Retrospective Study on Tooth Shell Technique Using Endodontically Treated Teeth in Lateral Ridge Augmentation
Abstract
:1. Introduction
2. Materials and Methods
- -
- Age > 18 years;
- -
- Alveolar crest augmentation of a lateral bony defect with the tooth shell technique;
- -
- Lateral alveolar crest defect of at least 4 mm in the region of implant placement prior to augmentation;
- -
- Restoration with a fixed denture is intended;
- -
- Edentulous region of maximum of two missing teeth.
- -
- Age < 18 years;
- -
- Untreated or residual periodontitis;
- -
- Uncontrolled diabetes mellitus with HbA1c > 7%;
- -
- A malignant neoplasm;
- -
- A history of therapy with bisphosphonates or other antiresorptive medication (e.g., RANKL inhibitors);
- -
- A history of radiotherapy in the head and neck region;
- -
- Immunosuppression or immunosuppressant therapy;
- -
- A lateral alveolar crest defect of less than 4 mm in the region of implant placement prior to augmentation;
- -
- The restoration of the implant with a removable denture is intended.
- Group 1:
- Group 2 (control group):
- -
- Demographic data: Age and gender;
- -
- The endodontic status of the tooth used for grafting;
- -
- Data on restoration and maintenance therapy;
- -
- Complications: The loss of the graft or implant, dehiscence, and infections/inflammation;
- -
- Implant data: type, length/diameter, and region;
- -
- After augmentation with simultaneous implantation, a CBCT image was taken to assess the surgical procedure. These X-rays were analyzed;
- -
- At the time of implant exposure—3 months after augmentation—CBCT was performed to assess osseointegration, the buccal lamella, and horizontal hard tissue loss. These CBCT images were analyzed.
- -
- The dehiscence of the wound;
- -
- Infection with or without suppuration;
- -
- Severe peri-implant bone loss;
- -
- Implant loss;
- -
- Others.
2.1. Clinical Complications
2.2. Clinical Procedure of the Tooth Preparation
2.3. General Surgical Procedure of the Tooth Shell Technique (TST)
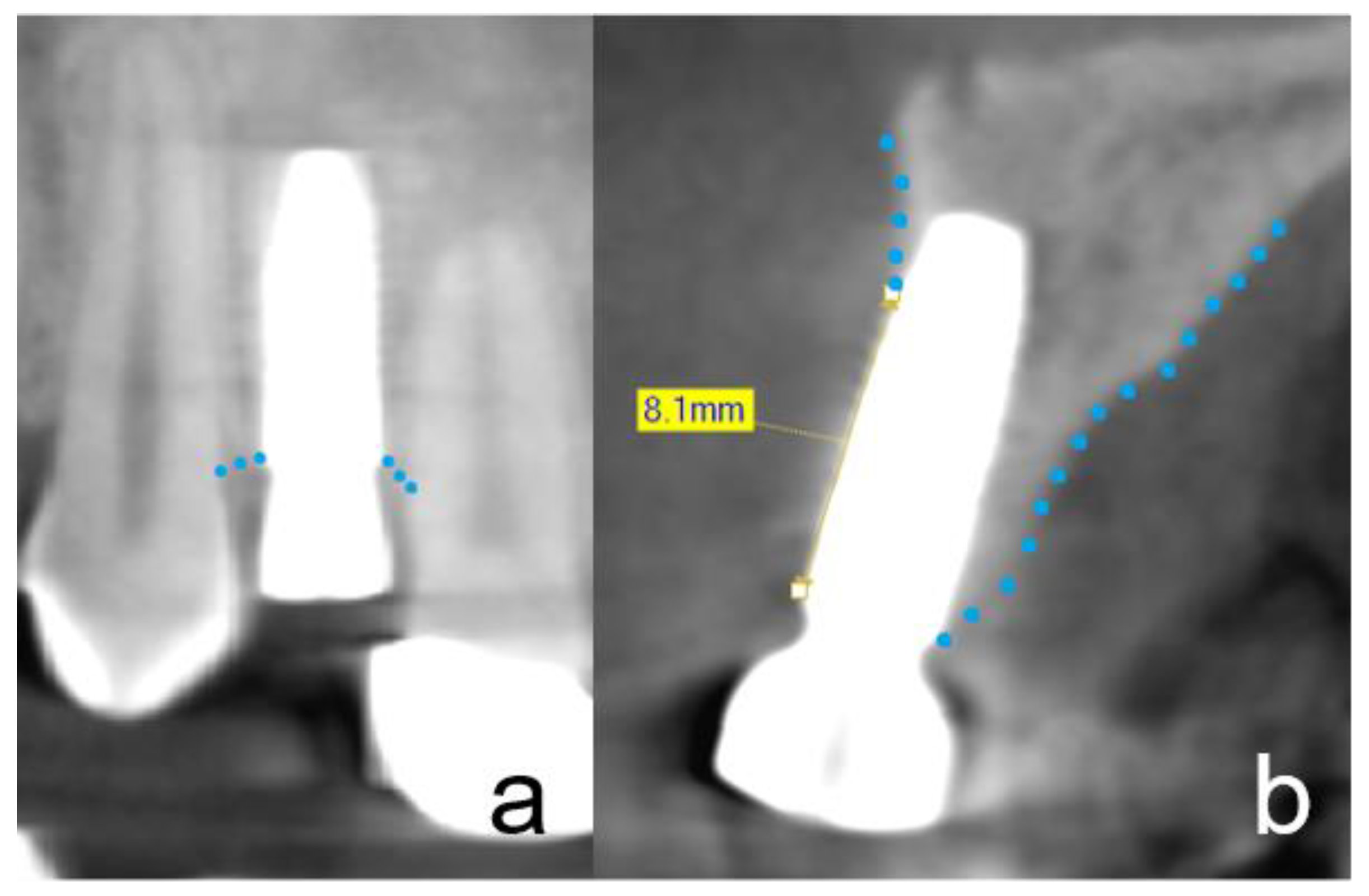
2.4. Radiographic Evaluation
2.5. Osseointegration
- -
- A peri-implant bone loss less than 1 mm at the four measuring points;
- -
- An ISQ value over 60;
- -
- A implant covered by a radio-opaque structure in CBCT;
- -
- The integrity of the buccal lamella being preserved in the CBCT (no more than 1 mm loss).
2.6. Prosthetic Restoration
2.7. Statistical Analyses
3. Results
3.1. Severe Clinical Complications
3.2. Non-Severe Clinical Complications
3.3. Radiographic Evaluation
3.4. Peri-Implant Tissue Probing
3.5. Implant Stability
3.6. Osseointegration
3.7. Prosthetic Restoration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Nkenke, E.; Radespiel-Troger, M.; Wiltfang, J.; Schultze-Mosgau, S.; Winkler, G.; Neukam, F.W. Morbidity of harvesting of retromolar bone grafts: A prospective study. Clin. Oral Implant. Res. 2002, 13, 514–521. [Google Scholar] [CrossRef]
