The Response of Halophyte (Tetragonia tetragonioides (Pallas) Kuntz.) and Glycophyte (Lactuca sativa L.) Crops to Diluted Seawater and NaCl Solutions: A Comparison between Two Salinity Stress Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Plant Material and Growth Conditions
2.2. Plant Growth and Biomass Yield
2.3. Water Consumption and Water Use Efficiency
2.4. Leaf Gas-Exchange Parameters
2.5. Concentration of Mineral Elements, Phenolics and Proline in Edible Leaves
2.6. Statistical Analysis
3. Results
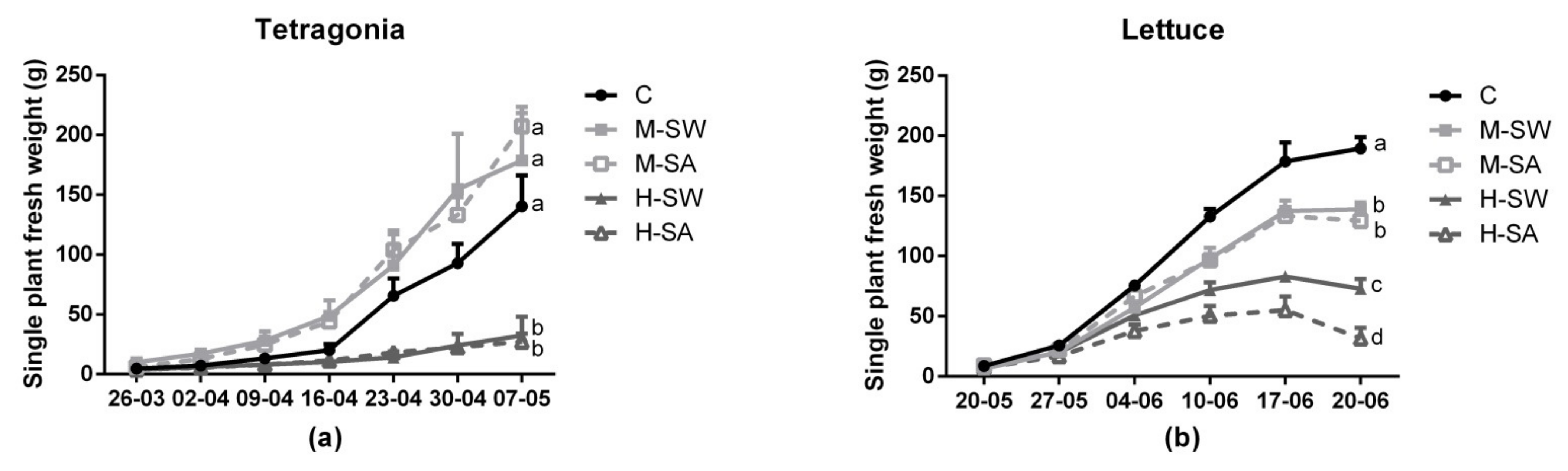
3.1. Plant Growth and Biomass Yield
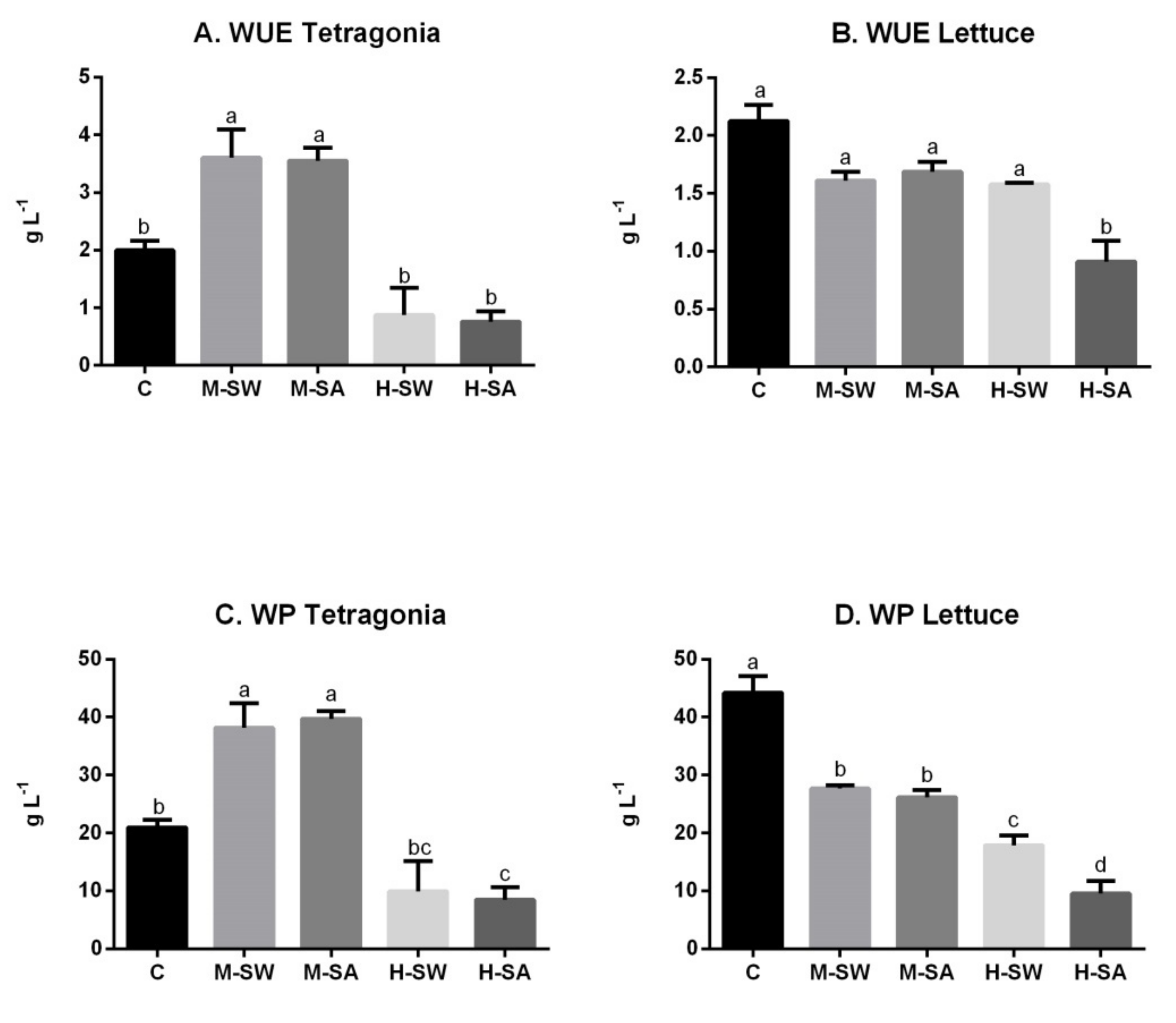
3.2. Water Consumption, Water Use Efficiency and Water Productivity
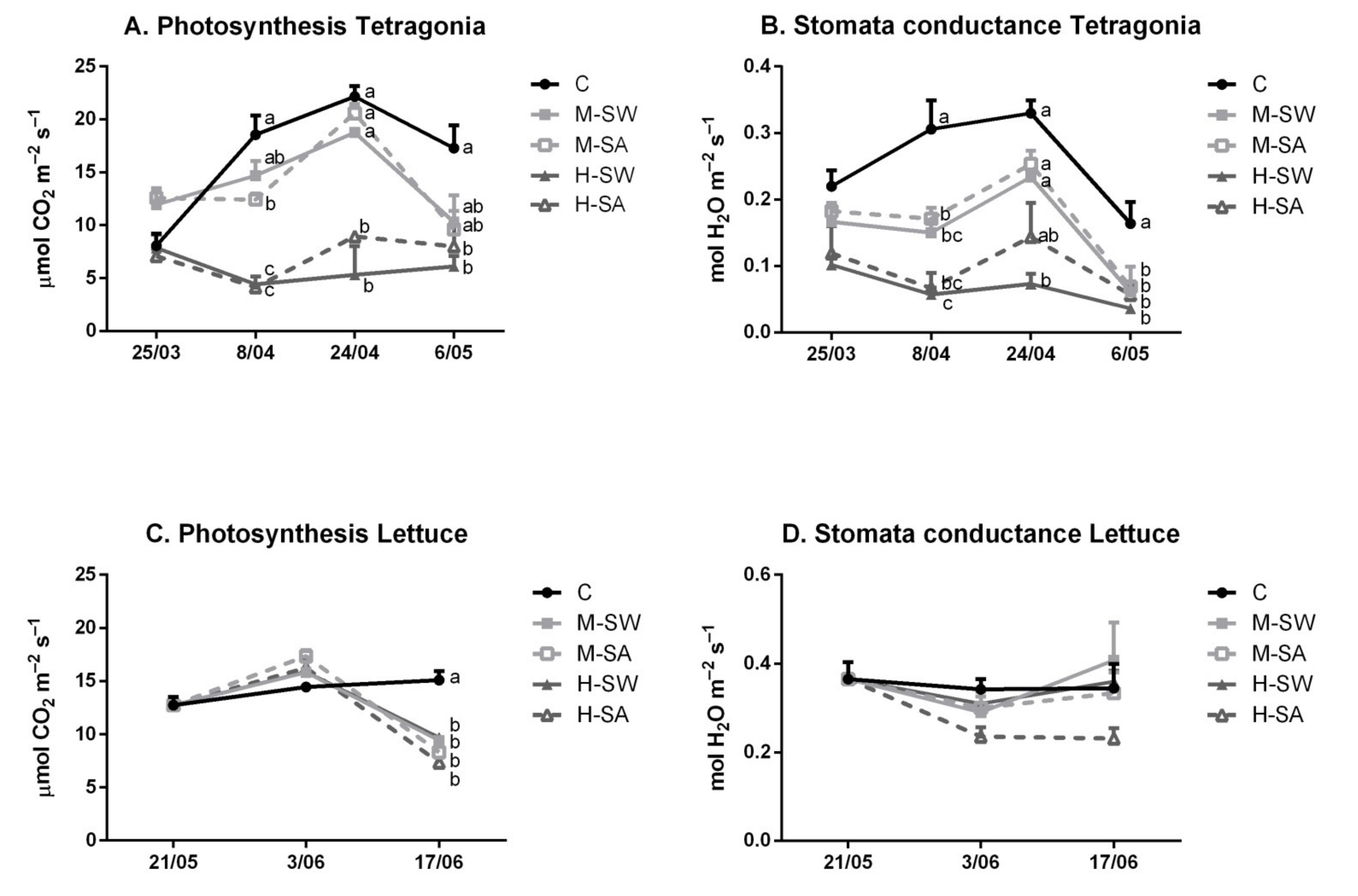
3.3. Leaf Gas-Exchange Parameters
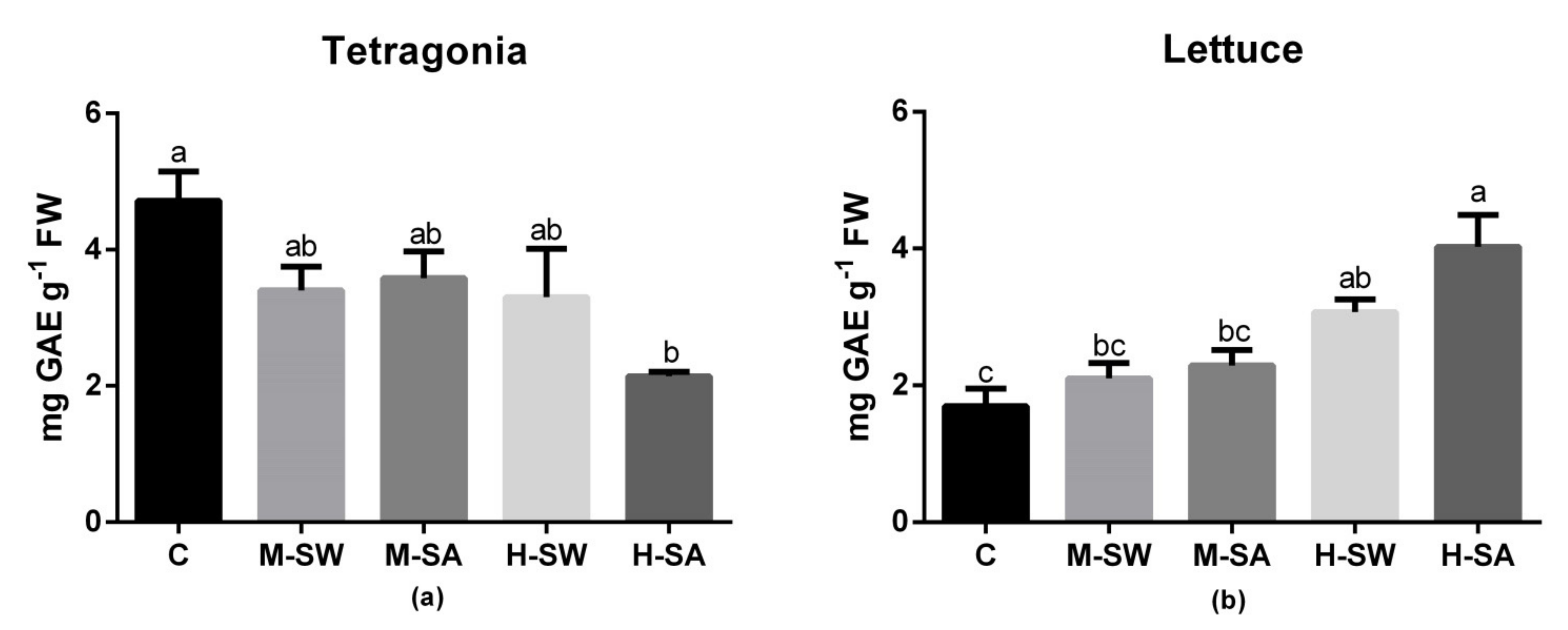
3.4. Element, Phenolic Compounds and Proline Concentration in Edible Leaves
4. Discussion
4.1. Salinity Type Affected Biomass Growth Only in Salt-Sensitive Species
4.2. Water Relations Are Differently Affected by Seawater and NaCl in Both Species
4.3. Physiological Insights
4.4. Effects of the Type of Salt Stress on the Nutritional Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béné, C.; Oosterveer, P.; Lamotte, L.; Brouwer, I.D.; de Haan, S.; Prager, S.D.; Talsma, E.F.; Khoury, C.K. When food systems meet sustainability—Current narratives and implications for actions. World Dev. 2019, 113, 116–130. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzori, G. The potential of edible halophytes as new crops in saline agriculture—The ice plant (Mesembryanthemum crtstallinum L.) case study. In Future of Sustainable Agriculture in Saline Environments; Negacz, K., Vellinga, P., Barrett-Lennard, E., Choukr-Allah, R., Elzenga, T., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 443–460. ISBN 9781003112327. