Experimental Study of Coflow Propane—Air Laminar Diffusion Flames at Subatmospheric Pressures
Abstract
:1. Introduction
2. Experimental Apparatus
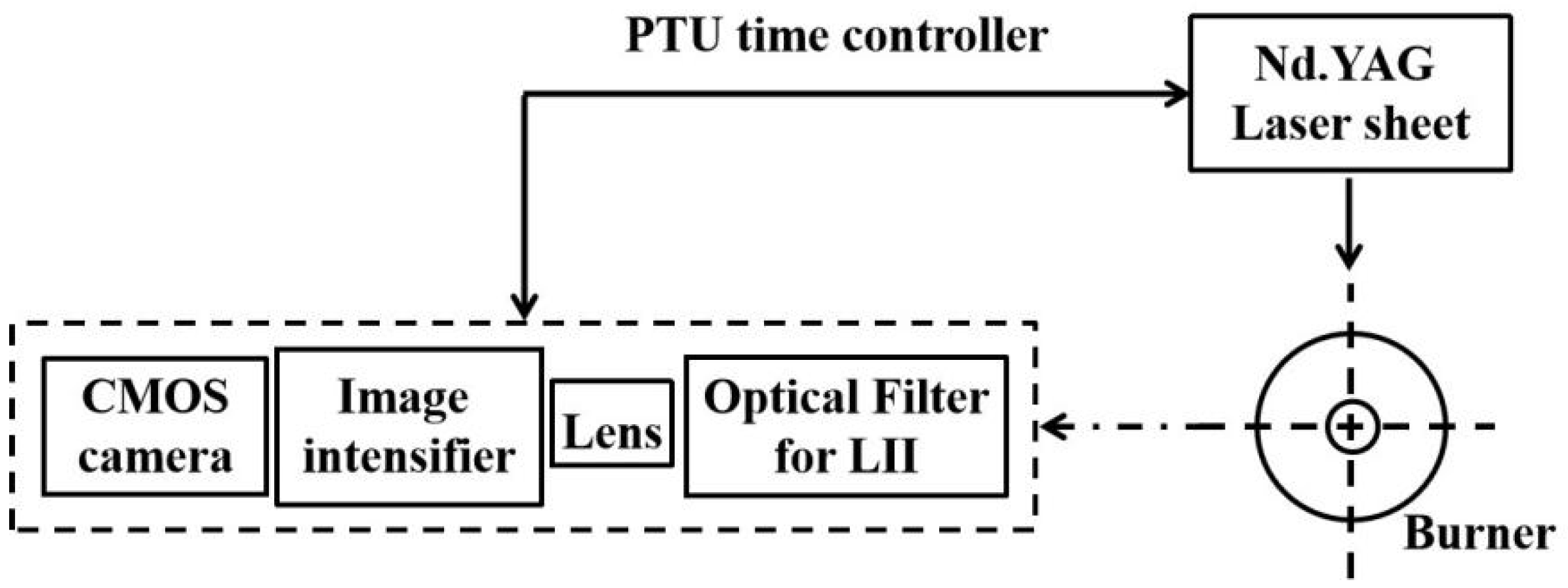
2.1. Optical Apparatus
- (1)
- A pulsed laser and a light sheet. An Nd:YAG double-cavity laser, 135 mJ/pulse, was used. To avoid LIF signals, a 1064-nm laser was applied. A 0.48 mJ/mm2 laser fluence was applied.
- (2)
- A CMOS camera and an image intensifier. A CMOS digital camera was used together with a 25-mm intensifier with a gate–delay controller. The duration of the camera gate was 100 ns with a delay of 40 ns after the occurrence of the laser pulse. Besides, a 500-nm filter with half bandwidth of 10 nm was applied to remove other noise signals.
- (3)
- A programmable timing unit.