- Sakkas, A.; Schramm, A.; Winter, K.; Wilde, F. Risk factors for post-operative complications after procedures for autologous bone augmentation from different donor sites. J. Craniomaxillofac. Surg. 2018, 46, 312–322. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.; Um, I.W.; Kim, K.W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Kabir, M.A.; Um, I.W.; Minamida, Y.; Kim, K.-W.; Kim, Y.-K.; Sun, Y.; Qin, Y.S.A.C. Autograft of Dentin Materials for Bone Regeneration. IntechOpen 2013, 2013, 391–402. [Google Scholar]
- Al-Asfour, A.; Andersson, L.; Kamal, M.; Joseph, B. New bone formation around xenogenic dentin grafts to rabbit tibia marrow. Dent. Traumatol. 2013, 29, 455–460. [Google Scholar] [CrossRef]
- Al-Asfour, A.; Farzad, P.; Al-Musawi, A.; Dahlin, C.; Andersson, L. Demineralized Xenogenic Dentin and Autogenous Bone as Onlay Grafts to Rabbit Tibia. Implant. Dent. 2017, 26, 232–237. [Google Scholar] [CrossRef]
- Andersson, L. Dentin xenografts to experimental bone defects in rabbit tibia are ankylosed and undergo osseous replacement. Dent. Traumatol. 2010, 26, 398–402. [Google Scholar] [CrossRef]
- Bono, N.; Tarsini, P.; Candiani, G. Demineralized dentin and enamel matrices as suitable substrates for bone regeneration. J. Appl. Biomater. Funct. Mater. 2017, 15, e236–e243. [Google Scholar] [CrossRef] [Green Version]
- Bormann, K.H.; Suarez-Cunqueiro, M.M.; Sinikovic, B.; Kampmann, A.; von See, C.; Tavassol, F.; Binger, T.; Winkler, M.; Gellrich, N.-C.; Rücker, M. Dentin as a suitable bone substitute comparable to ss-TCP-an experimental study in mice. Microvasc. Res. 2012, 84, 116–122. [Google Scholar] [CrossRef]
- Jun, S.H.; Ahn, J.S.; Lee, J.I.; Ahn, K.J.; Yun, P.Y.; Kim, Y.K. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J. Adv. Prosthodont. 2014, 6, 528–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Kim, Y.K.; Park, Y.H.; Park, J.C.; Ku, J.K.; Um, I.W.; Kim, J.-Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials 2017, 10, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Yun, P.Y.; In-Woong Um, I.W.; Lee, H.J.; Yi, Y.J.; Bae, J.H.; Lee, J. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: Prospective case series. J. Adv. Prosthodont. 2014, 6, 521–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, F.; Golubovic, V.; Becker, K.; Mihatovic, I. Extracted tooth roots used for lateral alveolar ridge augmentation: A proof-of-concept study. J. Clin. Periodontol. 2016, 43, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Mihatovic, I.; Golubovic, V.; Becker, J. Dentointegration of a titanium implant: A case report. Oral Maxillofac. Surg. 2013, 17, 235–241. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Nevins, M.; Schupbach, P. New Bone Formation Using an Extracted Tooth as a Biomaterial: A Case Report with Histologic Evidence. Int. J. Periodontics Restor. Dent. 2019, 39, 157–163. [Google Scholar] [CrossRef]