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.V.; Hoffman, G.J. Crop Salt Tolerance—Current Assessment. J. Irrig. Drain. Div. Am. Soc. Civ. Eng. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Tanji, K.K.; Kielen, N.C. Agricultural Drainage Water Management in Arid and Semi-Arid Areas; FAO Irrigation and Drainage Paper 61; FAO: Rome, Italy, 2002. [Google Scholar]
- De Vos, A.; Bruning, B.; van Straten, G.; Oosterbaan, R.; Rozema, J.; van Bodegom, P. Crop Salt Tolerance under Controlled Field Conditions in The Netherlands, Based on Trials Conducted at Salt Farm Texel; Salt Farm Texel: Den Burg, The Netherlands, 2016. [Google Scholar]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Han, G.; Guo, J.; Meng, Z.; Chen, M. Progress in the Study and Use of Seawater Vegetables. J. Agric. Food Chem. 2020, 68, 5998–6006. [Google Scholar] [CrossRef] [PubMed]
- Atzori, G.; Nissim, W.G.; Macchiavelli, T.; Vita, F.; Azzarello, E.; Pandolfi, C.; Masi, E.; Mancuso, S. Tetragonia tetragonioides (Pallas) Kuntz. as promising salt-tolerant crop in a saline agriculture context. Agric. Water Manag. 2020, 240, 106261. [Google Scholar] [CrossRef]
- Atzori, G.; Mancuso, S.; Masi, E. Seawater potential use in soilless culture: A review. Sci. Hortic. 2019, 249, 199–207. [Google Scholar] [CrossRef]
- Atzori, G.; de Vos, A.C.; van Rijsselberghe, M.; Vignolini, P.; Rozema, J.; Mancuso, S.; van Bodegom, P.M. Effects of increased seawater salinity irrigation on growth and quality of the edible halophyte Mesembryanthemum crystallinum L. under field conditions. Agric. Water Manag. 2017, 187, 37–46. [Google Scholar] [CrossRef]
- De Vos, A.C.; Broekman, R.; de Almeida Guerra, C.C.; van Rijsselberghe, M.; Rozema, J. Developing and testing new halophyte crops: A case study of salt tolerance of two species of the Brassicacea, Diplotaxis tenuifoli and Cochlearia officinalis. Environ. Exp. Bot. 2013, 92, 154–164. [Google Scholar] [CrossRef]
