A Benchmark Study of Burning Rate of Selected Thermites through an Original Gasless Theoretical Model
Abstract
:1. Introduction
2. Methodology
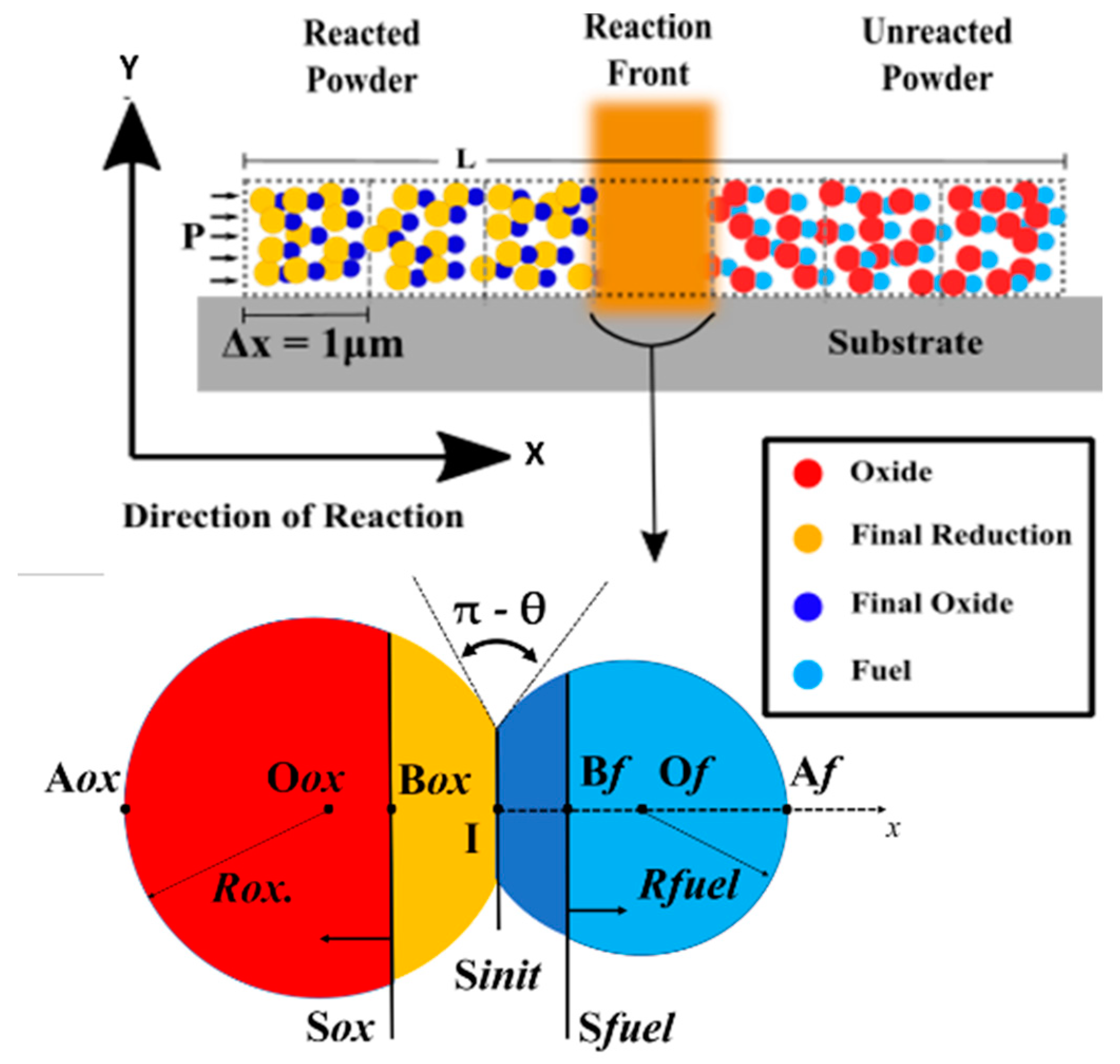
2.1. System Model
2.2. Diffusion/Reaction Model
2.3. System and Simulation Set-Up
2.4. Effective Conduction Coefficient Calculation
3. Results and Discussion
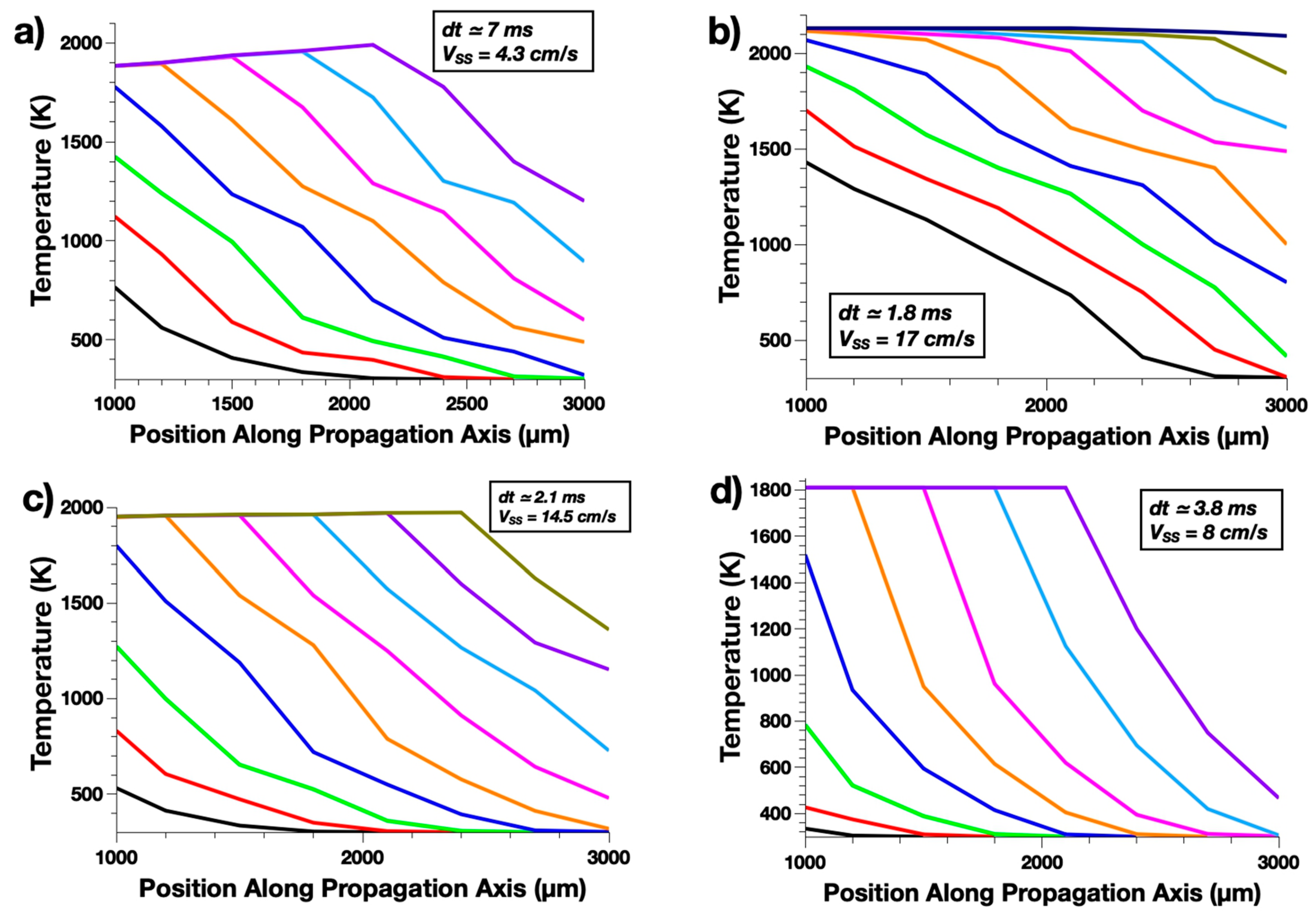
3.1. Benchmark Study
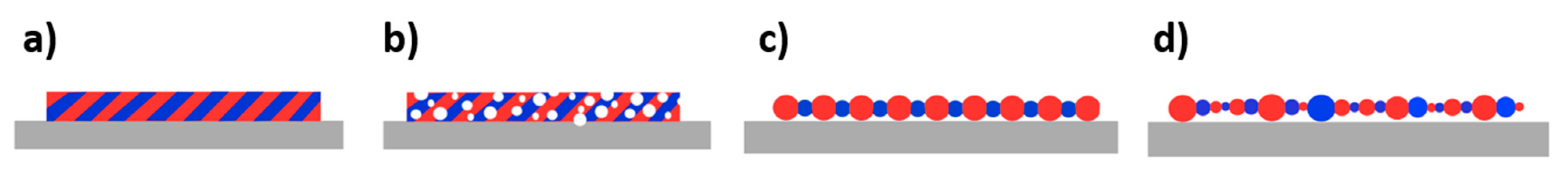
3.2. Effective Thermal Conductivity Formulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koch, E.C.; Knapp, S. Thermites-versatile materials. Propell. Explos. Pyrot. 2019, 44, 7. [Google Scholar] [CrossRef] [Green Version]
- Aumann, C.E.; Skofronick, G.L.; Martin, J.A. Oxidation behavior of aluminum nanopowders. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1994, 13, 1178–1183. [Google Scholar] [CrossRef]
- Rossi, C. Engineering of Al/CuO reactive multilayer thin films for tunable initiation and actuation. Propellants Explos. Pyrotech. 2019, 44, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Chiou, W.-A.; Fiore, R.; Zachariah, M.R. In situ microscopy of rapidly heated nano-Al and nano-Al/WO3 thermites. Appl. Phys. Lett. 2010, 97, 133104. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, E.R.; Sullivan, K.T.; Grapes, M.D. Designer direct ink write 3D-printed thermites with tunable energy release rates. Adv. Eng. Mater. 2020, 22, 1901196. [Google Scholar] [CrossRef]
- Pantoya, M.L.; Granier, J.J. Combustion behavior of highly energetic thermites: Nano versus micron composites. Propellants Explos. Pyrotech. Int. J. Deal. Sci. Technol. Asp. Energetic Mater. 2005, 30, 53–62. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducere, J.M.; Baijot, V.; Pinon, S.; Calais, T.; Esteve, A.; Rouhani, M.D.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Wang, L.; Luss, D.; Martirosyan, K.S. The behavior of nanothermite reaction based on Bi2O3/Al. J. Appl. Phys. 2011, 110, 74311. [Google Scholar] [CrossRef] [Green Version]