- Xu, X.; Sohn, D.S.; Kim, H.G.; Lee, S.J.; Moon, Y.S. Comparative Histomorphometric Analysis of Maxillary Sinus Augmentation With Deproteinized Bovine Bone and Demineralized Particulate Human Tooth Graft: An Experimental Study in Rabbits. Implant. Dent. 2018, 27, 324–331. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Sahin, D.; Sader, R.; Becker, J.; Schwarz, F. Efficacy of autogenous teeth for the reconstruction of alveolar ridge deficiencies: A systematic review. Clin. Oral Investig. 2019, 23, 4263–4287. [Google Scholar] [CrossRef]
- Parvini, P.; Sader, R.; Sahin, D.; Becker, J.; Schwarz, F. Radiographic outcomes following lateral alveolar ridge augmentation using autogenous tooth roots. Int. J. Implant. Dent. 2018, 4, 31. [Google Scholar] [CrossRef]
- Schwarz, F.; Hazar, D.; Becker, K.; Sader, R.; Becker, J. Efficacy of autogenous tooth roots for lateral alveolar ridge augmentation and staged implant placement. A prospective controlled clinical study. J. Clin. Periodontol. 2018, 45, 996–1004. [Google Scholar] [CrossRef]
- Li, P.; Zhu, H.; Huang, D. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant. Dent. Relat Res. 2018, 20, 923–928. [Google Scholar] [CrossRef]
- Korsch, M. Tooth shell technique: A proof of concept with the use of autogenous dentin block grafts. Aust. Dent. J. 2020, 66, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Golubovic, V.; Mihatovic, I.; Becker, J. Periodontally diseased tooth roots used for lateral alveolar ridge augmentation. A proof-of-concept study. J. Clin. Periodontol. 2016, 43, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Del Fabbro, M.; Samaranayake, L.; Chang, J.W.; Corbella, S. Microbial invasion of dentinal tubules: A literature review and a new perspective. J. Investig. Clin. Dent. 2014, 5, 163–170. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.J.; Kasper, E.L.; Hatton, J.F.; Murray, B.E.; Nallapareddy, S.R.; Gillespie, M.J. Enterococcus faecalis adhesin, Ace, mediates attachment to particulate dentin. J. Endod. 2006, 32, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M. Enterococcus faecalis—A mechanism for its role in endodontic failure. Int. Endod. J. 2001, 34, 399–405. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Three-Dimensional Vertical Alveolar Ridge Augmentation in the Posterior Maxilla: A 10-year Clinical Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 471–480. [Google Scholar] [CrossRef]
- Korsch, M.; Kasprzyk, S.; Walther, W.; Bartols, A. Lateral alveolar ridge augmentation with autogenous block grafts fixed at a distance vs. resorbable poly-D-L-lactide foil fixed at a distance: 5-year results of a single-blind, randomised controlled trial. Int. J. Oral Implantol. 2019, 12, 299–312. [Google Scholar]