- De Vos, A.C.; Broekman, R.; Groot, M.P.; Rozema, J. Ecophysiological response of Crambe maritima to airborne and soil-borne salinity. Ann. Bot. 2010, 105, 925–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozema, J.; Cornelisse, D.; Zhang, Y.; Li, H.; Bruning, B.; Katschnig, D.; Broekman, R.; Ji, B.; van Bodegom, P. Comparing salt tolerance of beet cultivars and their halophytic ancestor: Consequences of domestication and breeding programmes. AoB Plants 2015, 7, plu083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Status of the World’s Soil Resources Main Report; FAO: Rome, Italy, 2015. [Google Scholar]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW). Managing Systems at Risk; The Food and Agriculture Organization of the United Nations: Rome, Italy; Earthscan: London, UK, 2011. [Google Scholar]
- Cheeseman, J.M. Food Security in the Face of Salinity, Drought, Climate Change, and Population Growth. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 111–124. ISBN 9780128018545. [Google Scholar]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Boyko, H. (Ed.) Salinity and Aridity New Approaches to Old Problems; Dr. W. Junk Publishers: The Hague, The Netherlands, 1966. [Google Scholar]
- Boyko, H.; Boyko, E. Experiments of Plant Growing under Irrigation with Saline Waters from 2000 mg/L T.D.S. (Total Diluted Solids) up to Sea-water of Oceanic Concentration, without Desalination. In Salinity and Aridity New Approaches to Old Problems; Boyko, H., Ed.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1966; pp. 214–282. [Google Scholar]
