Abstract
The number and the binding affinity, measured as the mean fluorescent intensity (MFI) of HLA-specific IgG antibodies, formed in the sera of end-stage organ disease patients and allograft recipients, referred to as sensitization, may restrict the availability of a donor organ and/or lead to graft failure after transplantation. The MFI of HLA Abs in sera is monitored with the Luminex-based single-antigen bead (SAB) immunoassay. The following two factors may impact the reliable measurement of MFI: one, the HLA structural variants on the SAB, namely, trimeric HLA (closed conformers, CC) and monomeric heavy chains (open conformers, OC); and two, the nature of the detection Abs, namely, IgG heavy-chain binding polyclonal-Fab (IgHPolyFab) or Fc-binding monoclonal-IgG (FcMonoIgG). Anti-CC Abs correlate with positive flow cross-matches, and are considered to be pathogenic and damaging to the graft, whereas anti-OC Abs appear to have little relevance to graft attrition. The presence of both CC and OC on beads may impair the reliability of monitoring the nature and MFI of pathogenic Abs. Our objective is to compare the MFI of the HLA Abs in the sera of 20 sensitized patients in two different SAB assays, with the two detection Abs. Our data reveal that the admixture of OC with CC on beads will affect the reliability of the measurement of the pathogenic Abs, and that FcMonoIgG is the more sensitive and specific detection Ab for the accurate assessment of HLA sensitization.
1. Introduction
The presence of pre-existing anti-HLA antibodies (Abs) is referred to as sensitization. It is one of the significant immunological barriers to organ transplantation. Sensitization in patients waiting for transplantation is known to be 30% of kidney [,], 18% of heart [,], 29% of lung [,], and 20% of liver transplant candidates []. The level of sensitization is assessed based on the number of HLA antigens recognized by the serum IgG Abs, and on the MFI of the anti-HLA Abs at a particular dilution. These Abs may (a) restrict the availability of a given organ for a particular patient, and are a significant cause of long-term graft attrition post-transplantation [,,,,,,,]; (b) react with mismatched intact HLA antigens on a donor organ (donor-specific Abs, DSA); and (c) recognize non-donor-specific allo-HLA (NDSA). Although DSA are associated with reduced allograft survival [,], it is increasingly observed that not all DSA result in graft failure [,,,,,,,]. These observations suggest possible pitfalls in the immunoassay, and raise concerns about the specificity and sensitivity of the assay for the reliable measurement of the levels of HLA Abs.
HLA molecules on the cell surface occur as hetero-dimers for HLA-I (α-chain and β2-microglobulin β2m) and HLA-II (α- and β-chains), complexed with a short peptide. These trimeric structures are designated as “HLA closed conformers (HLA CC)” []. These heteromeric HLA are involved in antigen presentation. In addition to trimers, HLA molecules on the cell surface also occur as monomeric heavy chains (HC), called “HLA open conformers (HLA OC)” []. HLA OC are expressed on the surface of metabolically activated cells, including human T lymphocytes, EBV-transformed B cells, CD19+ B cells, CD8+ T cells, CD56+ NK cells, CD14+ monocytes, extravillous trophoblasts and monocytes, B-cell lines (RAJI, NALM6), and the myeloid cell line (KG-1A) [,,,,,,]. These studies also show that the tyrosyl and seryl residues, in the extended cytoplasmic tail of naturally occurring HLA-I OC, can be phosphorylated and involved in signal transduction [].
HLA Abs, associated with pre- and post-transplant sensitization [,,,,], are monitored with Luminex-based multiplex single-antigen bead immunoassay (SAB immunoassay), in which SABs are coated with individual HLA molecules. A vast majority of reports have observed HLA Abs using single-antigen SABs (LABScreen, LS) from a vendor. Some of the studies aimed to distinguish Abs binding to HLA-I OC, induced by acid or heat treatment, from those binding to HLA CC (regular beads), which showed that Abs against intact HLA CC, but not those against denatured HLA OC, are predictive of graft failure [,,,,,,,].
In addition to LS-SAB, another SAB (LIFECODES, LC) from a different vendor is used routinely outside the USA [,,,,,,,,,,]. We have compared the structural variants of the HLA-I molecules on LS-SAB and LC-SAB using the following two monoclonal Abs: mAb W6/32 (HLA trimer/CC-specific) and mAb TFL-006 (HLA monomer/OC-specific) []. The LS-SABs were positive for both W6/32 and TFL-006 [,,], which is indicative of the presence of HLA CC admixed with HLA OC. In contrast, the LC-SABs were positive for W6/32, but negative for TFL-006, confirming the absence of HLA OC on LC-SAB [,,]. Similarly, HLA-II-coated LS-SABs also carry an admixture of OC with CC. Monomeric HLA-II OC was documented when the anti-HLA-II IgG reactivity was detected in sera (n = 141) using the LS-SAB coated with HLA-DRB1*09:01, DRB3*01:01, DRB3*02:02, DRB3*03:01, DPB1*02:01, DPB1*20:01, and DPB1*28:01 []. The sera reacting to the LS-SAB failed to react with the native cell surface HLA (e.g., HLA-DRB1*09:01) in a flow cytometry crossmatch (FCXM) and in absorption/elution experiments, suggesting that some HLA-II alleles on the LS-SAB may be present as OC [].
Several reports have documented that many DSA monitored with LS-SABs in pre-or post-transplant sera were not associated with graft failure [,,,,,,,,,]. These studies suggest that (1) only specific monitoring of CC-specific Abs would elucidate the causal relationship between the levels of DSAs and NDSAs with graft failure or rejection, and (2) the reactivity of the sera to naturally occurring OC that are admixed with CC in SAB assay may result in the denial of an otherwise compatible organ, or may result in inappropriate pre- and post-transplantation therapies. Therefore, there is a need to monitor HLA Ab binding to CC, without the interference of OC, during the sensitization of end-stage organ disease patients.
Another salient factor of Ab, which may impact the precise measurement of the MFI or density of serum HLA Abs, is the clonality of the detection Abs []. The most commonly used detection antibody in SAB immunoassay is IgHPolyFab [], which is recommended by histopathologists for better resolution and localization of the surface antigens on cells and tissue sections, due to the signal amplification caused by the IgG heavychain binding polyclonal Fab fragments, binding to an antigen-bound primary Ab (Cusabio Catalog, Cusabio Product Center. Introductory Notes for Secondary Antibodies. Available at: http://www.cusabio.com/catalog-21-1.html, accessed on 12 October 2015.). For the detection of IgG subclasses, Fc-specific monoclonal IgG (FcMonoIgG) is used as a detection Ab []. For the detection of anti-HLA IgG, FcMonoIgG has not been used to compare or validate the sensitivity and reliability of the different detection Abs for monitoring Abs in the Luminex SAB assay. We have documented that FcMonoIgG may provide a better quantitative assessment (MFI or titer) of anti-HLA IgG measurements, as it binds to a single Fc domain of the primary anti-HLA IgG, in a one-to-one ratio [,]. The studies further revealed that when the serum Ab density was low, the binding of IgHPolyFab was amplified. When the serum density was high, the binding of IgHPolyFab diminished. No such variations were observed with FcMonoIgG []. However, it remains to be elucidated whether the nature (mono- versus poly-clonality, and whole IgG versus Fab fragments) of the detection Abs impacts the assessment of the sensitization of patients before transplantation.
These reports led to the following hypotheses: (1) the number and the MFI of anti-HLA CC Abs may differ due to the presence or absence of OC on the SAB, and (2) the sensitivity of the assay may differ depending on the clonality of the detection Abs, i.e., IgHPolyFab and FcMonoIgG. The present investigation is aimed at testing these hypotheses in a clinically defined sensitized ESRD patient cohort waiting for donor organs.
2. Material and Methods
2.1. Patients Sera
Both hypotheses were validated on the sera of 20 sensitized ESRD patients (16 females and four males). The possible causes of sensitization vary among these patients. Ten patients (2DM, 6FG, 7HN, 8DM, 10DM, 12DM, 13LE, 15NA, 16HN and 20HN) were possibly sensitized by pregnancy, five (1DM, 3LE, 11DM, 18PK and 19HN) by transfusion, three (9NA, 14HN, 17LE) due to prior transplantation and two (4LE and 5DM) for reasons unknown. All these patients were waiting for donor organs. The patients 1DM, 3LE, 4LE, and 7HN were transplanted within a year after sample collection for this study. The nature of ESRD is provided in the legend for Figure 1. Sera were randomly selected from the Downstate pre-transplant clinic at Downstate Health Sciences University, University Hospital of Brooklyn (UHB) by Prof. Dr. Allen Norin and Dr. Ballabh Das. SAB/DSA study protocols were reviewed and approved by the Downstate Institutional Review Board (IRB), (IRB #1232938-3 and #341403-1). Sera were examined with LS- and LC-SABs using IgHPolyFab and FcMonoIgG. All assays were carried out simultaneously on a single day by one HLA technologist (Mr. Vadim Jucaud) at Terasaki Foundation Laboratory (TFL).
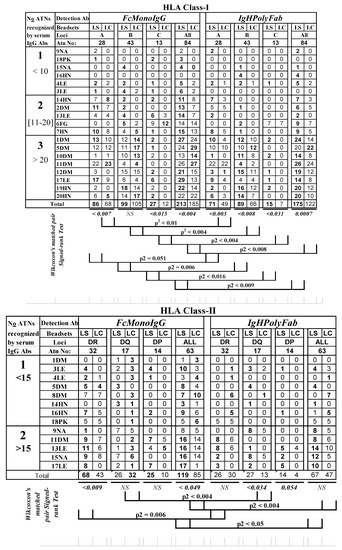
Figure 1.