- Bartols, A.; Kasprzyk, S.; Walther, W.; Korsch, M. Lateral alveolar ridge augmentation with autogenous block grafts fixed at a distance versus resorbable Poly-D-L-Lactide foil fixed at a distance: A single-blind, randomized, controlled trial. Clin. Oral Implant. Res. 2018, 29, 843–854. [Google Scholar] [CrossRef]
- Korsch, M.; Peichl, M. Retrospective Study: Lateral Ridge Augmentation Using Autogenous Dentin: Tooth-Shell Technique vs. Bone-Shell Technique. Int. J. Environ. Res. Public Health 2021, 18, 3174. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, T.A.; Wu, S.Y.; Lin, C.P.; Chang, H.H. Regeneration of Tooth with Allogenous, Autoclaved Treated Dentin Matrix with Dental Pulpal Stem Cells: An In Vivo Study. J. Endod. 2020, 46, 1256–1264. [Google Scholar] [CrossRef]
- Kastel, I.; de Quincey, G.; Neugebauer, J.; Sader, R.; Gehrke, P. Does the manual insertion torque of smartpegs affect the outcome of implant stability quotients (ISQ) during resonance frequency analysis (RFA)? Int. J. Implant. Dent. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, P.B.; Moura, C.C.; Claudino, M.; Carvalho, V.F.; Rocha, F.S.; Zanetta-Barbosa, D. Influence of Implant Surfaces on Osseointegration: A Histomorphometric and Implant Stability Study in Rabbits. Braz. Dent. J. 2015, 26, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, S.A.; Maté Sánchez de Val, J.E.; Ramírez Fernández, M.P.; Shibli, J.A.; Rossetti, P.H.; Calvo Guirado, J.L. Stability and Crestal Bone Behavior Following Simultaneous Placement of Multiple Dental Implants (Two or More) with the Bone Splitting Technique: A Clinical and Radiographic Evaluation. Clin. Implant. Dent. Relat Res. 2017, 19, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.; Drescher, D.; Honscheid, R.; Golubovic, V.; Mihatovic, I.; Schwarz, F. Biomechanical, micro-computed tomographic and immunohistochemical analysis of early osseous integration at titanium implants placed following lateral ridge augmentation using extracted tooth roots. Clin. Oral Implant. Res. 2017, 28, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Raj, R.M.; Liao, X.F.; Shi, W.; Ma, B.; Gong, S.Q.; Chen, W.-M.; Zhou, B. Using rigidly fixed autogenous tooth graft to repair bone defect: An animal model. Dent. Traumatol. 2014, 30, 380–384. [Google Scholar] [CrossRef]



| Study Group | Sign | |||
|---|---|---|---|---|
| Baseline Data of Participants | Total | ETT | NETT | p-Value |
| Age (years) | ||||
| Mean (SD) | 61.7 (10.5) | 62.6 (8.6) | 60.8 (12.4) | n.s. |
| Range | 28–80 | 49–80 | 28–76 | |
| Gender (male) | ||||
| n (%) | 15 of 34 (44) | 6 of 17 (35) | 9 of 17 (52) | n.s. |
| Study Group | Fisher’s Exact Test (2-Sided) | |||
|---|---|---|---|---|
| Clinical Complication | Total | ETT | NETT | p-Value |
| Total severe complications | ||||
| n (%) on PL | 0 of 34 (0) | 0 of 17 (0) | 0 of 17 (0) | n.s. |
| n (%) on RL | 0 of 40 (0) | 0 of 18 (0) | 0 of 22 (0) | n.s. |
