, Carlo Franchini, and Maria Stefania Sinicropi. 2021. "Simple Thalidomide Analogs in Melanoma: Synthesis and Biological Activity" Applied Sciences 11, no. 13: 5823. https://doi.org/10.3390/app11135823
APA StyleBarbarossa, A., Catalano, A., Ceramella, J., Carocci, A., Iacopetta, D., Rosano, C., Franchini, C., & Sinicropi, M. S. (2021). Simple Thalidomide Analogs in Melanoma: Synthesis and Biological Activity. Applied Sciences, 11(13), 5823. https://doi.org/10.3390/app11135823