Profiles of sensitization estimated by the number of HLA class I and class II antigens, recognized by the IgG antibodies in the ESRD patients’ sera, significantly differ between the two different SABs, LABScreen (LS) and LIFECODES (LC), and with two different detection Abs (FcMonoIgG and IgHPolyFab). The nature of disease in the ESRD are identified as follows: seven DM (diabetes mellitus), four LE (lupus erythematosus), one FG (focal glomerulonephritis), five HN (hypertensive nephrosclerosis), one PK (polycystic kidney) and two NA (not available). MFI < 500 is shown as zero. The sera were tested at 1/10 dilution and the cut-off of the normalized trimmed mean MFI is 500. Note that the number of antigens recognized with LS-SABS are frequently higher (numbers in bold) than that recognized by LC-SABs. FcMonoIgG recognized greater number of antigens than the conventionally used IgHPolyFab.
2.2. Luminex Multiplex SAB Assay
2.2.1. SABs from Different Vendors
Abs were monitored using SABs from the following two vendors on the Luminex platform:
- (i)
- LS-SAB class I (Cat. # LS1A04, Lot#10) and class II (Cat. # LS2A01, Lot#12) (Thermo Fisher/One Lambda, Canoga Park, CA, USA);
- (ii)
- LC-SAB class I (Cat. # 265100R, Lot # 3005613) and class II (Cat. # 265200R, Lot # 3005537) (Immucor, Norcross, GA, USA).
The panel of molecules coated on LS-SAB and LC-SAB can be common to both LC-SAB and LS-SAB or “unique” to either SAB. The number of common antigens are as follows: HLA-A (n = 28), HLA-B (n = 43), HLA-C (n = 13), HLA-DR (n = 32), HLA-DQA1/DQB1 (n = 17) and HLA-DPA1/DPB1 (n = 13). The pane of molecules unique to each SAB and their Ab reactivities are not included in this report.
2.2.2. The HLA-I Polyreactive mAb TFL-006 as Quality Control Reagent to Monitor HLA-I OC on the SABs
At Terasaki Foundation Laboratory, the mAb TFL-006 was generated by immunizing mice with a recombinant heavy chain of HLA-ER107 [,]. To verify the specificity of the mAb TFL-006 and other mAbs generated against HLA-E, they were tested using LS-SAB coated with 31 HLA-A, 50 HLA-B, 16 HLA-C, one HLA-G, two HLA-E, and one HLA-F antigen. The mAbs were categorized into eight groups based on their affinity. Group 1 constituted the HLA-E monospecific mAbs that did not react with any HLA class I antigens other than HLA-E. Group 8 (which included TFL-006 and TFL-007, previously called PTER-006/007) reacted with all of the abovementioned HLA-I antigen-coated beads on the LS-SAB (U.S patent 10,800,847; 13 October 2020). The epitope specificity of the polyreactive anti-HLA-E mAbs was monitored by dosimetric inhibition studies using several synthetic peptides, as reported earlier [,]. The binding of mAbs TFL-006 and TFL-007 to HLA-I antigens was inhibited by a synthetic peptide 117AYDGKDY123 shared by all alleles of HLA class I loci [,], but remain cryptic in native HLA-I as they are masked by β2m (Table 4 in []). TFL-006 was tested on LS-SAB (Lots # 10 and 11) and on LC-SAB (lot # 3005613) at different concentrations ranging from 10 to 40 μg/mL. The diluents used are the same as reported [,].
2.2.3. The HLA-II Polyreactive mAb TFL-FJ5109 Identifies HLA-II-OC on LS-SAB
The presence of the OC among HLA-II antigens was examined with an HLA-II polyreactive mAb.FJ5109, (source: One Lambda, Canoga Park, CA, USA). The mAb was used on LS-SAB (HLA-II LS2A01009, lot 9) after passing through a protein G column. Due to the restricted availability of the mAb, we have tested LS-SAB only.
2.2.4. Modifications in the Assay Protocol for Comparing the Different SABs
To remove the protocol differences between the vendors as a confounding variable, we used the LS vendor protocol (using 2 μL of beads instead of 5 μL/test, a standard procedure at Terasaki Foundation Laboratory (TFL) and Terasaki Research Institute (TRI)). To keep the number of beads consistent, the LC-SABs were spun down to remove 835 μL of the fluid volume from the stock to concentrate their beads (the initial volume of LC beads is 960 μL). The final volume of the SABs from both vendors is therefore maintained at 125 μL. Our standard operating procedure (SOP) was extensively used at TFL and we had obtained concordant results when performing proficiency testing for the UCLA serum exchange program. While designing the assay, we kept the antibody-to-bead ratio consistent between the vendors. For the assay, neat sera were avoided. The sera (20 µL) were diluted (1/10) and then incubated with 2 μL of beads (for both LS and LC) for 30 min at room temperature (RT) on a shaker. The beads were then washed (3×) with LS wash buffer. The Ab binding to beads was assessed with two PE-conjugated secondary Abs (see below) by incubating the detection Ab (50 μL at 5 μg/mL) for 30 min at RT on a shaker. After washing, the beads were suspended in 1× PBS before acquisition on the Luminex platform. At least 100 beads were counted for each antigen for the SAB from different vendors. On the rare occasion that any variation occurred in the bead count, it was <5%. The assay includes a positive control bead (coated with human IgG) and a negative control bead (no antigen). In addition, we have used negative control serum (demonstrated by other methods considered to be free of anti-HLA IgG) as well as positive control serum, prepared by the vendor by pooling sera from several individuals carrying anti-HLA IgG.
In this investigation, we have recorded MFI after normalizing the trimmed mean MFI. Since we are comparing MFIs of different SABs with potentially differing composition panels (LS with CC admixed with OC and LC with CC only), we normalized as follows:
- Step 1.
- We subtracted MFI of PBS only from trimmed MFI. This is an essential step because when antibodies were tested on beads with PBS alone, differential MFI values were observed with different HLA molecules.
- Step 2.
- We used (trim mean MFI-PBS MFI) to subtract the following:
- Negative control MFI (NC MFI);
- Negative sera value obtained with LS-SABs (NGS-LS);
- Negative sera value obtained with LC-SABs (NGS-LC);
- B and C were added and divided by 2 for correction.
The following formula explains the steps used:
Normalized MFI = [(Trim. Mean MFI − PBS MFI) − (NC MFI)] − (NGS-LS-SABS+ NGS-LC)/2).
These steps are critical for comparing the two different kinds of SABs and very useful for considering the diverse composition of the panels and the different frequencies of the target epitopes on the beads. In the above protocol, no background noise was observed for any secondary antibody, whether IgHPolyFab or FcMonoIgG. Note that the protocol considers, while calculating normalized trimmed means, the factors that may cause background noise, such as PBS alone or negative controls.
2.2.5. The Diversity of the Secondary Detection Abs
The rationale for comparing two different detection Abs was elaborated in earlier reports [,]. The following two PE-conjugated detection Abs were used:
IgHPolyFab: PE-conjugated human IgG heavy chain (IgH)-specific polyclonal goat anti-Human IgG Ab fragments [F(ab)2], supplied as 0.5 mg/mL in PBS pH 7.6, by One Lambda Inc (Canoga Park, CA) Cat # LS-A82. The label on the box of the vial (the product literature) states that the product is “PE-conjugated Goat-anti-Human IgG; R-phycoerythrin-coated AffiniPure F(ab)2; goat X human IgG 1 mL (100×)”.
FcMonoIgG: PE-conjugated human IgG Fc-specific mouse monoclonal IgG, a whole purified IgG, supplied as 0.5 mg of purified IgG in 1 mL of borate-buffered saline (pH 8.2) by Southern Biotech (Birmingham, AL Cat # 9040-09). The product insert states that the subtype of the IgG is IgG1k, derived from clone JDC-10 and reacts specifically with human/rhesus/chimpanzee IgG Fc (MW 150 kDa). FcPolyIgG, recommended by LC vendor, was not used for the reasons reported in our earlier report [].
All anti-HLA Ab analyses for HLA-I and -II were performed by the same TFL HLA technologist (VJ) on the same day and the same run on a single tray. We performed experiments using sera diluted 1:10 to avoid falsely low values in the case of antibody excess (the “prozone effect”). The binding affinity of any detected Ab as measured by normalized MFI was compared, using an MFI cut-off for positivity of 500, at serum dilution 1:10. The MFI cut-off was determined for each SAB with each detection Ab based on our earlier report [] after studying their titration profiles.
2.3. cPRA Calculations
Panel-reactive antibodies (PRA) values have been the measurement of preformed HLA Abs (sensitization), which are considered as a formidable barrier to transplantation []. United Network for Organ Sharing (UNOS) implemented a strategy using unacceptable HLA antigens and a calculated PRA (cPRA) to award sensitization points. For example, a patient with 80% cPRA would be crossmatched incompatible with 80% of donors. The MFI obtained with Luminex SAB assay is utilized for calculating % of cPRA. For this experimental investigation, the cPRAs for each patient were calculated for each vendor’s SAB with each of the detection Abs, using the cPRA calculator on the UNOS website (https://optn.transplant.hrsa.gov/resources/allocation-calculators/cpra-calculator, accessed on 30 May 2021).
2.4. Statistical Analysis
Jeff Gornbein of UCLA/SBCC performed the statistical analyses. Paired comparisons of the number of alleles recognized and the antibody MFI responses were made between LC-SAB and LS-SAB for FcMonoIgG and IgHPolyFab, as well as between FcMonoIgG and IgHPolyFab for LC-SAB and for LS. Since the MFI data did not follow a normal (Gaussian) distribution, the non-parametric p-value for these comparisons was computed using the Wilcoxon signed-rank test for paired data.