| n (%) on IL | 0 of 45 (0) | 0 of 21 (0) | 0 of 24 (0) | n.s. |
| Wound dehiscence | ||||
| n (%) on PL | 2 of 34 (6) | 1 of 17 (6) | 1 of 17 (6) | 1.000 |
| n (%) on RL | 2 of 40 (8) | 1 of 18 (6) | 1 of 22 (5) | 0.884 |
| n (%) on IL | 2 of 45 (4) | 1 of 21 (5) | 1 of 24 (4) | 0.923 |
| Inflammation (pus) | ||||
| n (%) on PL | 0 of 34 (0) | 0 of 17 (0) | 0 of 17 (0) | n.s. |
| n (%) on RL | 0 of 40 (0) | 0 of 18 (0) | 0 of 22 (0) | n.s. |
| n (%) on IL | 0 of 45 (0) | 0 of 21 (0) | 0 of 24 (0) | n.s. |
| Total complications at all | ||||
| n (%) on PL | 3 of 34 (9) | 1 of 17 (6) | 2 of 17 (12) | 0.545 |
| n (%) on RL | 3 of 40 (8) | 1 of 18 (6) | 2 of 22 (9) | 0.673 |
| n (%) on IL | 3 of 45 (9) | 1 of 21 (5) | 2 of 24 (8) | 0.632 |
| Study Group | Two-Sample t-Test | |||
|---|---|---|---|---|
| Time of Measurement | Mean | ETT | NETT | (p-Value) |
| T1 | ||||
| Mean bucco-palatal alveolar ridge width (mm) | ||||
| PL, n = 34 (SD) | 9.1 (1.5) | 9.4 (1.7) | 8.7 (1.2) | 0.146 |
| RL, n = 40 (SD) | 9.0 (1.5) | 9.3 (1.8) | 8.7 (1.1) | 0.205 |
| IL, n = 45 (SD) | 9.0 (1.5) | 9.3 (1.8) | 8.8 (1.3) | 0.229 |
| Mean buccal lamella width L0 (mm) | ||||
| PL, n = 34 (SD) | 2.7 (0.9) | 2.6 (1.0) | 2.7 (0.8) | 0.917 |
| RL, n = 40 (SD) | 2.6 (0.9) | 2.6 (1.0) | 2.7 (0.8) | 0.742 |
| IL, n = 45 (SD) | 2.6 (0.9) | 2.6 (1.0) | 2.7 (0.9) | 0.702 |
| Mean buccal lamella width L2 (mm) | ||||
| PL, n = 34 (SD) | 3.2 (0.9) | 3.4 (1.0) | 3.0 (0.8) | 0.187 |
| RL, n = 40 (SD) | 3.1 (0.9) | 3.3 (1.1) | 3.0 (0.8) | 0.314 |
| IL, n = 45 (SD) | 3.2 (0.9) | 3.3 (1.0) | 3.0 (0.8) | 0.391 |
| Mean buccal lamella width L4 (mm) | ||||
| PL, n = 34 (SD) | 3.5 (1.3) | 3.9 (1.5) | 3.1 (1.0) | 0.079 |
| RL, n = 40 (SD) | 3.4 (0.9) | 3.8 (1.5) | 3.1 (1.0) | 0.096 |
| IL, n = 45 (SD) | 3.4 (1.3) | 3.8 (1.5) | 3.2 (1.1) | 0.118 |
| T2 | ||||
| Mean bucco-palatal alveolar ridge width (mm) | ||||
| PL, n = 34 (SD) | 8.7 (1.4) | 9.1 (1.7) | 8.2 (1.0) | 0.073 |
| RL, n = 40 (SD) | 8.6 (1.4) | 9.0 (1.7) | 8.2 (1.0) | 0.101 |
| IL, n = 45 (SD) | 8.6 (1.4) | 9.0 (1.7) | 8.3 (1.0) | 0.077 |
| Mean buccal lamella width L0 (mm) | ||||
| PL, n = 34 (SD) | 2.2 (1.0) | 2.2 (1.1) | 2.2 (0.9) | 0.904 |
| RL, n = 40 (SD) | 2.2 (1.0) | 2.2 (1.0) | 2.1 (0.9) | 0.810 |
| IL, n = 45 (SD) | 2.2 (1.0) | 2.3 (1) | 2.2 (0.9) | 0.798 |
| Mean buccal lamella width L2 (mm) | ||||
| PL, n = 34 (SD) | 3.0 (1.0) | 3.2 (1.0) | 2.7 (0.8) | 0.093 |
| RL, n = 40 (SD) | 2.9 (1.0) | 3.2 (1.0) | 2.6 (0.9) | 0.093 |
| IL, n = 45 (SD) | 2.9 (1.0) | 3.2 (1.0) | 2.7 (0.9) | 0.093 |
| Mean buccal lamella width L4 (mm) | ||||
| PL, n = 34 (SD) | 3.2 (1.3) | 3.7 (1.4) | 2.8 (1.0) | 0.029 |
| RL, n = 40 (SD) | 3.2 (1.3) | 3.6 (1.5) | 2.8 (1.0) | 0.038 |
| IL, n = 45 (SD) | 3.2 (1.3) | 3.6 (1.4) | 2.8 (1.0) | 0.035 |
| Study Group | Two-Sample t-Test | |||
|---|---|---|---|---|
| Mean Resorption in mm | Mean | ETT | NETT | (p-Value) |