- Piekiel, N.W.; Zhou, L.; Sullivan, K.T.; Chowdhury, S.; Egan, G.C.; Zachariah, M.R. Initiation and reaction in Al/Bi2O3Nanothermites: Evidence for the predominance of condensed phase chemistry. Combust. Sci. Technol. 2014, 186, 1209–1224. [Google Scholar] [CrossRef]
- Rodríguez, G.A.A.; Suhard, S.; Rossi, C.; Estève, D.; Fau, P.; Sabo-Etienne, S.; Mingotaud, A.F.; Mauzac, M.; Chaudret, B. A microactuator based on the decomposition of an energetic material for disposable lab-on-chip applications: Fabrication and test. J. Micromechanics Microeng. 2008, 19, 015006. [Google Scholar] [CrossRef]
- Nicollet, A.; Salvagnac, L.; Baijot, V.; Estève, A.; Rossi, C. Fast circuit breaker based on integration of Al/CuO nanothermites. Sens. Actuators A Phys. 2018, 273, 249–255. [Google Scholar] [CrossRef]
- Piercey, D.G.; Klapooke, T.M. Nanoscale aluminum-metal oxide (thermite) reactions for application in energetic materials. Cent. Eur. J. Energ. Mat. 2010, 7, 115–129. [Google Scholar]
- Staley, C.S.; Raymond, K.E.; Thiruvengadathan, R.; Apperson, S.J.; Gangopadhyay, K.; Swaszek, S.M.; Taylor, R.J.; Gangopadhyay, S. Fast-impulse nanothermite solid-propellant miniaturized thrusters. J. Propul. Power. 2015, 31, 483. [Google Scholar] [CrossRef]
- Chaalane, A.; Rossi, C.; Esteve, D. The formulation and testing of new solid propellant mixture (DB plus x%BP) for a new MEMS-based microthruster. Sensor Actuat. A Phys. 2007, 138, 161–166. [Google Scholar] [CrossRef]
- Puchades, I.; Hobosyan, M.; Fuller, L.F.; Liu, F.; Thakur, S.; Martirosyan, K.S.; Lyshevski, S.E. MEMS microthrusters with nanoenergetic solid propellants. In Proceedings of the 14th IEEE International Conference on Nanotechnology, Toronto, ON, Canada, 18–21 August 2014. [Google Scholar]
- Bezmelnitsyn, A.; Thiruvengadathan, R.; Barizuddin, S.; Tappmeyer, D.; Apperson, S.; Gangopadhyay, K.; Gangopadhyay, S.; Redner, P.; Donadio, M.; Kapoor, D.; et al. Modified Nanoenergetic Composites with Tunable Combustion Characteristics for Propellant Applications. Propellants Explos. Pyrotech. 2010, 35, 384–394. [Google Scholar] [CrossRef]