2.2. Pressure Combustion Chamber
3. Results and Discussion
3.1. Physical Flame Structure
3.2. Soot Formation
4. Conclusions
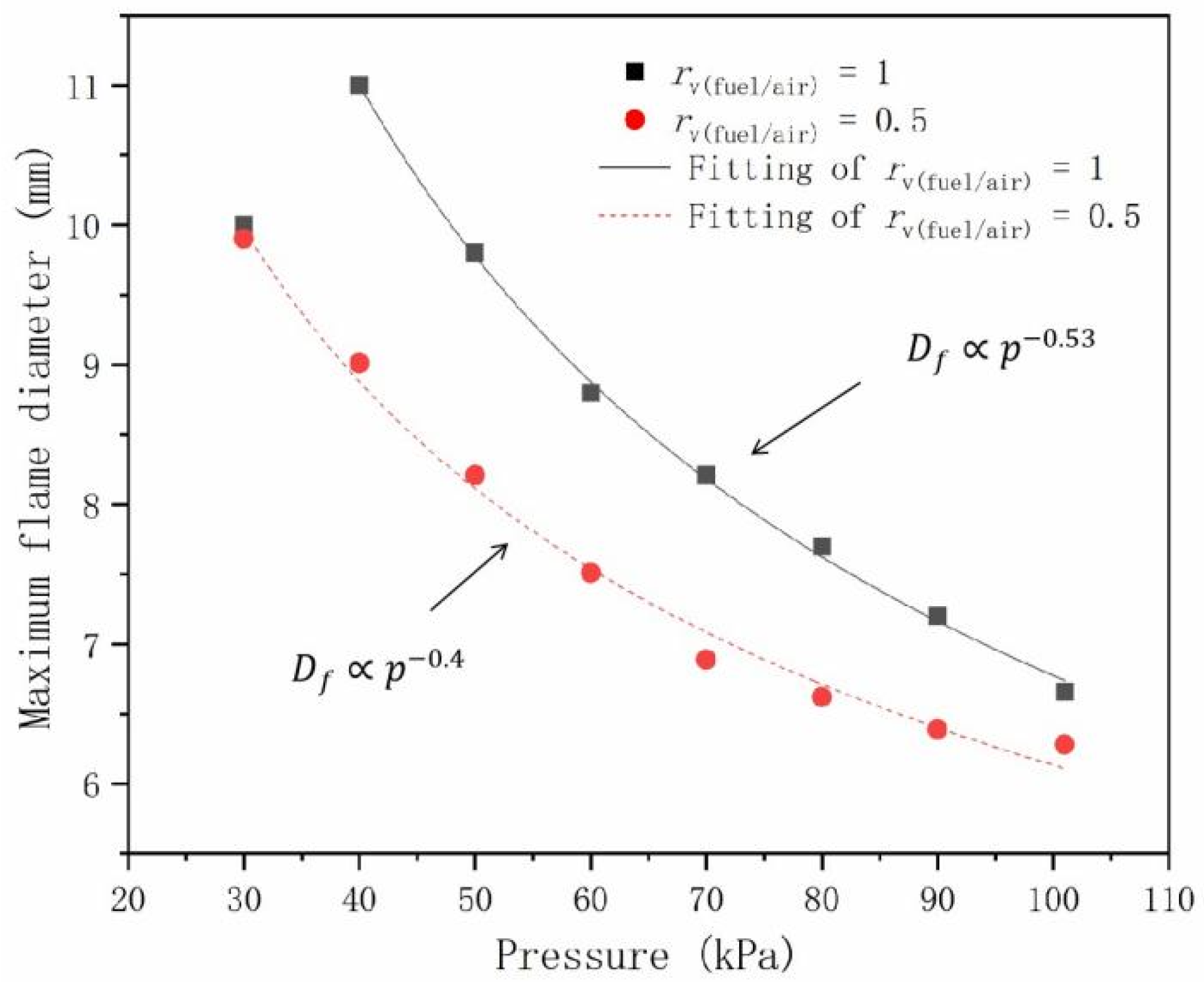
- As pressure decreases, the visible flame height decreases and flame diameter expands. The maximum flame diameter was found to scale with pressure, with and for and 0.5, respectively.
- 2.