| bucco-oral alveolar ridge | ||||
| PL, n = 34 (SD) | 0.39 (0.68) | 0.34 (0.35) | 0.45 (0.90) | 0.637 |
| RL, n = 40 (SD) | 0.40 (0.66) | 0.33 (0.36) | 0.45 (0.83) | 0.551 |
| IL, n = 45 (SD) | 0.40 (0.67) | 0.30 (0.35) | 0.49 (0.85) | 0.340 |
| L0 | ||||
| PL, n = 34 (SD) | 0.45 (0.72) | 0.41 (0.68) | 0.49 (0.77) | 0.770 |
| RL, n = 40 (SD) | 0.47 (0.72) | 0.38 (0.68) | 0.54 (0.75) | 0.467 |
| IL, n = 45 (SD) | 0.42 (0.70) | 0.33 (0.64) | 0.51 (0.76) | 0.398 |
| L2 | ||||
| PL, n = 34 (SD) | 0.24 (0.56) | 0.17 (0.43) | 0.31 (0.68) | 0.483 |
| RL, n = 40 (SD) | 0.26 (0.58) | 0.14 (0.44) | 0.36 (0.67) | 0.247 |
| IL, n = 45 (SD) | 0.25 (0.55) | 0.12 (0.41) | 0.36 (0.64) | 0.149 |
| L4 | ||||
| PL, n = 34 (SD) | 0.28 (0.61) | 0.19 (0.55) | 0.37 (0.69) | 0.416 |
| RL, n = 40 (SD) | 0.26 (0.60) | 0.18 (0.54) | 0.32 (0.65) | 0.473 |
| IL, n = 45 (SD) | 0.26 (0.58) | 0.16 (0.50) | 0.34 (0.63) | 0.302 |
| Study Group | Two-Sample t-Test | |||
|---|---|---|---|---|
| Ratio from T1 to T2 | Mean | ETT | NETT | (p-Value) |
| bucco-oral alveolar ridge | ||||
| PL, n = 34 (SD) | 0.96 (0.08) | 0.97 (0.04) | 0.96 (0.10) | 0.680 |
| RL, n = 40 (SD) | 0.96 (0.07) | 0.97 (0.04) | 0.95 (0.09) | 0.568 |
| IL, n = 45 (SD) | 0.96 (0.07) | 0.97 (0.04) | 0.95 (0.09) | 0.402 |
| L0 | ||||
| PL, n = 34 (SD) | 0.85 (0.29) | 0.85 (0.26) | 0.84 (0.33) | 0.917 |
| RL, n = 40 (SD) | 0.84 (0.29) | 0.87 (0.26) | 0.81 (0.31) | 0.521 |
| IL, n = 45 (SD) | 0.86 (0.30) | 0.88 (0.25) | 0.84 (0.34) | 0.588 |
| L2 | ||||
| PL, n = 34 (SD) | 0.93 (0.18) | 0.96 (0.13) | 0.91 (0.22) | 0.457 |
| RL, n = 40 (SD) | 0.93 (0.19) | 0.97 (0.15) | 0.89 (0.22) | 0.199 |
| IL, n = 45 (SD) | 0.93 (0.18) | 0.98 (0.14) | 0.89 (0.21) | 0.125 |
| L4 | ||||
| PL, n = 34 (SD) | 0.93 (0.19) | 0.97 (0.17) | 0.90 (0.20) | 0.286 |
| RL, n = 40 (SD) | 0.94 (0.19) | 0.97 (0.17) | 0.91 (0.20) | 0.363 |
| IL, n = 45 (SD) | 0.94 (0.18) | 0.97 (0.16) | 0.91 (0.19) | 0.239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsch, M.; Peichl, M. Retrospective Study on Tooth Shell Technique Using Endodontically Treated Teeth in Lateral Ridge Augmentation. Appl. Sci. 2021, 11, 5882. https://doi.org/10.3390/app11135882
Korsch M, Peichl M. Retrospective Study on Tooth Shell Technique Using Endodontically Treated Teeth in Lateral Ridge Augmentation. Applied Sciences. 2021; 11(13):5882. https://doi.org/10.3390/app11135882
Chicago/Turabian StyleKorsch, Michael, and Marco Peichl. 2021. "Retrospective Study on Tooth Shell Technique Using Endodontically Treated Teeth in Lateral Ridge Augmentation" Applied Sciences 11, no. 13: 5882. https://doi.org/10.3390/app11135882
APA StyleKorsch, M., & Peichl, M. (2021). Retrospective Study on Tooth Shell Technique Using Endodontically Treated Teeth in Lateral Ridge Augmentation. Applied Sciences, 11(13), 5882. https://doi.org/10.3390/app11135882