- Swiston, A.J.; Besnoin, E.; Duckham, A.; Knio, O.M.; Weihs, T.P.; Hufnagel, T.C. Thermal and microstructural ef-fects of welding metallic glasses by self-propagating reactions in multilayer foils. Acta Mater 2005, 53, 3713–3719. [Google Scholar] [CrossRef]
- Duckham, A.; Spey, S.J.; Wang, J.; Reiss, M.; Weihs, T.P.; Besnoin, E.; Knio, O.M. Reactive nanostructured foil used as a heat source for joining titanium. J. Appl. Phys. 2004, 96, 2336–2342. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, R.Q.; Ye, Y.H.; Zhou, X.; Hu, Y.; Wu, L.Z. Energetic Igniters Based on Al/CuO/B/Ti Reactive Multilayer Films. Theory and Practice of Energetic Materials (Vol Ix). In Proceedings of the 2011 International Autumn Seminar on Propellants, Explosives and Pyrotechnics, Nanjing, China, 20–23 September 2011. [Google Scholar]
- Nicollet, A.; Lahiner, G.; Belisario, A.; Assié-Souleille, S.; Djafari-Rouhani, M.; Estève, A.; Rossi, C. Investigation of Al/CuO multilayered thermite ignition. J. Appl. Phys. 2017, 121, 034503. [Google Scholar] [CrossRef] [Green Version]
- Glavier, L.; Nicollet, A.; Jouot, F.; Martin, B.; Barberon, J.; Renaud, L.; Rossi, C. Nanothermite/RDX-Based Miniature Device for Impact Ignition of High Explosives. Propell. Explos. Pyrot. 2017, 42, 307–316. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, R.; Ye, Y.; Fu, S.; Li, D. Characterization of Al/CuO nanoenergetic multilayer films integrated with semiconductor bridge for initiator applications. J. Appl. Phys. 2013, 113, 184505. [Google Scholar] [CrossRef]
- Sun, J.; Pantoya, M.L.; Simon, S.L. Dependence of size and size distribution on reactivity of aluminum nanoparticles in reactions with oxygen and MoO3. Thermochim. Acta 2006, 444, 117–127. [Google Scholar] [CrossRef]
- Sanders, V.E.; Asay, B.W.; Foley, T.J.; Tappan, B.C.; Pacheco, A.N.; Son, S.F. Reaction propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO, and Bi2O3). J. Propul. Power 2007, 23, 707–714. [Google Scholar] [CrossRef]
- Rossi, C. Two decades of research on nano-energetic materials. Propellants Explos. Pyrotech. 2014, 39, 323–327. [Google Scholar] [CrossRef]