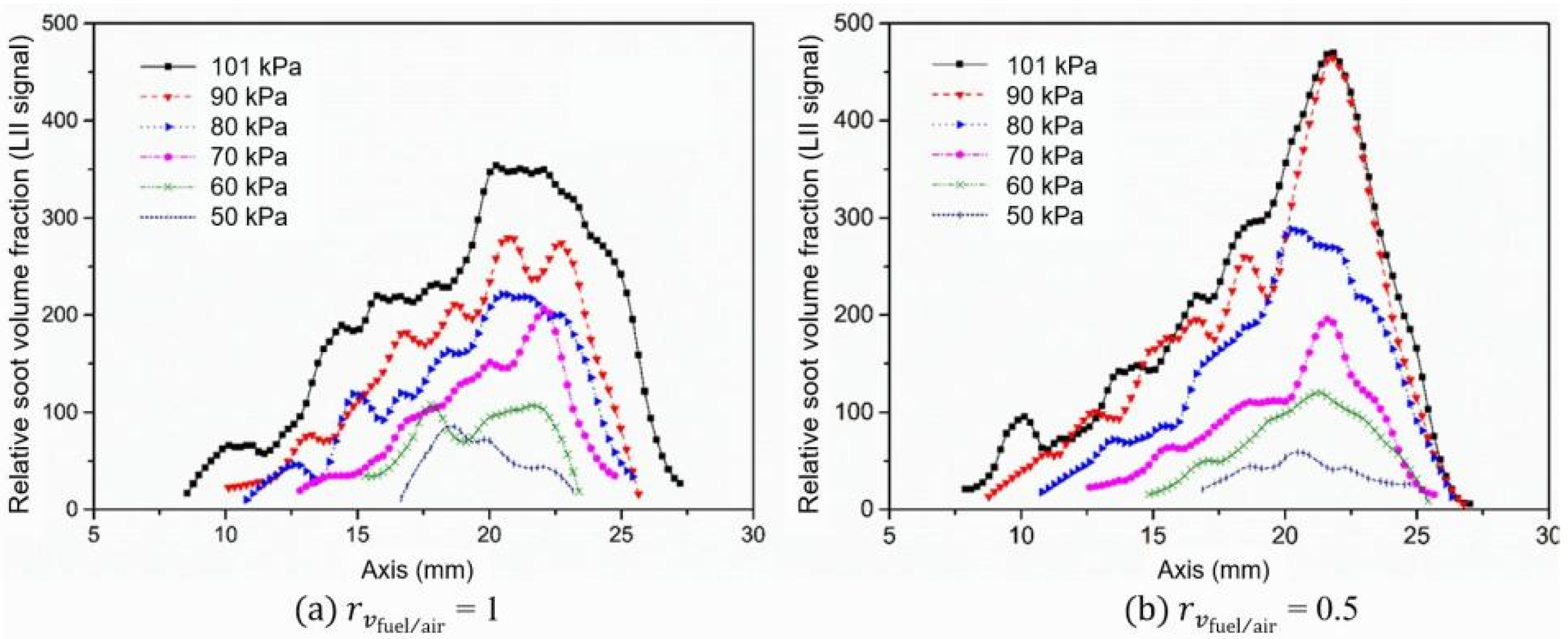
- The relative soot volume fraction represented by the LII signal intensity () decreases at lower pressures. However, at a lower air-to-fuel ratio, the soot volume fraction decreases more quickly with the decrease in pressure.
- 3.
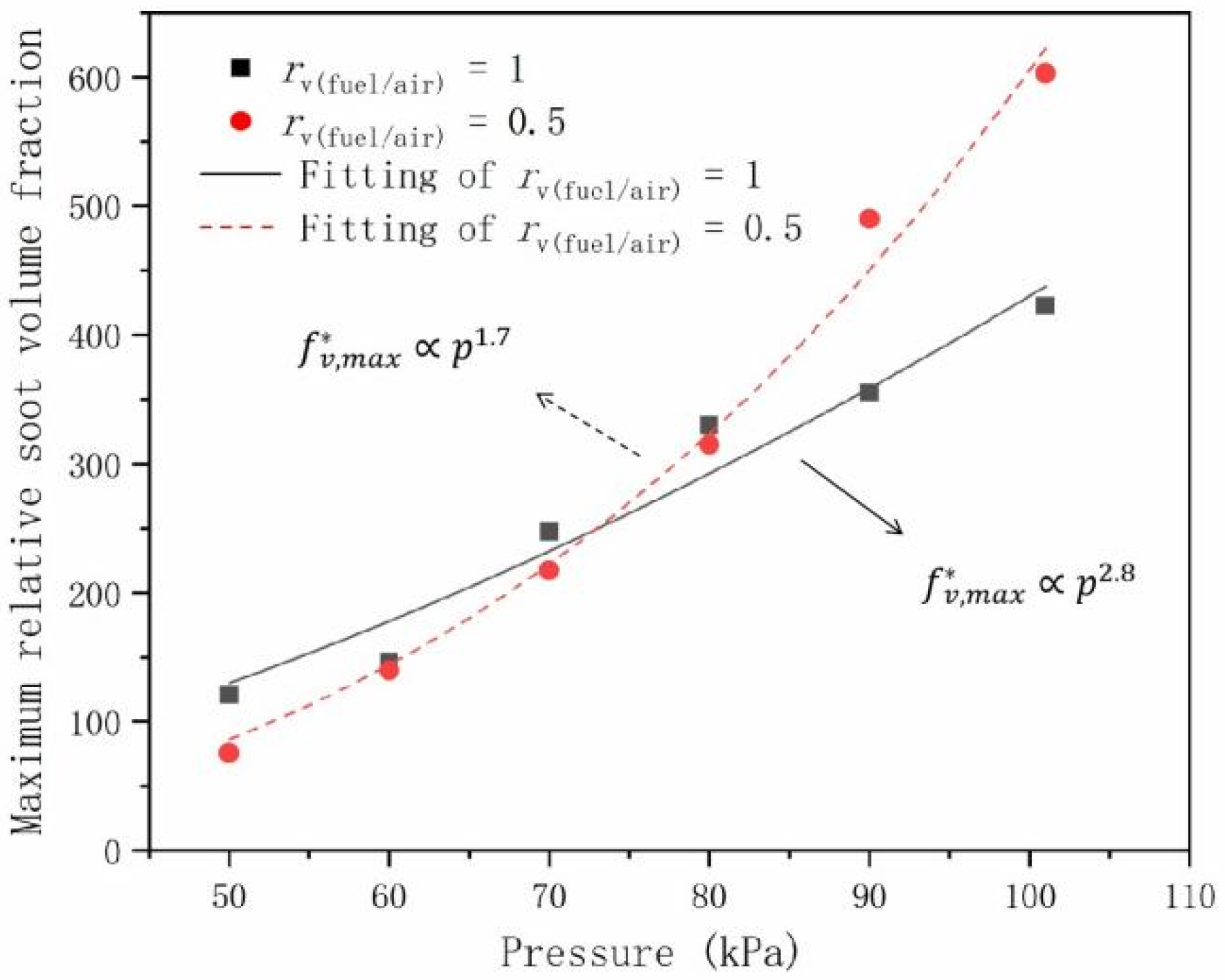
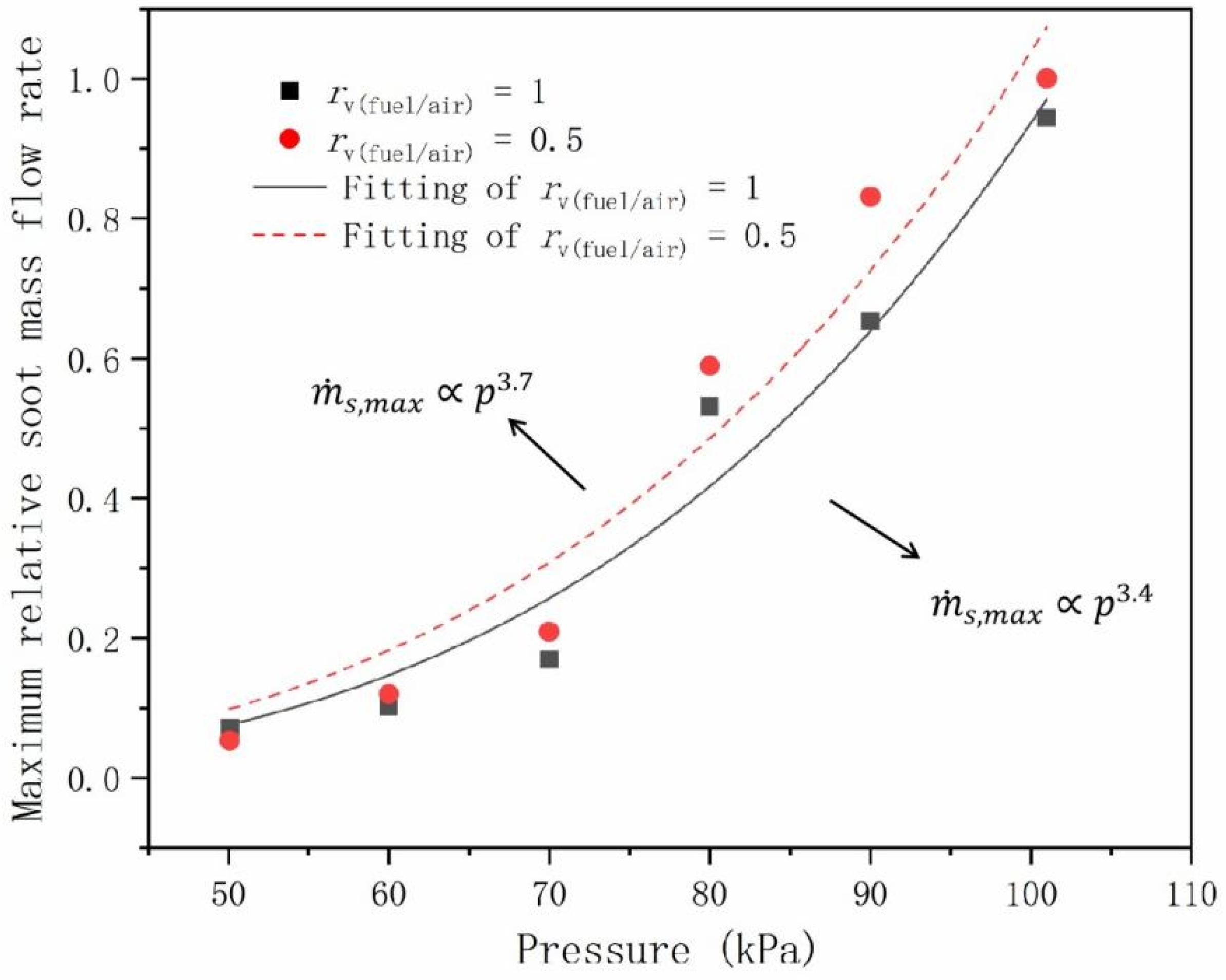
- The maximum relative soot volume fraction and the maximum relative soot mass flow rate have a power law relationships with pressure. The exponents were 1.7 and 2.8 for and 3.4 and 3.7 for , with and 0.5, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartholet, D.L.; Arellano-Trevino, M.A.; Chan, F.L.; Lucas, S.; Zhu, J.Q.; John, P.C.S.; Alleman, T.L.; McEnally, C.S.; Pfefferle, L.D.; Ruddy, D.A.; et al. Property predictions demonstrate that structural diversity can improve the performance of polyoxymethylene ethers as potential bio-based diesel fuels. Fuel 2021, 295, 120509. [Google Scholar] [CrossRef]
- Jiang, B.; Jia, P.H.; Liu, D. Comparative study on soot characteristics of non-swirling and swirling inverse diffusion iso-octane flames with biofuel 2,5-dimethylfuran addition. J. Energy Inst. 2020, 93, 2108–2123. [Google Scholar] [CrossRef]
- Karataş, A.E.; Gülder, Ö.L. Soot formation in high pressure laminar diffusion flames. Prog. Energy Combust. Sci. 2012, 38, 818–845. [Google Scholar] [CrossRef]
- Glassman, I.; Yetter, R.A. Chapter 8—Environmental combustion considerations. In Combustion, 5th ed.; Glassman, I., Yetter, R.A., Glumac, N.G., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 393–475. [Google Scholar]