3. Results
3.1. HLA Sensitization (Number of Abs against HLA Alleles and the MFI of Abs in the Patients Differ between LS-SAB and LC-SAB and between Two Detection Abs
The level of sensitization in the sera of waitlist patients is assessed by the number of HLA antigens that are recognized by the serum IgG Abs, and by the MFI of anti-HLA Abs on LS-SAB and LC-SAB, using two different detection Abs.
3.1.1. Differences in the HLA Antigens Recognized by Sera HLA Abs
Figure 1A shows the following three levels of sensitization based on the number of anti-HLA-I Abs on both the LS- and LC-SABs tested with two different detection Abs:
- Group 1 (low sensitization): sera IgG reacting to <10 HLA antigens (n = 6);
- Group 2 (moderate sensitization): sera IgG reacting to 11–20 HLA antigens (n = 5);
- Group 3 (high sensitization): sera IgG reacting to >20 HLA antigens (n = 8).
Figure 1B shows the following two levels of sensitization based on the number of anti-HLA-II Abs:
- Group 1 (low sensitization): sera IgG reacting to <15 HLA antigens (n = 8);
- Group 2 (high sensitization): sera IgG reacting to >15 HLA antigens (n = 5).
The MFI of both HLA-I and HLA-II Abs differed between LS-SAB, LC-SABS, and the detection Ab. The salient findings are as follows:
- (I)
- The number of HLA-I and HLA-II alleles recognized by sera Abs is significantly higher on the LS-SAB than on LC-SAB, with both the detection Abs (numbers in bold in Figure 1);
- (II)
- The FcMonoIgG recognized a significantly (two-tailed t-test) higher number of Abs than IgHPolyFab, for both the HLA-I and II antigens (number in bold in Figure 2), from moderate-to-high sensitization groups;
- (III)
- Sera IgG of several patients (2DM, 6FG, 10DM, 12DM, 19HN, and 20HN) did not react with any of the HLA-II antigens, with both the detection-Abs, while these sera reacted moderately (2DM and 6FG) or highly (10DM, 12DM, 19HN and 20 HN) with HLA-I antigens.
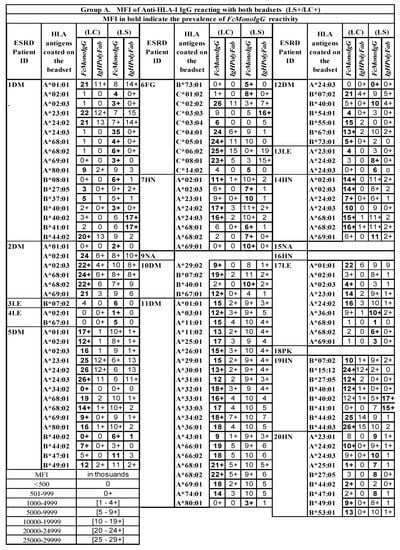
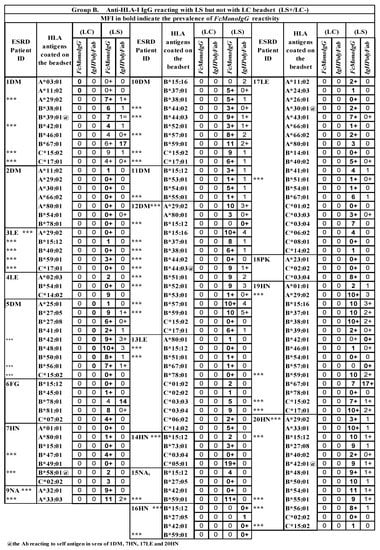
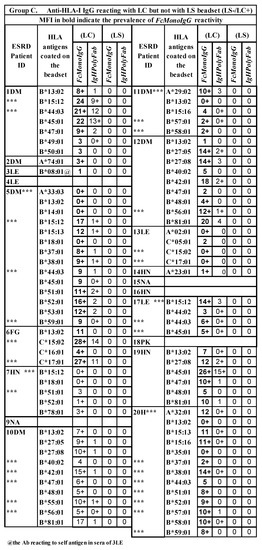
Figure 2.
Three groups of serum IgG antibodies in ESRD patients reacting to HLA-I antigens on either LS or LC, or to both SABs, when tested with two different detection Abs (FcMonoIgG and IgHPolyFab). MFI values are expressed as thousands. Group A: Abs binding to both SABs; Group B: Abs binding to LS-SAB only; Group C: Abs binding to LC-SAB only. IgG antibodies recognizing antigens unique to the SABs are excluded. The sera were tested at 1/10 dilution and the cut-off of the normalized/trimmed mean MFI is 500. Sera of patient 8DM showed no anti-HLA-I Ab reactivity. HLA antigen with @ sign refer to patient’s self-antigen recognized by the patient’s IgG Ab. *** Two different kinds of Ab reactivity against a particular allele. One kind of Ab binds LS only (B) and another kind binds to LC only (C), suggesting that it may be due Abs binding LS only (B) may be binding to the heavy chain only or open conformers, OC) and another class of Ab binding LC only(C) may be binding to intact HLA (closed conformers, CC). MFI values are indicated in numbes, each number refering to thousands. For example 10 implies ten thousand and 10+ implies 10,500–10,999.
Inferences: (1) More HLA antigens are recognized in LS- than in LC-SABs. (2) In moderately or highly sensitized groups, FcMonoIgG recognized more HLA alleles than IgHPolyFab, for both HLA-I and HLA-II. (3) The HLA sensitization in several ESRD patients may differ between HLA-I and HLA-II antigens.
3.1.2. Differential Binding Patterns of anti-HLA IgG Abs on LS- and LC-SABs
The MFI of sera HLA Abs tested at a 1:10 dilution can be categorized into the following three groups based on the differential binding to LS and LC (Figure 2 and Figure 3):
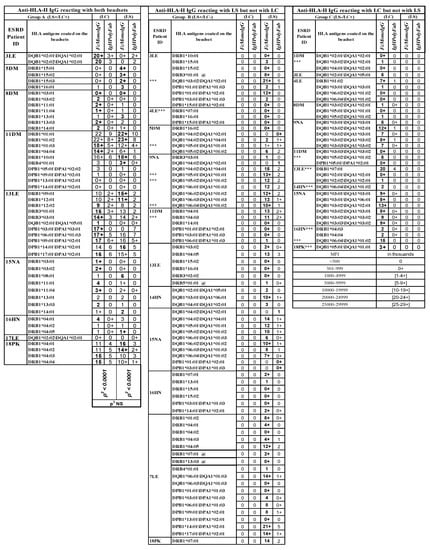
Figure 3.
Three groups of serum IgG antibodies in ESRD patients reacting to HLA-II antigens on either LS or LC, or to both SABs, when tested with two different detection Abs (FcMonoIgG and IgHPolyFab). MFI values are expressed as thousands. Group A: Abs binding to both SABs; Group B: Abs binding to LS-SAB only; Group C: Abs binding to LC-SAB only. IgG antibodies recognizing antigens unique to the SABs are excluded. Sera of patients 2DM, 6FG, 10DM, 12DM, 19HN and 20 HN showed no anti-HLA-II Ab reactivity. The sera were tested at 1/10 dilution and the cut-off of the normalized/trimmed mean MFI is 500. HLA antigen with @ sign refer to patient’s self-antigen recognized by the patient’s IgG Ab. *** Two different kinds of Ab reactivity against a particular allele. One kind of Ab binds LS only (B) and another kind binds to LC only (C), suggesting that it may be due Abs binding LS only (B) may be binding to the heavy chain only or open conformers, OC) and another class of Ab binding LC only(C) may be binding to intact HLA (closed conformers, CC). MFI values are indicated in numbes, each number refering to thousands. For example 10 implies ten thousand and 10+ implies 10,500–10,999.
All the three groups of IgGs reacting to HLA-I alleles can be found in one patient’s serum. For example, the sera IgG of patient 1DM reacted with 34 HLA-I alleles, of which 17 are located on both LS and LC (Group A), ten are observed on LS only (Group B), and seven are noted with LC only (Group C).
The following Abs reacted with HLA-I alleles on both the SABs (Figure 2A):
- A*01:01, 02:03, 11:02, 23:01, 24:03, 29:01, and A*80:01 (7 out of 28 HLA-A alleles);
- B*15:12, 40:02, 44:02, 47:01, 49:01, and B*54:01 (6 out of 43 HLA-B alleles);
- C*01:02, 02:02, 03:03, 03:04, 06:02, 08:01, and C*14:02 (6 out of 13 HLA-C alleles).
Similarly, all three groups of IgGs reacting to HLA-II alleles can be found in one patient’s serum (Figure 3). For example, the sera Abs of 11DM responded with 10 HLA-II alleles on both LS and LC (Group A), six alleles on LS only (Group B), and three alleles on LC only (Group C). In patient 13LE, the serum IgG reacted with 11 HLA-II alleles on both LS and LC (Group A), six alleles on LS only (Group B), and three alleles on LC only (Group CI).
Some serum Abs are directed against self-HLA antigens (with a low MFI). They include HLA-A, HLA-B, and HLA-DRB alleles, and are most often on LS beads (these antigens and their respective MFIs are marked @ in the row of the alleles in Figure 3 and Figure 4). Since such auto-Abs are rarely observed on LC-SAB (e.g., 3LE), it is suggested that the auto-Abs may be binding to OC, due to the exposure of shared epitopes.
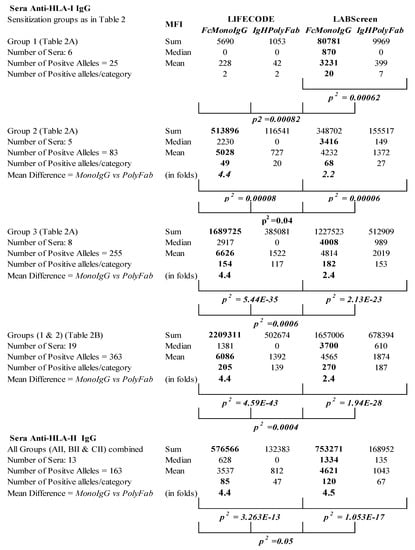
Figure 4.