- Zhou, X.; Torabi, M.; Lu, J.; Shen, R.; Zhang, K. Nanostructured energetic composites: Synthesis, ignition/combustion modeling, and applications. ACS Appl. Mater. Interfaces 2014, 6, 3058–3074. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.; Klaumünzer, M.; Comet, M.; Martin, C.; Vidal, L.; Schäfer, M.; Kryschi, C.; Spitzer, D. Insights into combustion mechanisms of variable aluminum-based iron oxide/-hydroxide nanothermites. Combust. Flame 2017, 184, 186–194. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Kuntz, J.; Gash, A.E. The role of fuel particle size on flame propagation velocity in thermites with a nanoscale oxidizer. Propellants, Explos. Pyrotech. 2014, 39, 407–415. [Google Scholar] [CrossRef]
- Zohari, N.; Keshavarz, M.H.; Seyedsadjadi, S.A. The advantages and shortcomings of using nano-sized energetic materials. Cent. Eur. J. Energ. Mat. 2013, 10, 135–147. [Google Scholar]
- Zapata, J.; Nicollet, A.; Julien, B.; Lahiner, G.; Esteve, A.; Rossi, C. Self-propagating combustion of sputter-deposited Al/CuO nanolaminates. Combust. Flame 2019, 205, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Lahiner, G.; Tenailleau, C.; Reig, B.; Hungria, T.; Esteve, A.; Rossi, C. Unexpected enhanced reactivity of aluminized nanothermites by accelerated aging. Chem. Eng. J. 2021, 418, 129432. [Google Scholar] [CrossRef]
- Julien, B.; Wang, H.; Tichtchenko, E.; Pelloquin, S.; Estève, A.; Zachariah, M.R.; Rossi, C. Elucidating the dominant mechanisms in burn rate increase of thermite nanolaminates incorporating nanoparticle inclusions. Nanotechnology 2021, 21, 215401. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Julien, B.; Kline, D.J.; Alibay, Z.; Rehwoldt, M.C.; Rossi, C.; Zachariah, M.R. probing the reaction zone of nanolaminates at similar to mu s time and similar to mu m spatial resolution. J. Phys. Chem. C 2020, 124, 13679–13687. [Google Scholar] [CrossRef]
- Mursalat, M.; Huang, C.; Julien, B.; Schoenitz, M.; Esteve, A.; Rossi, C.; Dreizin, E.L. Low-temperature exothermic reactions in Al/CuO nanothermites producing copper nanodots and accelerating combustion. ACS Appl. Nano Mater. 2021, 4, 3811–3820. [Google Scholar] [CrossRef]