- McCrain, L.L.; Roberts, W.L. Measurements of the soot volume field in laminar diffusion flames at elevated pressures. Combust. Flame 2005, 140, 60–69. [Google Scholar] [CrossRef]
- Joo, H.I.; Gülder, Ö.L. Soot formation and temperature field structure in co-flow laminar methane–air diffusion flames at pressures from 10 to 60atm. Proc. Combust. Inst. 2009, 32, 769–775. [Google Scholar] [CrossRef]
- Mandatori, P.M.; Gülder, Ö.L. Soot formation in laminar ethane diffusion flames at pressures from 0.2 to 3.3 MPa. Proc. Combust. Inst. 2011, 33, 577–584. [Google Scholar] [CrossRef]
- Bento, D.S.; Thomson, K.A.; Gülder, Ö.L. Soot formation and temperature field structure in laminar propane–air diffusion flames at elevated pressures. Combust. Flame 2006, 145, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Commodo, M.; Karata, A.E.; Falco, G.D.; Minutolo, P.; D’Anna, A.; Gülder, Ö.L. On the effect of pressure on soot nanostructure: A Raman spectroscopy investigation. Combust. Flame 2020, 219, 13–19. [Google Scholar] [CrossRef]
- Gülder, Ö.L.; Intasopa, G.; Joo, H.I.; Mandatori, P.M.; Bento, D.S.; Vaillancourt, M.E. Unified behaviour of maximum soot yields of methane, ethane and propane laminar diffusion flames at high pressures. Combust. Flame 2011, 158, 2037–2044. [Google Scholar] [CrossRef]
- Mi, X.T.; Saylam, A.; Endres, T.; Schulz, C.; Dreier, T. Near-threshold soot formation in premixed flames at elevated pressure. Carbon 2021, 181, 143–154. [Google Scholar] [CrossRef]
- Dou, Y.L.; Liu, H.Q.; Liu, B.; Zhang, Y.; Liu, Y.Q.; Cheng, X.Z.; Tao, C.F. Effects of carbon dioxide addition to fuel on flame radiation fraction in propane diffusion flames. Energy 2021, 218, 119552. [Google Scholar] [CrossRef]