- Eyster, C. Seawater as a Source of Plant Nutrients. Nature 1968, 220, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kogi, M.; Yanagisawa, T. Effects of Salinity and Nutrients in Seawater on Hydroponic Culture of Red Leaf Lettuce. Environ. Control Biol. 2014, 52, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; Muscolo, A.; Farooq, M.; Ahmad, W. Sustainable use and management of non-conventional water resources for rehabilitation of marginal lands in arid and semiarid environments. Agric. Water Manag. 2019, 221, 462–476. [Google Scholar] [CrossRef]
- Yousif, B.S.; Liu, L.Y.; Nguyen, N.T.; Masaoka, Y.; Saneoka, H. Comparative Studies in Salinity Tolerance between New Zealand Spinach (Tetragonia tetragonioides) and chard (Beta vulgaris) to salt stress. Agric. J. 2010, 5, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. Nutrient Solutions for Hydroponic Culture; University of California Agricultural Experimental Station Circular: Berkeley, CA, USA, 1938; Volume 347. [Google Scholar]
- Atzori, G.; Guidi Nissim, W.; Caparrotta, S.; Masi, E.; Azzarello, E.; Pandolfi, C.; Vignolini, P.; Gonnelli, C.; Mancuso, S. Potential and constraints of different seawater and freshwater blends as growing media for three vegetable crops. Agric. Water Manag. 2016, 176, 255–262. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma 2016, 253, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Downton, W.J.S.; Grant, W.J.R.; Robinson, S.P. Photosynthetic and Stomatal Responses of Spinach Leaves to Salt Stress. Plant Physiol. 1985, 77, 85–88. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; De Pascale, S. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Atzori, G.; Guidi Nissim, W.; Caparrotta, S.; Santantoni, F.; Masi, E. Seawater and water footprint in different cropping systems: A chicory (Cichorium intybus L.) case study. Agric. Water Manag. 2019, 211, 172–177. [Google Scholar] [CrossRef]
- Katerji, N.; Van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. Agric. Water Manag. 2003, 62, 37–66. [Google Scholar] [CrossRef]