- Wang, H.; Kline, D.J.; Zachariah, M.R. In-operando high-speed microscopy and thermometry of reaction propagation and sintering in a nanocomposite. Nat. Commun. 2019, 10, 3032. [Google Scholar] [CrossRef]
- Chakraborty, P.; Zachariah, M.R. Do nanoenergetic particles remain nano-sized during combustion? Combust. Flame 2014, 161, 1408–1416. [Google Scholar] [CrossRef]
- Monk, I.; Schoenitz, M.; Dreizin, E.L. The effect of heating rate on combustion of fully dense nanocomposite thermite particles. Combust. Sci. Technol. 2017, 190, 1–19. [Google Scholar] [CrossRef]
- Brotman, S.; Rouhani, M.D.; Rossi, C.; Esteve, A. A condensed phase model of the initial Al/CuO reaction stage to interpret experimental findings. J. Appl. Phys. 2019, 125. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Ma, B.; Tang, C.-M.; Cheng, X.-L. Molecular dynamic simulation of thermite reaction of Al nanosphere/Fe2O3 nanotube. Phys. Lett. A 2016, 380, 194–199. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wang, M.-J.; Chang, C.-R.; Xu, K.; Ma, H.-X.; Zhao, F.-Q. A DFT study on the enthalpies of thermite reactions and enthalpies of formation of metal composite oxide. Chem. Phys. 2018, 507, 19–27. [Google Scholar] [CrossRef]
- Xiong, G.; Yang, C.; Feng, S.; Zhu, W. Ab initio molecular dynamics studies on the transport mechanisms of oxygen atoms in the adiabatic reaction of Al/CuO nanothermite. Chem. Phys. Lett. 2020, 745, 137278. [Google Scholar] [CrossRef]
- Lanthony, C.; Ducere, J.M.; Esteve, A.; Rossi, C.; Djafari-Rouhani, M. Formation of Al/CuO bilayer films: Basic mechanisms through density functional theory calculations. Thin Solid Film. 2012, 520, 4768–4771. [Google Scholar] [CrossRef]
- Lanthony, C.; Guiltat, M.; Ducere, J.M.; Verdier, A.; Hemeryck, A.; Djafari-Rouhani, M.; Rossi, C.; Chabal, Y.J.; Esteve, A. Elementary surface chemistry during CuO/Al nanolaminate-thermite synthesis: Copper and oxygen deposition on aluminum (111) surfaces. ACS Appl. Mater. Inter. 2014, 6, 15086–15097. [Google Scholar] [CrossRef]