- Tao, C.F.; Liu, B.; Dou, Y.L.; Qian, Y.J.; Zhang, Y.; Meng, S. The experimental study of flame height and lift-off height of propane diffusion flames diluted by carbon dioxide. Fuel 2021, 290, 119958. [Google Scholar] [CrossRef]
- Charest, M.R.J.; Groth, C.P.T.; Gülder, Ö.L. A numerical study on the effects of pressure and gravity in laminar ethylene diffusion flames. Combust. Flame 2011, 158, 1933–1945. [Google Scholar] [CrossRef]
- Panek, N.; Charest, M.R.; Gülder, Ö.L. Simulation of microgravity diffusion flames using sub-atmospheric pressures. AIAA J. 2012, 50, 976–980. [Google Scholar] [CrossRef]
- Kim, C.H.; El-Leathy, A.M.; Xu, F.; Faeth, G.M. Soot surface growth and oxidation in laminar diffusion flames at pressures of 0.1–1.0 atm. Combust. Flame 2004, 136, 191–207. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhou, Z.H. Examination of radiative fraction of small-scale pool fires at reduced pressure environments. Fire Saf. J. 2019, 110, 102894. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.H.; Zhang, D.M.; Chen, G.F.; Wang, J. Experimental study of reduced pressure effect on radiation feedback to the fuel surface of pools fires. J. Therm. Anal. Calorim. 2021, 144, 883–893. [Google Scholar] [CrossRef]
- Wang, J.W.; Fang, J.; Guan, J.F.; Zeng, Y.; Zhang, Y.M. Flame volume and radiant fraction of jet diffusion methane flame at sub-atmospheric pressures. Fuel 2016, 167, 82–88. [Google Scholar] [CrossRef]