Summary of the profiles of MFI of anti-HLA IgG Abs monitored in the sera of ESRD patients with LABScreen (LS) and LIFECODES (LC), and with two different detection Abs (FcMonoIgG and IgHPolyFab). Note (1) higher sum and mean MFI values for different sensitization groups with FcMonoIgG than with IgHPolyFab (by minimum two- to maximum four-fold) and (2) the higher number of folds of increase in sum and mean MFI with LIFECODES compared to LABScreen.
Inference: The sera IgG reacting to the HLA alleles on LS failed to recognize the same molecules on LC, suggesting that these antibodies are bound to the OCs on LS. The sera IgG reacting to the HLA alleles on LC were unable to recognize the same molecules on LS, implying that the OC on LS may interfere with, or cause steric hindrance to, the binding with the CC on LS.
3.1.3. Differences in the Resolution with and the Sensitivity of the Detection Abs
The MFI levels of the sera Abs tested at a particular dilution (1:10) measure the binding affinity of the anti-HLA Abs. The MFI of the anti-HLA Abs that are reactive on both the SABs (Figure 2) also differs with different detection Abs. The MFI values obtained using FC-binding monoclonal IgG are consistently higher than the MFI obtained with IgG heavy chain-binding polyclonal Fab fragments.
- The MFI obtained for anti-HLA Abs against HLA-I alleles are frequently higher with FcMonoIgG;
- Several sera Abs (e.g., 3LE, 7HN, 13LE, 14HN), with a high MFI with FcMonoIgG, were too low or negative with IgHPolyFab, with both LS and LC;
- Some sera Abs (e.g., 14HN, 5DM, 2DM, 6FC), though positive with IgHPolyFab (Figure 2), had MFIs with FcMonoIgG that were consistently higher with LC-SAB;
- Several sera (e.g., 3LE, 11DM, 13LE 15NA, 17LE, 18PK) anti-HLA-II Abs (Figure 3), when positive on both SABs, also showed a very high MFI with FcMonoIgG (bold values), while the reactivity with IgHPolyFab was low or negative with both LC and LS.
In addition to HLA antigens that are common to both SABs, few antigens are unique to either LS or LC (data not presented).
- For HLA-I antigens on LS-SAB, 38 MFI values were obtained, of which 30 were higher with FcMonoIgG. On LC-SAB, 38 MFI values were obtained, of which 34 were higher with FcMonoIgG;
- For HLA-II antigens on LS-SABs, 12 sera tested positive. Of the 45 MFI values obtained with these sera, 43 MFIs were markedly higher with FcMonoIgG. On LC-SABs, all the 27 MFI values obtained with the nine sera were remarkably higher, only with FcMonoIgG.
Inference: FcMonoIgG enhances the resolution and sensitivity of the detection of HLA Abs on both the SABs much better than IgHPolyFab.
Rarely, higher MFIs with IgHPolyFab were observed, only on LS-SAB. The sera may be poorly sensitized, as in 16HN (B*15:12/27:5/42:01/59:01, Figure 2B), or highly sensitized, as in 6FG (C:03:03, Figure 2A), 19HN (B*40:02/41:01, Figure 2A; B*67:01, Figure 2B), 1DM (A*01:01/23:01/24:02/40:02/41:01, Figure 2A; B* 67:01, Figure 2B), and 5DM (A*23:01/24:02/24:03 in the serum of 5DM, Figure 2A). Similarly, the positive MFIs for Abs against HLA-II, with a IgHPolyFab concomitant with low or negative values, were observed with FcMonoIgG on LS-SAB for DQB1*04:02\DQA1*02:01 (e.g., sera # 5F47, 9M33 and 15F43).
Inference: These exceptions observed on LS-SAB could be due to the presence of an OC on the LS, and a lack of the same in LC.
Figure 4 summarizes the disparity based on the total sum and mean MFI of IgGs reacting to HLA-I antigens in the four different categories of tests (LC and FcMonoIgG; LC and IgHPolyFab; LS and FcMonoIgG; and LS and IgHPolyFab).
- (a)
- Both the sum and mean MFI values of the anti-HLA-I Abs in group 2 and 3 (moderately or highly sensitized groups) are persistently higher with LC than with LS when tested with FcMonoIgG. All three groups combined are strikingly higher with LC than with LS when tested with FcMonoIgG;
- (b)
- With LC-SAB, the sum and mean MFI values for HLA-I Abs assessed using FcMonoIgG are 4.4-fold higher than with IgHPolyFab; whereas, with LS-SAB, the reactivity with FcMonoIgG is just 2.2 to 2.4 higher than that of IgHPolyFab;
- (c)
- In poorly sensitized patients, the sum and mean MFIs are higher with LS than with LC when tested with FcMonoIgG or IgHPolyFab. The MFIs with both the SABs with IgHPolyFab, though almost half of the values were obtained with FcMonoIgG, were higher with LS than with LC;
- (d)
- Similarly, both the sum and mean MFIs of all anti-HLA-II reactivity with FcMonoIgG are significantly enhanced four-fold, relative to IgHPolyFab;
- (e)
- The maximum MFI observed with FcMonoIgG, compared to IgHPolyFab, is higher for LS than for LC.
Inference: It is evident that FcMonoIgG enhances the sensitivity of the SAB immunoassay, particularly while monitoring anti-HLA Abs in the sera of highly sensitized patients. The higher sum and mean MFI values with LS could be due to the presence of an OC.
3.2. Documentation of the Open Conformers (OC) on the Different Lots of LS and Lack of the Same in LC-SAB
The presence of an OC on LS-SAB, and the absence of the same on LC-SAB, were shown using the monoclonal antibody TFL-006, which binds to the OC of all HLA class I molecules [,]. The presence of lot-to-lot variations in a vendor SAB is known, as every vendor may change the profile of the HLA coated on new lots. In the present study, the lots of SABs used are different from those used in previous reports, and hence they were assessed for the presence or absence of an OC using TFL-006. Figure 5A shows that mAb TFL-006 did not bind to HLA-A (n = 31 alleles), HLA-B (n = 50), and HLA-C (n = 16) molecules on the lot of LC-SABs (lot 3005613), even at 20 μg/mL, while the mAb showed positivity for all the HLA-I antigens coated on the two lots of LS-SABs (#10 and #11).
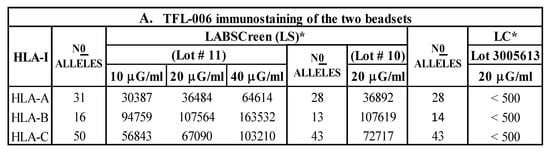
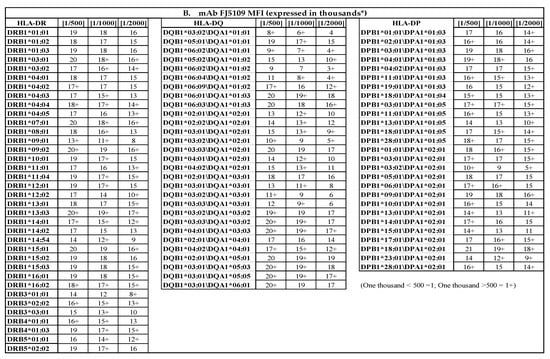
Figure 5.
(A) The HLA-I polyreactive mAb TFL-006, which recognizes shared epitopes found on β2m-free heavy chains of HLA-I alleles (open conformers, OC), binds to most of the HLA-I molecules coated on LABScreen (LS), but not those on LIFECODES (LC) SABs. The binding affinity of antibodies is measured as normalized MFI. Note LC-SABs are totally negative (MFI <500). (B) The HLA-II polyreactive mAb FJ5109 binds to most of the HLA-II molecules coated on LABScreen (LS). The binding affinity of antibodies is measured as normalized MFI on the SABs with the mAb. Since the mAb binds to all of the three loci of HLA-II molecules, presence of a shared epitopes among all HLA-II molecules is indicated.
Inference: The findings confirm that LS carries admixtures of HLA-I CC with OC, while LC only has HLA-I CC.
Similarly, mAb FJ5109 may bind to several HLA-II OC. Figure 5B confirms the positivity of HLA-II-coated LS-SABs, with the mAb at different dilutions. All 36 DR alleles, 29 DQA/DQB alleles, and 27 DPA/DPB alleles were highly positive. There are 36 DRB, 12 DQA, 14 DQB, 4 DPA, and 19 DPB heavy chains (HC). The positive MFI indicates the binding of the mAb to a public epitope on HLA-II heavy chains. The mAb-binding confirms the presence of HLA-II OC on the LS-SABs. However, the presence or absence of HLA-II OC on LC-SAB could not be confirmed, due to the non-availability of the mAb.
3.3. Admixture of OC with CC on LS-SAB and Use of IgHPolyFab Impacts a Reliable Estimation of % cPRA of Serum HLA Abs of Waitlist Patients
Figure 6 shows that the high % of cPRA for both the anti-HLA-I Abs and anti-HLA-II Abs correlates with FcMonoIgG, for both the SABs. The results obtained with % cPRA of anti-HLA-I Abs confirm the following:
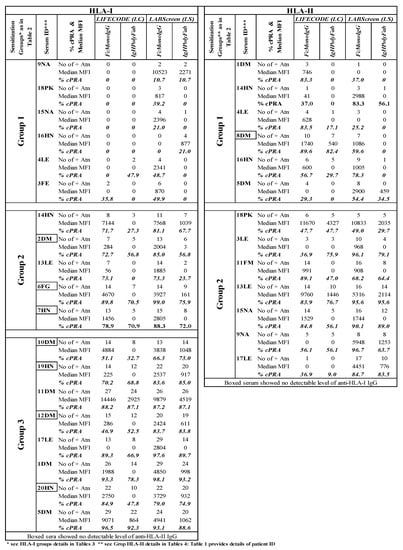
Figure 6.