- Neves, M.A.; Miguel, M.G.; Marques, C.; Panagopoulos, T.; Beltrao, J. The Combined Effects of Salts and Calcium on Growth and Mineral Accumulation of Tetragonia tetragonioides—A Salt Removing Species. WSEAS Trans. Environ. Dev. 2008, 4, 1–5. [Google Scholar]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Bartha, C.; Fodorpataki, L.; Martinez-Ballesta, M.d.C.; Popescu, O.; Carvajal, M. Sodium accumulation contributes to salt stress tolerance in lettuce cultivars. J. Appl. Bot. Food Qual. 2015, 88, 42–48. [Google Scholar]
- Caparrotta, S.; Masi, E.; Atzori, G.; Diamanti, I.; Azzarello, E.; Mancuso, S.; Pandolfi, C. Growing spinach (Spinacia oleracea) with different seawater concentrations: Effects on fresh, boiled and steamed leaves. Sci. Hortic. 2019, 256, 1–7. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Falleh, H.; Jalleli, I.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Magné, C.; Abdelly, C. Effect of salt treatment on phenolic compounds and antioxidant activity of two Mesembryanthemum edule provenances. Plant Physiol. Biochem. 2012, 52, 1–8. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Huang, J.; Gruber, M.Y.; Kaddour, R.; Lachaâl, M.; Ouerghi, Z.; Hannoufa, A. The impact of genotype and salinity on physiological function, secondary metabolite accumulation, and antioxidative responses in lettuce. J. Agric. Food Chem. 2010, 58, 5122–5130. [Google Scholar] [CrossRef]
- Khani, A.; Barzegar, T.; Nikbakht, J.; Ghahremani, Z. Effect of foliar spray of calcium lactate on the growth, yield and biochemical attribute of lettuce (Lactuca sativa L.) under water deficit stress. Adv. Hortic. Sci. 2020, 34, 11–24. [Google Scholar]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Waskiewicz, A.; Muzolf-Panek, M.; Golinski, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Springer: Berlin/Heidelberg, Germany, 2013; pp. 283–314. ISBN 9781461447474. [Google Scholar]