- Jacob, R.J.; Hill, K.J.; Yang, Y.; Pantoya, M.L.; Zachariah, M.R. Pre-stressing aluminum nanoparticles as a strategy to enhance reactivity of nanothermite composites. Combust. Flame 2019, 205, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, Y.C.; Focke, W.W.; Kelly, C. Measurement and modelling of pyrotechnic time delay burning rates: Method and model development. Propellants Explos. Pyrotech. 2017, 42, 1161–1167. [Google Scholar] [CrossRef]
- Tichtchenko, E.; Estève, A.; Rossi, C. Modeling the self-propagation reaction in heterogeneous and dense media: Application to Al/CuO thermite. Combust. Flame 2021, 228, 173–183. [Google Scholar] [CrossRef]
- Epps, J.M.; Hickey, J.-P.; Wen, J.Z. Modelling reaction propagation for Al/CuO nanothermite pellet combustion. Combust. Flame 2021, 229, 111374. [Google Scholar] [CrossRef]
- Lahiner, G.; Nicollet, A.; Zapata, J.; Marin, L.; Richard, N.; Rouhani, M.D.; Rossi, C.; Esteve, A. A diffusion-reaction scheme for modeling ignition and self-propagating reactions in Al/CuO multilayered thin films. J. Appl. Phys. 2017, 122, 155105. [Google Scholar] [CrossRef]
- Baijot, V.; Mehdi, D.R.; Rossi, C.; Esteve, A. A multi-phase micro-kinetic model for simulating aluminum based thermite reactions. Combust. Flame 2017, 180, 10–19. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General relationship for the thermal oxidation of silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.; Piekiel, N.; Wu, C.; Chowdhury, S.; Kelly, S.; Hufnagel, T.; Fezzaa, K.; Zachariah, M. Reactive sintering: An important component in the combustion of nanocomposite thermites. Combust. Flame 2012, 159, 2–15. [Google Scholar] [CrossRef]
- Baijot, V.; Glavier, L.; Ducere, J.M.; Rouhani, M.D.; Rossi, C.; Esteve, A. Modeling the pressure generation in aluminum-based thermites. Propell. Explos. Pyrot. 2015, 40, 402–412. [Google Scholar] [CrossRef]
- Jian, G.; Chowdhury, S.; Sullivan, K.; Zachariah, M.R. Nanothermite reactions: Is gas phase oxygen generation from the oxygen carrier an essential prerequisite to ignition? Combust. Flame 2013, 160, 432–437. [Google Scholar] [CrossRef]