Estimations of cPRA values of ESRD patients for diseased donor organ selection differ between LABScreen and LIFECODES SABs and between the two different detection Abs FcMonoIgG and IgHPolyFab. The cPRA (panel-reactive antibodies) calculator on the following UNOS website was used: (https://optn.transplant.hrsa.gov/resources/allocation-calculators/cpra-calculator, accessed on 30 May 2021).
- (a)
- The % cPRA may not be reliable with LS-SAB, due to the presence of an admixture of OC with CC, as negative cPRA was observed with LC (e.g., 9NA, 18PK, 15NA, and 16HN);
- (b)
- LS-SAB with IgHPolyFab showed negative cPRA, yet the same SAB (LS) with FcMonoIgG showed distinct positive cPRA in the following patients: 4LE and 3LE;
- (c)
- The reliability of FcMonoIgG is inferred when the cPRA is positive (35–50%) or highly positive (>50%), only with FcMonoIgG, but not with IgHPolyFab, with both the SABs in the following patients: 3LE and 13LE.
The results obtained with the % of cPRA of anti-HLA-I Abs reveal the following:
- (a)
- LS-SAB with IgHPolyFab showed negative cPRA, while the % cPRA was observed with FcMonoIgG on both the SABs (1DM, 4LE, 8DM, and 16HN);
- (b)
- The % cPRA may not be reliable with LS, possibly due to the presence of an admixture of OC with CC, due to the negative cPRA observed with LC in the following patient: 5DM.
Based on the negative (total zero) cPRA obtained with LS using IgHPolyFab, the patients could have transplanted an organ without recognizing the presence of unacceptable antigens. This can be avoided by using FcMonoIgG with LC-SABs. In contrast, an inaccurately inflated cPRA, due to the OC, could result in declined offers for potentially compatible organs in high % cPRA patients.
Inference: HLA Abs monitored on LC using FcMonoIgG are more reliable for monitoring cPRA than the conventional protocol of using LS with IgHPolyFab.
4. Discussion
4.1. LC-SABs with FcMonoIgG Is Better for Monitoring anti-HLA CC IgG Abs
While both the SABs carry identical alleles of HLA CC, several sera IgG Abs bind differentially to the two SABs (Figure 2A–C and Figure 3) when monitored with two different detection Abs; LC-SABs are devoid of HLA OC (Figure 5A), and hence are considered CC-specific. A noteworthy finding regarding detection Abs emerges when both the SABs are positive for sera Abs (Figure 2A for anti-HLA-I, and the first column of Figure 3 for anti-HLA-II). Most of the beads are positive only with FcMonoIgG (see values in bold), and the reactivity is significantly higher with LC than with LS. IgHPolyFab showed low or no binding with both the SABs. Evidently, LC-SABs with FcMonoIgG as the detection Ab are reliable for monitoring intact HLA or CC. FcMonoIgG provides greater sensitivity and specificity (resolution) than IgHPolyFab, with a more accurate estimation of binding affinity (MFI). This is important because the Luminex SAB assay is semi-quantitative. Our present and previous results [] caution that LS-SAB and polyclonal detection Abs should be avoided for calculating % cPRA. If not, potentially compatible organs may be denied, or inappropriate administration of costly and possibly toxic desensitization procedures may result.
Several HLA alleles are recognized by the patients’ sera on CC-specific LC-SABs, but not on LS-SABs (Figure 7). The failure of these CC-specific IgG Abs to recognize LS-SABs could be either due to (1) the low density of CC on LS-SAB, or (2) the steric hindrance caused by the admixture of OC with CC on LS. Figure 7 also highlights a unique profile in that several sera IgGs bind only with LS-SABs, with both IgHPolyFab and FcMonoIgG. The failure of these IgG Abs to recognize LC-SABs could be due to the lack of OC on LC. Those IgG binding only to LS are likely to be OC-specific IgG Abs. These findings explain why not all donor-specific Abs (DSAs) observed with LS-SABs result in graft failure [,,,,,,,,,].
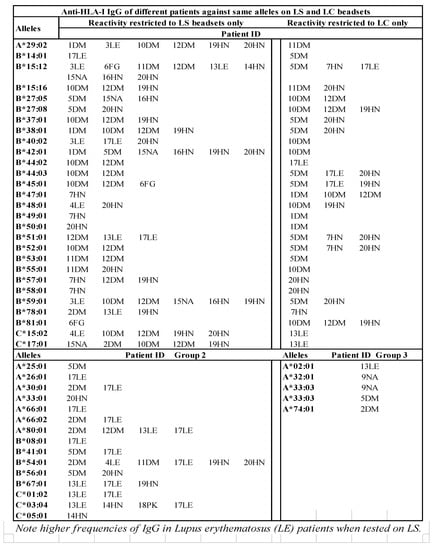
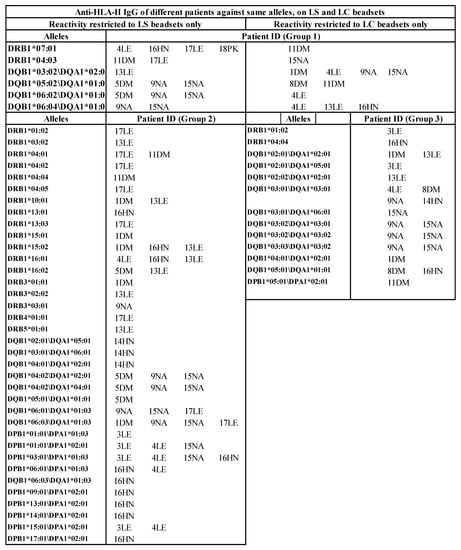
Figure 7.
HLA antigens (HLA-I and HLA-II) recognized by the sera IgG (MFI >500) of different ESRD patients against same alleles differ between LABScreen and LIFECODES SABs. Several IgG reactivities are restricted only to LS (Group 2). Similarly, reactivities of several IgGs are restricted only to LC (Group 3).
It is known that the results of SAB assays often fail to correlate with the results of FCXM tests. Recently, we have reported (data submitted for publication []) that sera reacted strongly with LS-SABs (>13,000 MFI), but weakly or not at all with LC-SABs (<1000 MFI), which gave negative T- and B-cell FCXMs []. In contrast, sera that reacted with LC-SABs (>13,000 MFI), but weakly with LS-SAB (<2100 MFI), exhibited positive FCXMs []. The detection of Abs directed against the OC in SAB assays may lead to an inappropriate listing of unacceptable antigens, pre- or post-transplant desensitization procedures, or the decision not to XM.
The findings reported in this investigation gain support from the works of Gao et al. [] and Porcheray et al. [], who have developed a unique B-cell clone (4G10) from an explanted kidney graft of a transplant recipient. The reactivity of the clone supernatants and the patient’s serum to HLA-I were assessed using regular LS-SABs, iBeads (enzymatically treated LS-SAB to remove OC- and CC-specific), and the acid-treated LS (denatured OC). The mAb 4G10 showed no reactivity to iBeads, but reacted with a majority of B and C locus antigens on regular LS-SAB and acid-treated LS, confirming the affinity of the mAb 4G10 for OC on the LS. Notably, both mAb 4G10 and the patient’s sera were negative by complement-dependent crossmatch (CDCXM) testing, suggesting the lack of pathogenic relevance of anti-OC Abs.
Similarly, Michel et al. [] confirmed the presence of OC on LS-SAB, using mAb HC-10. Indeed, no patients in their study had Ab-mediated rejection, and had antibodies reactive to β2m-free HLA (OC). Further, they emphasized that “reactions due to the presence of β(2)-m-fHLA [OC] … can lead to the inappropriate assignment of unacceptable antigens during transplant listing and possibly inaccurate identification of DSA in the post-transplant period”(p. 356, []).
Susal and co-investigators [,] observed that the sera Abs that reacted with LS-SABs were non-reactive with complement-dependent crossmatch (CDCXM) testing. They further examined ten sera of waiting list patients who responded positively for anti-HLA Abs on LS-SAB with LC-SAB. All the ten sera that were positive with LS were reactive to A*24:02, B*08:01, B*15:12, B*44:02, B*37:01 C*05:01, and C*17:01. However, with LC-SAB, eight of the ten patients failed to show HLA Abs reactivity. Of the different HLA Abs detected by LS, only A*24:02, B*08:01, and B*15:12 were detected with LC for the two LC-positive sera. They noted that the sensitized patients that were positive for five alleles with LC were negative with LS-SAB. In conclusion, there was no association of kidney graft loss with the HLA Abs detected exclusively using LS-SAB with IgHPolyFab. They state that “HLA mismatches defined according to the HLA antibody specificities in a potential recipient’s serum, the smaller the likelihood that a donor kidney will be considered suitable for this patient. The erroneous assignment of HLA antibodies, and consequently of ‘‘unacceptable HLA mismatches, can therefore have dire consequences” (p. 2080, []). Despite these reports [,,,,,], and after direct documentation of the presence of an OC on LS-SAB [,], many clinical laboratories continue to use LS-SAB to monitor sensitization in end-stage disease patients and post-transplantation.
Similar findings are also observed for HLA-II. An HLA-II polyreactive mAb FJ5109 (Figure 5B) revealed the OC of HLA-II on the LS-SAB. The existence of an HLA-II OC on LS-SAB was also supported by earlier reports [,]. This study documented (Figure 5B) that the LS-SAB may carry OC of HLA-II. More in-depth studies are needed, using FJ5109 or similar mAbs, as was done for HLA-I.