- Michelsen, H.A.; Schulz, C.; Smallwood, G.J.; Will, S. Laser-induced incandescence: Particulate diagnostics for combustion, atmospheric, and industrial applications. Prog. Energy Combust. Sci. 2015, 51, 2–48. [Google Scholar] [CrossRef] [Green Version]
- Thomson, K.A.; Gülder, Ö.L.; Weckman, E.J.; Fraser, R.A.; Snelling, D.R. Soot concentration and temperature measurements in co-annular, nonpremixed CH4/air laminar flames at pressures up to 4 MPa. Combust. Flame 2005, 140, 222–232. [Google Scholar] [CrossRef]
- Roper, F.G. The prediction of laminar jet diffusion flame sizes: Part I. Theoretical model. Combust. Flame 1977, 29, 219–226. [Google Scholar] [CrossRef]
- Takahashi, F.; Katta, V.R. A reaction kernel hypothesis for the stability limit of methane jet diffusion flames. Proc. Combust. Inst. 2000, 28, 2071–2078. [Google Scholar] [CrossRef]
- Yelverton, T.L.B.; Roberts, W.L. Effect of dilution, pressure, and velocity on smoke point in laminar jet flames. Combust. Sci. Technol. 2008, 180, 1334–1346. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Liu, J.; Wang, J. Experimental Study of Coflow Propane—Air Laminar Diffusion Flames at Subatmospheric Pressures. Appl. Sci. 2021, 11, 5979. https://doi.org/10.3390/app11135979
Yao J, Liu J, Wang J. Experimental Study of Coflow Propane—Air Laminar Diffusion Flames at Subatmospheric Pressures. Applied Sciences. 2021; 11(13):5979. https://doi.org/10.3390/app11135979
Chicago/Turabian StyleYao, Jiajie, Jiahao Liu, and Jian Wang. 2021. "Experimental Study of Coflow Propane—Air Laminar Diffusion Flames at Subatmospheric Pressures" Applied Sciences 11, no. 13: 5979. https://doi.org/10.3390/app11135979