- Smirnoff, N.; Cumbes, J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemisrty 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Radyukina, N.L.; Kartashov, A.V.; Ivanov, Y.V.; Shevyakova, N.I.; Kuznetsov, V.V. Functioning of defense systems in halophytes and glycophytes under progressing salinity. Russ. J. Plant Physiol. 2007, 54, 806–815. [Google Scholar] [CrossRef]

| Na | K | NO2–N | Silicates | PO4 | NO3–N | pH | EC |
|---|---|---|---|---|---|---|---|
| mg L−1 | µg L−1 | dS m−1 | |||||
| 11.500 | 450 | 0.015 | 0.051 | 0.01 | 0.387 | 7.8 | 52 |
| Treatment | Tetragonia g | Lettuce g |
|---|---|---|
| C | 12.6 ± 0.6 a | 8.2 ± 0.3 a |
| M-SW | 17.4 ± 3.4 a | 6.7 ± 0.4 a |
| M-SA | 16 ± 1.9 a | 6.8 ± 0.4 a |
| H-SW | 2.5 ± 1.2 b | 4.4 ± 0.2 b |
| H-SA | 2.1 ± 0.5 b | 2.4 ± 0.6 c |
| Treatment | Tetragonia | Lettuce | ||
|---|---|---|---|---|
| Cumulative (L) | Daily Average (L day−1) | Cumulative (L) | Daily Average (L day−1) | |
| C | 5.3 ± 0.6 a | 0.12 ± 0.01 a | 3.9 ± 0.1 a | 0.12 ± 0.003 a |
| M-SW | 4.6 ± 0.3 a,b | 0.11 ± 0.01 a,b | 4.2 ± 0.1 a | 0.13 ± 0.003 a |
| M-SA | 4.5 ± 0.2 a,b | 0.10 ± 0.01 a,b | 4 ± 0.1 a | 0.13 ± 0.002 a |
| H-SW | 4 ± 0.8 a,b | 0.09 ± 0.02 a,b | 3 ± 0.1 b | 0.09 ± 0.003 b |
| H-SA | 2.8 ± 0.2 b | 0.07 ± 0.004 b | 3 ± 0.1 b | 0.09 ± 0.005 b |
| Treatment | Tetragonia | Lettuce | ||||
|---|---|---|---|---|---|---|
| Na | K | Ca | Na | K | Ca | |
| mg g−1 DW | mg g−1 DW | |||||
| C | 5.9 ± 2.2 d | 63.8 ± 6 a | 5.3 ± 0.4 a | 1.5 ± 0.6 d | 67.3 ± 7.1 a | 5.5 ± 0.6 a |
| M-SW | 84.4 ± 6.4 c | 40.1 ± 4 b | 3.7 ± 0.2 b,c | 31.8 ± 1.6 c | 54.5 ± 2.9 a,b | 1.9 ± 0.2 b |
| M-SA | 104.1 ± 4.9 b,c | 34 ± 2.5 b | 3.3 ± 0.1 c | 46.5 ± 2.1 b,c | 43.7 ± 1.2 b,c | 1.6 ± 0.04 b |
| H-SW | 126.6 ± 12.5 a,b | 26 ± 2.6 b,c | 5 ± 0.7 a,b | 49.9 ± 1.7 b | 43.8 ± 2.4 b,c | 2.5 ± 0.1 b |