Figure 4 summarizes the entire findings of this investigation. The median or mean of the pooled MFIs of all alleles are persistently higher with FcMonoIgG than with IgHPolyFab, for both LS- and LC-SABs. The MFI values with FcMonoIgG for LC-SAB are significantly greater than those obtained with LS. The above observations, on the clonality of the detection Abs, do not support the contention of Hilton and Parham []. They also compared the binding of polyreactive mAbs (MA2.1 and BB7.1) using LS- and LC-SABs. The mAb MA2.1 bound to A*02:01/02/03/B*57:01/B*58:01 (see Figure 1 in []), and mAb BB7.1 bound to HLA-B*42:01, B7 and B27. These mAbs reacted well with LS-SABs, but poorly with LC-SAB. Therefore, they attributed “the lower affinity of MA2.1 and BB7.1 for HLA class I allotypes presented on the Gen-Probe (LC) SAB” “is (due to) the lower antigen density present on these beads” (page 215, []). Our study clarifies that the lower affinity of these mAbs for LC could be due to the absence of OC on LC-SAB. In several previous reports [,,,,], we have documented that the HLA polyreactive monoclonal antibodies (TFL-006 and TFL-007) bind to shared epitopes found on the HLA OC, which are otherwise cryptic due to the dimerization of the OC with β2-microglobulin.
This study and our previous reports [,] stress the need to eliminate IgHPolyFab and replace it with FcMonoIgG, for the monitoring HLA Abs on the Luminex-based SAB assay. This should be the standard protocol for monitoring an array of antigens on Luminex multiplex SAB assays.
4.2. Limitations of This Investigation
IgM and four subclasses of IgG are found in normal subjects’ sera and patients’ sera. The OC generated as a consequence of inflammation are immunogenic, for they expose otherwise cryptic epitopes and are capable of eliciting IgM initially. The failure of the detection Abs to bind to LS-SABs could be due to IgM binding to the OC, causing steric hindrance for IgG binding to CC. Selective binding of non-immunoglobulin serum components (proteins, peptide repertoire, chemo- and cytokines, oligosaccharides, saturated and unsaturated fatty acids, and cholesterol) to HLA OC cannot be ruled out. Lower MFIs observed with IgHPolyFab, in contrast to those of FcMonoIgG, could be due to such steric hindrances.
4.3. Conclusions
The objective of an immunoassay is to monitor the MFI of the sera Abs against a target antigen coated on a solid matrix. In the enzyme-linked immunosorbent assay (ELISA), the binding affinity of the Abs is measured quantitatively as titers, after serially diluting the sera. The solid matrix in the Luminex multiplex immunoassay is single-antigen beads. The assay enables the measuring of Abs against a hundred HLA antigens simultaneously, by using the antigen-coated beads. Titration can be conducted []; however, titration on every serum of SABs coated with the two classes of HLA is cost-prohibitive. Hence, the sera are not diluted serially. Often undiluted serum is used, which results in a “prozone effect”. In this study, the sera are tested after a 1:10 dilution, and hence the assay remains semi-quantitative. There is a need for a reliable and accurate measurement of the MFI of the antibody. This can be achieved only after a stringent and empirical assessment of the SAB assay protocol. As frequently observed [,,,,,], the accuracy may be affected by an admixture of OC with CC on the SAB, as well as by the use of polyclonal Fab as the detection antibody (IgHPolyFab). This study indicates that the accuracy in measurement can be attained using SAB coated with HLA CC only, and with Fc-specific monoclonal IgG (FcMonoIgG).
The costs of LS-SAB and LC-SAB do not differ much. However, clinicians in the USA tend to use LS more often than LC, despite several compelling reports [,,,,,]. Most strikingly, Battle et al. [] from Scotland compared the so-called “prozone effect” on LS- and LC-SABs, and documented that the “prozone effect” was specific for LS-SAB and not observed with LC-SAB. A recent report [] comparing the FCXMs of sera that are reactive against HLA OCs, with sera that are reactive against only HLA CCs, showed that the sera reacting strongly with LS-SAB, but not with LC-SAB, gave negative T- and B-cell FCXMs. In contrast, the sera that reacted strongly with LC-SAB, but poorly with LS-SAB, exhibited positive FCXMs. The detection of Abs directed against HLA OCs in LS-SAB assays may lead to an inappropriate listing of unacceptable antigens and pre- or post-transplant desensitization procedures. Susal et al. [] and Michel et al. [] have emphasized that such HLA antibodies on OC-coated (β2-microglobulin-free HLA) SAB can lead to “dire consequences,” namely, “inappropriate assignment of unacceptable antigens during transplant listing and possibly inaccurate identification of DSA post-transplant.”
In conclusion, we recommend LC-SAB for monitoring HLA antibodies for end-stage organ disease patients, both before and after transplantation. The sensitivity of detection can be much improved with FcMonoIgG. In the interest of avoiding the “dire consequences” stated above, and to eliminate the “prozone effect” once and for all, the vendors should seriously consider manufacturing the HLA-I SAB to be negative for mAb TFL-006, and HLA-II SAB to be non-reactive to mAb FJ5109. The inclusion of FcMonoIgG with the vendors’ kits would provide extraordinary service and be cost-worthy for the patients.
Author Contributions
M.H.R. formulated the overall concept and experimental design and supervised all experiments performed by his research assistants at Terasaki Foundation Laboratory. N.M.R. significantly contributed to the development of the idea since 2017, regularly discussed the progress of the experiments, data analysis, and contributed significantly in drafting and critically reviewing the manuscript at every level of its development. C.J.A.-M. carried out cPRA analysis for all the patients and contributed to the sections about cPRA. All authors have read and agreed to the published version of the manuscript.
Funding
Mepur H. Ravindranath received a research grant from Immucor Inc. (July 2017–July 2018), while at the Terasaki Foundation Laboratory. Mark Terasaki, the first son of Late Paul I Terasaki, provided necessary funds while completing and writing the project.
Institutional Review Board Statement
This study involves sera obtained from end-stage renal disease patients; consent forms and the Institutional Review Board (IRB) approval were obtained by Professor Allen J. Norin, at in the department of Medicine and Cell Biology Division of Transplant Immunology and Immunogenetics, SUNY Downstate Medical Center, 450 Clarkson Ave. Brooklyn, NY 11203. Sera were randomly selected from the Downstate pre-transplant clinic at Downstate Health Sciences University, University Hospital of Brooklyn (UHB) by Allen Norin and Ballabh Das. SAB/DSA study protocols were reviewed and approved by the Downstate Institutional Review Board (IRB), (IRB #1232938-3 and #341403-1).
Informed Consent Statement
Reviewed and approved by the Downstate Institutional Review Board (IRB), (IRB #1232938-3 and #341403-1).
Data Availability Statement
All datasets generated for this study are included in the manuscript. The raw data are available with M.H.R and stored also with V.J at Terasaki Research Institute, Los Angeles.
Acknowledgments
The authors dedicate this paper to the late Paul Ichiro Terasaki, Director of Terasaki Foundation Laboratory and Terasaki Research Institute, and CEO of One Lambda Inc. We sincerely thank Vadim Jucaud, Research Scientist at Terasaki Foundation Laboratory, for meticulously carrying out SAB assays for this investigation. We also thank Jeff Gornbein of UCLA/SBCC for performing the statistical analyses, Edward J. Filippone, Division of Nephrology, Dept. of Medicine, Sidney Kimmel Medical College at Thomas Jefferson University. Philadelphia, Fernando A. Arosa, Department of Medical Sciences, Health Sciences Research Center (CICS-UBI) University of Beira Interior, Covilhã. Portugal, Grace Mahowald, Department of Pathology, Massachusetts General Hospital, Harvard Medical School, Boston, for reviewing the manuscript, and Ray Bryan, Transplantation Research, Immucor Inc. Waukesha, Wisconsin, for their valuable discussion pertaining to the data and for critically reviewing the manuscript at different stages of its development. We are indebted to Allen J. Norin, Director, Histocompatibility and Immunogenetics, SUNY Downstate Health Sciences University, and Ballabh Das, Transplant Immunology and Histocompatibility Laboratory, SUNY DownState Medical Center, Brooklyn, NY for obtaining IRB approval of sera for the study and for sending the sera for this investigation. In addition, we are thankful to both for reviewing the manuscript and offering invaluable suggestions. Thanks are also due to Stephen Hardy at TRI and to Professor Shahab Asgharzhadeh, Children’s Hospital, Los Angeles, for providing the facilities to complete this investigation. MHR is particularly thankful to Laurel Nelson for her support. This investigation, carried out between July 2017 and June 2018 at TFL, is primarily supported by Terasaki Family Foundation (a non-profit organization) and a research grant from Immucor Inc. Norcross, GA. The theme of this study emanates from a discussion with late Professor Terasaki regarding the problems encountered while purifying dimeric HLA for coating the SABs. Terasaki is the founder and CEO of One Lambda Inc., in Los Angeles, which manufactures LABScreen SAB and associated kits. MHR was a research scientist at Terasaki Foundation Laboratory, under the tutelage of Terasaki (2008–2018). M.H.R. and N.M.R. are indebted to his first son Mark Terasaki for his constant research support and encouragements.
Conflicts of Interest
Mepur H. Ravindranath received a research grant from Immucor Inc. (July 2017–July 2018) at the Terasaki Foundation Laboratory, and a supply of LS-SAB for this research from One Lambda Inc. Carly J. Amato-Menker (formerly Callender) was an employee of Immucor Inc. until 2018. Neither Immucor nor one Lambda Inc. were involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Contribution to the Field
Antibodies against intact HLA (CC) limit organ availability for patients and contribute to allograft failure. Accurate determination of the presence of CC Abs is critical for donor organ selection and graft acceptance. The MFI of sera, using two different vendors’ SABs (LS- and LC) and two different detection antibodies, were compared using a common protocol. The LS-SABs carried an admixture of OC with CC, whereas the LC-SABs carried only CC, suggesting that LC-SAB is specific for monitoring the CC Abs. A greater number of positive antigens and a higher MFI of Abs with FcMonoIgG on either SAB suggest that FcMonoIgG enhances the sensitivity of the assay.