- Zhou, L.; Piekiel, N.; Chowdhury, S.; Zachariah, M.R. Time-resolved mass spectrometry of the exothermic reaction between nanoaluminum and metal oxides: The role of oxygen release. J. Phys. Chem. C 2010, 114, 14269–14275. [Google Scholar] [CrossRef]
- Gao, Y.; Mathilde, I.; Mattson, E.; Lucero, A.T.; Kim, J.; Djafari Rouhani, M.; Chabal, Y.; Rossi, C.; Esteve, A. Al interaction with ZnO surfaces. J. Phys. Chem. C 2018, 122, 17856. [Google Scholar] [CrossRef]
- Lanthony, C.; Ducere, J.M.; Rouhani, M.D.; Hemeryck, A.; Esteve, A.; Rossi, C. On the early stage of aluminum oxidation: An extraction mechanism via oxygen cooperation. J. Chem. Phys. 2012, 137, 094707. [Google Scholar] [CrossRef] [Green Version]
- Fischer, S.; Grubelich, M. Theoretical Energy Release of Thermites, Intermetallics, and Combustible Metals; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 1998; pp. 231–286. [Google Scholar]
- Duraes, L.; Costa, B.F.O.; Santos, R.; Correia, A.; Campos, J.; Portugal, A. Fe2O3/aluminum thermite reaction intermediate and final products characterization. Mat. Sci. Eng. A Struct. 2007, 465, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Grapes, M.D.; Reeves, R.V.; Fezzaa, K.; Sun, T.; Densmore, J.M.; Sullivan, K.T. In situ observations of reacting Al/Fe2O3 thermite: Relating dynamic particle size to macroscopic burn time. Combust. Flame 2019, 201, 252–263. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, J.K.; Kim, J.W.; Moraes, C.A.M.; Kim, H.S.; Koo, K.K. Reaction characteristics of Al/Fe2O3 nanocomposites. J. Ind. Eng. Chem. 2012, 18, 1768–1773. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.; Siriwardane, R. Reduction of hematite (Fe2O3) to wüstite (FeO) by carbon monoxide (CO) for chemical looping combustion. Chem. Eng. J. 2014, 242, 204–210. [Google Scholar] [CrossRef]
- Montgomery, Y.C.; Focke, W.W.; Kelly, C. Measurement and modelling of pyrotechnic time delay burning rates: Application and prediction of a fast burning delay composition. Propell. Explos. Pyrot. 2017, 42, 1289–1295. [Google Scholar] [CrossRef]