| H-SA | 156.7 ± 7.2 a | 10.6 ± 1.2 c | 3.7 ± 0.2 b,c | 89.1 ± 8.5 a | 28.1 ± 2 c | 2.7 ± 0.1 b |
| Treatment | Tetragonia | Lettuce |
|---|---|---|
| µg g−1 FW | ||
| C | 221.2 ± 84.7 b | 122.4 ± 35.6 b |
| M-SW | 1006.3 ± 72.7 a | 123.3 ± 4.6 b |
| M-SA | 1226.2 ± 89 a | 427.2 ± 165.1 b |
| H-SW | 1067.8 ± 99.4 a | 816.2 ± 31 b |
| H-SA | 1289.8 ± 163.8 a | 3168.1 ± 580 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidi Nissim, W.; Masi, E.; Pandolfi, C.; Mancuso, S.; Atzori, G. The Response of Halophyte (Tetragonia tetragonioides (Pallas) Kuntz.) and Glycophyte (Lactuca sativa L.) Crops to Diluted Seawater and NaCl Solutions: A Comparison between Two Salinity Stress Types. Appl. Sci. 2021, 11, 6336. https://doi.org/10.3390/app11146336
Guidi Nissim W, Masi E, Pandolfi C, Mancuso S, Atzori G. The Response of Halophyte (Tetragonia tetragonioides (Pallas) Kuntz.) and Glycophyte (Lactuca sativa L.) Crops to Diluted Seawater and NaCl Solutions: A Comparison between Two Salinity Stress Types. Applied Sciences. 2021; 11(14):6336. https://doi.org/10.3390/app11146336
Chicago/Turabian StyleGuidi Nissim, Werther, Elisa Masi, Camilla Pandolfi, Stefano Mancuso, and Giulia Atzori. 2021. "The Response of Halophyte (Tetragonia tetragonioides (Pallas) Kuntz.) and Glycophyte (Lactuca sativa L.) Crops to Diluted Seawater and NaCl Solutions: A Comparison between Two Salinity Stress Types" Applied Sciences 11, no. 14: 6336. https://doi.org/10.3390/app11146336
APA StyleGuidi Nissim, W., Masi, E., Pandolfi, C., Mancuso, S., & Atzori, G. (2021). The Response of Halophyte (Tetragonia tetragonioides (Pallas) Kuntz.) and Glycophyte (Lactuca sativa L.) Crops to Diluted Seawater and NaCl Solutions: A Comparison between Two Salinity Stress Types. Applied Sciences, 11(14), 6336. https://doi.org/10.3390/app11146336