Ethics Statement
This study involves sera obtained from end-stage renal disease patients; consent forms and the Institutional Review Board (IRB) approval were obtained by Allen J. Norin, at in the department of Medicine and Cell Biology Division of Transplant Immunology and Immunogenetics, SUNY Downstate Medical Center, 450 Clarkson Ave. Brooklyn, NY 11203.
References
- Jordan, S.C.; Tyan, D.; Stablein, D.; McIntosh, M.; Rose, S.; Vo, A.; Toyoda, M.; Davis, C.; Shapiro, R.; Adey, D.; et al. Evaluation of Intravenous Immunoglobulin as an Agent to Lower Allosensitization and Improve Transplantation in Highly Sensitized Adult Patients with End-Stage Renal Disease: Report of the NIH IG02 Trial. J. Am. Soc. Nephrol. 2004, 15, 3256–3262. [Google Scholar] [CrossRef]
- Loupy, A.; Hill, G.S.; Jordan, S.C. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat. Rev. Nephrol. 2012, 8, 348–357. [Google Scholar] [CrossRef]
- Taylor, D.O.; Edwards, L.B.; Boucek, M.M.; Trulock, E.P.; Aurora, P.; Christie, J.; Dobbels, F.; Rahmel, A.O.; Keck, B.M.; Hert, M.I. Registry of the International Society for Heart and Lung Transplantation: Twenty-fourth official adult heart transplant report—2007. J. Heart Lung Transplant. 2007, 26, 769–781. [Google Scholar] [CrossRef]
- Potena, L.; Bontadini, A.; Iannelli, S.; Fruet, F.; Leone, O.; Barberini, F.; Borgese, L.; Manfredini, V.; Masetti, M.; Magnani, G.; et al. Occurrence of Fatal and Nonfatal Adverse Outcomes after Heart Transplantation in Patients with Pretransplant Noncytotoxic HLA Antibodies. J. Transplant. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Appel, J.Z.; Hartwig, M.G.; Cantu, E.; Palmer, S.M.; Reinsmoen, N.L.; Davis, R.D. Role of Flow Cytometry to Define Unacceptable HLA Antigens in Lung Transplant Recipients with HLA-Specific Antibodies. Transplantation 2006, 81, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Saini, D.; Steward, N.; Hachem, R.; Trulock, E.P.; Patterson, G.A.; Meyers, B.F.; Mohanakumar, T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann. Thorac. Surg. 2010, 90, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Skeans, M.A.; Leighton, T.R.; Ghimire, V.; Leppke, S.N.; Israni, A.K. OPTN/SRTR 2011 Annual Data Report: International Data. Am. J. Transplant. 2012, 13, 199–225. [Google Scholar] [CrossRef]
- Terasaki, P.I.; Ozawa, M. Predicting kidney graft failure by HLA antibodies: A prospective trial. Am. J. Transplant. 2004, 4, 438–443. [Google Scholar] [CrossRef] [PubMed]
- TTerasaki, P.; Cai, J. Humoral theory of transplantation: Further evidence. Curr. Opin. Immunol. 2005, 17, 541–545. [Google Scholar] [CrossRef]
- Terasaki, P.I.; Ozawa, M.; Castro, R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am. J. Transplant. 2007, 7, 408–415. [Google Scholar] [CrossRef]
- Terasaki, P.I.; Cai, J. Human leukocyte antigen antibodies and chronic rejection: From association to causation. Transplantation 2008, 86, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Cosio, F.G.; Gloor, J.M.; Sethi, S.; Dean, P.G.; Moore, S.B.; DeGoey, S.; Stegall, M.D. Transplant glomerulopathy: Risk and prognosis related to anti-human leukocyte antigen class ii antibody levels. Transplantation 2008, 86, 681–685. [Google Scholar] [CrossRef]
- Lachmann, N.; Terasaki, P.I.; Budde, K.; Liefeldt, L.; Kahl, A.; Reinke, P.; Pratschke, J.; Rudolph, B.; Schmidt, D.; Salama, A.; et al. Anti-human leukocyte antigen and donor-specific antibodies detected by Luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 2009, 87, 1505–1513. [Google Scholar] [CrossRef]
- Gao, B.; Rong, C.; Porcheray, F.; Moore, C.; Girouard, T.C.; Saidman, S.L.; Wong, W.; Fu, Y.; Zorn, E. Evidence to Support a Contribution of Polyreactive Antibodies to HLA Serum Reactivity. Transplantation 2016, 100, 217–226. [Google Scholar] [CrossRef]
- Süsal, C.; Fichtner, A.; Tönshoff, B.; Mehrabi, A.; Zeier, M.; Morath, C. Clinical Relevance of HLA Antibodies in Kidney Transplantation: Recent Data from the Heidelberg Transplant Center and the Collaborative Transplant Study. J. Immunol. Res. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Süsal, C.; Ovens, J.; Mahmoud, K.M.; Döhler, B.; Scherer, S.; Ruhenstroth, A.; Tran, T.H.; Heinold, A.; Opelz, G. No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: A collaborative transplant study report. Transplantation 2011, 91, 883–887. [Google Scholar] [CrossRef]
- Poli, F.; Benazzi, E.; Innocente, A.; Nocco, A.; Cagni, N.; Gianatti, A.; Fiocchi, R.; Scalamogna, M. Heart transplantation with donor-specific antibodies directed toward denatured HLA-A*02:01: A case report. Hum. Immunol. 2011, 72, 1045–1048. [Google Scholar] [CrossRef]
- PPereira, S.; Perkins, S.; Lee, J.-H.; Shumway, W.; Lefor, W.; Lopez-Cepero, M.; Wong, C.; Connolly, A.; Tan, J.C.; Grumet, F.C. Donor-specific antibody against denatured HLA-A1: Clinically nonsignificant? Hum. Immunol. 2011, 72, 492–498. [Google Scholar] [CrossRef]
- Salvadé, I.; Aubert, V.; Venetz, J.-P.; Golshayan, D.; Saouli, A.-C.; Matter, M.; Rotman, S.; Pantaleo, G.; Pascual, M. Clinically-relevant threshold of preformed donor-specific anti-HLA antibodies in kidney transplantation. Hum. Immunol. 2016, 77, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Courant, M.; Visentin, J.; Linares, G.; Dubois, V.; Lepreux, S.; Guidicelli, G.; Thaunat, O.; Merville, P.; Couzi, L.; Taupin, J.-L. The disappointing contribution of anti-human leukocyte antigen donor-specific antibodies characteristics for predicting allograft loss. Nephrol. Dial. Transplant. 2018, 33, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Kamburova, E.G.; Wisse, B.W.; Joosten, I.; Allebes, W.A.; Van Der Meer, A.; Hilbrands, L.B.; Baas, M.C.; Spierings, E.; Hack, C.E.; Van Reekum, F.E.; et al. Pretransplant C3d-Fixing Donor-Specific Anti-HLA Antibodies Are Not Associated with Increased Risk for Kidney Graft Failure. J. Am. Soc. Nephrol. 2018, 29, 2279–2285. [Google Scholar] [CrossRef]
- McCaughan, J.; Xu, Q.; Tinckam, K. Detecting donor-specific antibodies: The importance of sorting the wheat from the chaff. Hepatobiliary Surg. Nutr. 2019, 8, 37–52. [Google Scholar] [CrossRef]
- Arosa, F.A.; Esgalhado, A.J.; Padrão, C.A.; Cardoso, E.M. Divide, Conquer, and Sense: CD8+CD28—T Cells in Perspective. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Arosa, F.A.; Santos, S.G.; Powis, S.J. Open conformers: The hidden face of MHC-I molecules. Trends Immunol. 2007, 28, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, E.; Stockinger, H.; Majdic, O.; Gaugitsch, H.; Lindley, I.J.; Maurer, D.; Hajek-Rosenmayr, A.; Knapp, W. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J. Exp. Med. 1990, 171, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Heemels, M.T.; Neefjes, J.; Kast, W.; Melief, C.J.; Ploegh, H.L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell 1990, 62, 563–567. [Google Scholar] [CrossRef]
- Benjamin, R.J.; Madrigal, J.A.; Parham, P. Peptide binding to empty HLA-B27 molecules of viable human cells. Nat. Cell Biol. 1991, 351, 74–77. [Google Scholar] [CrossRef]
- Majdic, O.; Schnabl, E.; Stockinger, H.; Gadd, S.; Maurer, D.; Radaszkiewics, T. LA45, an activation-induced human lymphocyte antigen with strong homology to MHC class I molecules. In Leukocyte Typing IV; Oxford University Press: Oxford, UK, 1989; Volume 511, 8p. [Google Scholar]
- Madrigal, J.A.; Belich, M.P.; Benjamin, R.; Little, A.M.; Hildebrand, W.H.; Mann, D.L.; Parham, P. Molecular definition of a polymorphic antigen (LA45) of free HLA-A and -B heavy chains found on the surfaces of activated B and T cells. J. Exp. Med. 1991, 174, 1085–1095. [Google Scholar] [CrossRef]
- Raine, T.; Brown, D.; Bowness, P.; Gaston, J.S.H.; Moffett, A.; Trowsdale, J.; Allen, R. Consistent patterns of expression of HLA class I free heavy chains in healthy individuals and raised expression in spondyloarthropathy patients point to physiological and pathological roles. Rheumatology 2006, 45, 1338–1344. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Esgalhado, A.; Patrao, L.; Santos, M.; Neves, V.P.; Martinez, J.; Patto, M.A.V.; Silva, H.; Arosa, F.A. Distinctive CD8+ T cell and MHC class I signatures in polycythemia vera patients. Ann. Hematol. 2018, 97, 1563–1575. [Google Scholar] [CrossRef]
- Santos, S.G.; Powis, S.J.; Arosa, F.A. Misfolding of major histocompatibility complex class I molecules in activated T Cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J. Biol. Chem. 2004, 279, 53062–53070. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, R.; Porcelijn, L.; Lardy, N.; Briët, E.; Vrielink, H. Prevalence of leucocyte antibodies in the Dutch donor population. Vox Sang. 2010, 100, 327–335. [Google Scholar] [CrossRef]
- Hyun, J.; Park, K.D.; Yoo, Y.; Lee, B.; Han, B.Y.; Song, E.Y.; Park, M.H. Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplant. Proc. 2012, 44, 222–225. [Google Scholar] [CrossRef]
- Hilton, H.G.; Parham, P. Direct binding to antigen-coated beads refines the specificity and cross-reactivity of four monoclonal antibodies that recognize polymorphic epitopes of HLA class I molecules. Tissue Antigens 2013, 81, 212–220. [Google Scholar] [CrossRef]
- Oh, E.-J.; Park, H.; Park, K.U.; Kang, E.-S.; Kim, H.-S.; Song, E.Y. Interlaboratory comparison of the results of Lifecodes LSA class I and class II single antigen kits for human leukocyte antigen antibody detection. Ann. Lab. Med. 2015, 35, 321–328. [Google Scholar] [CrossRef][Green Version]
- Minucci, P.B.; Resse, M.; Sabia, C.; Esposito, A.; De Iorio, G.; Napoli, C. Anti-HLA Antibodies Testing on Solid Phase: Comparative Evaluation of Different Kit Vendors T\through Luminex Technology. Exp. Clin. Transplant. 2017, 15, 636–640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamburova, E.G.; Wisse, B.W.; Joosten, I.; Allebes, W.A.; Van Der Meer, A.; Hilbrands, L.B.; Baas, M.C.; Spierings, E.; Hack, C.E.; Van Reekum, F.E.; et al. Differential effects of donor-specific HLA antibodies in living versus deceased donor transplant. Am. J. Transplant. 2018, 18, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.; Nedelcu, D.; Toader, A.; Sora, M.; Tica, A.; Ferastraoaru, D.E.; Constantinescu, I. Cytotoxic antibodies—Valuable prognostic factor for long term kidney allograft survival. J. Med. Life 2010, 3, 390–395. [Google Scholar] [PubMed]
- Jung, S.; Oh, E.-J.; Yang, C.-W.; Ahn, W.-S.; Kim, Y.; Park, Y.-J.; Han, K. Comparative evaluation of ELISA and Luminex panel reactive antibody assays for HLA alloantibody screening. Ann. Lab. Med. 2009, 29, 473–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Battle, R.K.; Abel, A.A.; Turner, D.M. Prozone effect can be specific to single antigen bead kit manufacturers. Am. J. Transplant. 2017, 17, 1425–1426. [Google Scholar] [CrossRef][Green Version]
- Clerkin, K.J.; See, S.B.; Farr, M.A.; Restaino, S.W.; Serban, G.; Latif, F.; Li, L.; Colombo, P.C.; Vlad, G.; Ray, B.; et al. Comparative assessment of anti-HLA antibodies using two commercially available luminex-based assays. Transplant. Direct 2017, 3, e218. [Google Scholar] [CrossRef]
- Bertrand, D.; Farce, F.; Laurent, C.; Hamelin, F.; François, A.; Guerrot, D.; Etienne, I.; Hau, F. Comparison of Two Luminex Single-antigen Bead Flow Cytometry Assays for Detection of Donor-specific Antibodies after Renal Transplantation. Transplantation 2019, 103, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Jucaud, V.; Ferrone, S. Monitoring native HLA-I trimer specific antibodies in Luminex multiplex single antigen bead assay: Evaluation of beadsets from different manufacturers. J. Immunol. Methods 2017, 450, 73–80, Corrigendum to Monitoring native HLA-I trimer specific antibodies in Luminex multiplex single antigen bead assay: Evaluation of SABs from different manufacturers. J. Immunol. Methods 2018, 460, 125. [Google Scholar] [CrossRef] [PubMed]
- Jucaud, V.; Ravindranath, M.H.; Terasaki, P.I. Conformational Variants of the Individual HLA-I Antigens on Luminex Single Antigen Beads Used in Monitoring HLA Antibodies: Problems and Solutions. Transplantation 2017, 101, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Flippone, E.J.; Mahowald, G.; Callender, C.; Babu, A.; Saidman, S.; Ferrone, S. Significance of the intraindividual variability of HLA IgG antibodies in renal disease patients observed with different SABs monitored with two different secondary antibodies on a Luminex platform. Immunologic Res. 2018, 66, 584–604. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, L.; Sato, A.K.; Fischer, F.R.; Dorf, M.E.; Stern, L.J. Abundant empty class II MHC molecules on the surface of immature dendritic cells. Proc. Natl. Acad. Sci. USA 1999, 96, 15050–15055. [Google Scholar] [CrossRef]
- Grenzi, P.C.; De Marco, R.; Silva, R.Z.; Campos, E.F.; Gerbase-DeLima, M. Antibodies against denatured HLA class II molecules detected in luminex-single antigen assay. Hum. Immunol. 2013, 74, 1300–1303. [Google Scholar] [CrossRef]
- Michel, K.; Santella, R.; Steers, J.; Sahajpal, A.; Downey, F.X.; Thohan, V.; Oaks, M. Many de novo donor-specific antibodies recognize β 2 -microglobulin-free, but not intact HLA heterodimers. HLA 2016, 87, 356–366. [Google Scholar] [CrossRef]
- Gombos, P.; Süsal, C.; Opelz, G.; Scherer, S.; Morath, C.; Zeier, M.; Schemmer, P. Influence of test technique on sensitization status of patients on the kidney transplant waiting list. Am. J. Transplant. 2013, 13, 2075–2082. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Jucaud, V.; Banuelos, N.; Everly, M.J.; Cai, J.; Nguyen, A.; Terasaki, P.I. Nature and clonality of the fluoresceinated secondary antibody in Luminex multiplex bead assays are critical factors for reliable monitoring of serum HLA antibody levels in patients for donor organ selection, desensitization therapy, and assessment of the risk for graft loss. J. Immunol. 2017, 198, 4524–4538. [Google Scholar] [CrossRef]
- Cicciarelli, J.C.; Kasahara, N.; Lemp, N.A.; Adamson, R.; Dembitsky, W.; Browne, B.; Steinberg, S. Immunoglobulin G subclass analysis of HLA donor specific antibodies in heart and renal transplant recipients. Clin. Transplant. 2013, 2013, 413–422. [Google Scholar]
- Ravindranath, M.H.; Terasaki, P.I.; Pham, T.; Jucaud, V.; Kawakita, S. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E Abs that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood 2013, 121, 2013–2028. [Google Scholar] [CrossRef][Green Version]
- Ravindranath, M.H.; Zhu, D.; Pham, T.; Jucaud, V.; Hopfield, J.; Kawakita, S.; Terasaki, P.I. Anti-HLA-E monoclonal Abs reacting with HLA-la and lb alleles like IVIg as potential IVIg-immunomimetics: An evolving therapeutic concept. Clin. Transplant. 2013, 2013, 293–305. [Google Scholar]
- Ravindranath, M.H.; Pham, T.; El-Awar, N.; Kaneku, H.; Terasaki, P.I. Anti-HLA-E mAb 3D12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: Web-tools validate the immunogenic epitopes of HLA-E recognized by the Abs. Mol Immunol. 2011, 48, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Taniguchi, M.; Chen, C.W.; Ozawa, M.; Kaneku, H.; El-Awar, N.; Cai, J.; Terasaki, P.I. HLA-E monoclonal antibodies recognize shared peptide sequences on classical HLA class Ia: Relevance to human natural HLA antibodies. Mol. Immunol. 2010, 47, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Cecka, J.M.; Zhang, Q.; Reed, E.F. Preformed cytotozic antibodies in potential allograft recipients: Recent data. Hum. Immunol. 2005, 66, 343–349. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Filippone, E.J.; Callender, C.J.; Arosa, F.A.; Das, B.; Ou, Y.; Norin, A.J. Antibodies to Cryptic Epitopes on HLA Class I and Class II Heavy Chains Bound to Single Antigen Beads: Clinical Relevance. Transplant Immunol. 2021. (submitted). [Google Scholar]
- Porcheray, F.; DeVito, J.; Helou, Y.; Dargon, I.; Fraser, J.W.; Nobecourt, P.; Ferdman, J.; Germana, S.; Girouard, T.C.; Kawai, T.; et al. Expansion of polyreactive B Cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am. J. Transplant. 2012, 12, 2088–2097. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; El Hilali, F. Monospecific and Polyreactive MAbs against Human Leukocyte Antigen-E: Diagnostic and Therapeutic Relevance. In Monoclonal Antibodies; Resaei, N., Ed.; Intech Open: London, UK, 2021; 38p, ISBN 978-1-83968-370-1. [Google Scholar]
- Ravindranath, M.H. HLA Class Ia and Ib Polyreactive Anti-HLA-E IgG2a MAbs (TFL-006 and TFL-007) Suppress Anti-HLA IgG Production by CD19+ B-cells and Proliferation of CD4+ T-cells While Upregulating Tregs. J. Immunol. Res. 2017, 2017, 3475926. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).