- Huang, S.; Deng, S.; Jiang, Y.; Zheng, X. Experimental effective metal oxides to enhance boron combustion. Combust. Flame 2019, 205, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.; Young, G.; Zachariah, M.R. Enhanced reactivity of nano-B/Al/CuO MIC’s. Combust. Flame 2009, 156, 302–309. [Google Scholar] [CrossRef]
- Wainwright, E.R.; Dean, S.W.; Lakshman, S.V.; Weihs, T.P.; Gottfried, J.L. Evaluating compositional effects on the laser-induced combustion and shock velocities of Al/Zr-based composite fuels. Combust. Flame 2020, 213, 357–368. [Google Scholar] [CrossRef]

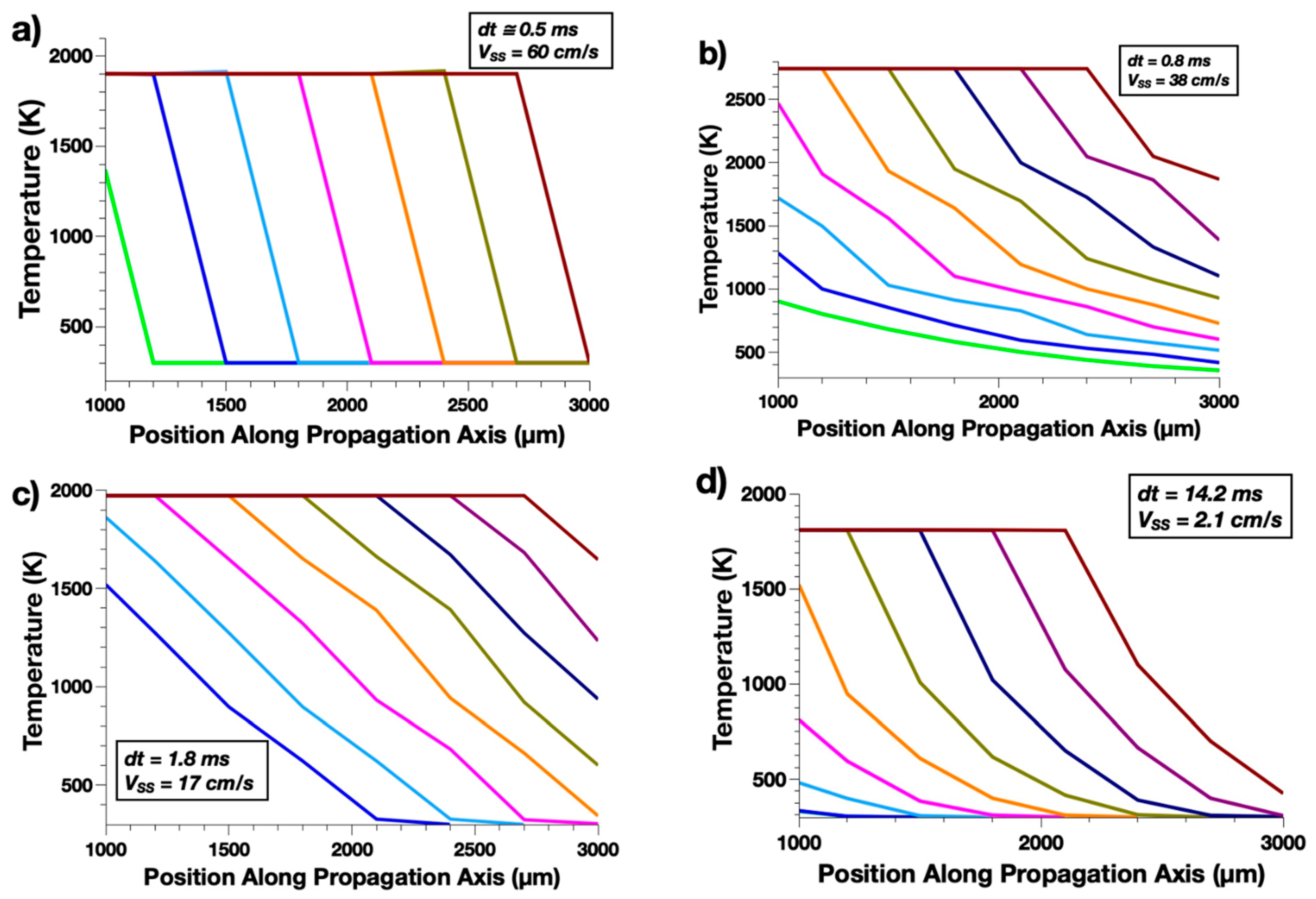

| Fuel Species | Oxide Species | Disruption Temperature in K/(Associated Vaporized Species) | Conductivity (W·m−1·K−1) | Initiation Temp (K) | Initiation Delay (ms) | Burn Rate (cm·s−1) |
|---|---|---|---|---|---|---|
| Al | CuO | 2070 (Cu2O) | 2.9 | 943 | 91 | 60 |
| Fe2O3 | 2743 (Al) | 6.7 | 1994 | 350 | 38 | |
| WO3 | 1973 (WO3) | 1.6 | 718 | 63 | 17 | |
| Pb3O4 | 1808 (PbO) | 1.6 | 1194 | 127 | 2.1 | |
| B | CuO | 2070 (Cu2O) | 2.4 | 1285 | 192 | 4.3 |
| Fe2O3 | 2130 (B2O3) | 5 | 1577 | 360 | 17 | |
| WO3 | 1973 (WO3) | 1.4 | 1228 | 186 | 14.5 | |
| Pb3O4 | 1808 (PbO) | 1.3 | 1192 | 165 | 8 | |
| Zr | CuO | 2070 (Cu2O) | 2.6 | 1643 | 270 | 6.7 |
| Fe2O3 | 3135 (Fe) | 6.8 | 1957 | 334 | 37 | |
| WO3 | 1973 (WO3) | 1.5 | 1862 | 245 | 14.3 | |
| Pb3O4 | 1808 (PbO) | 1.5 | no initiation | no initiation | no initiation |
| Formulation/Figure 6 Scheme | Effective Conduction Coefficient (W·mK−1) | Initiation Temperature (K) | Initiation Delay (ms) | Burn Rate (cm·s−1) |
|---|---|---|---|---|
| Equation (5)*/a) | 83.3 | 1003 | 85 | 22 |
| Equation (6)/b) | 50.2 | 997 | 69 | 11 |
| Equation (4)/c) | 2.9 | 944 | 59 | 0.6 |
| Equation (7)/d) | 0.6–1.2 | 943 | 23–42 | 0.2–0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brotman, S.; Djafari Rouhani, M.; Charlot, S.; Estève, A.; Rossi, C. A Benchmark Study of Burning Rate of Selected Thermites through an Original Gasless Theoretical Model. Appl. Sci. 2021, 11, 6553. https://doi.org/10.3390/app11146553
Brotman S, Djafari Rouhani M, Charlot S, Estève A, Rossi C. A Benchmark Study of Burning Rate of Selected Thermites through an Original Gasless Theoretical Model. Applied Sciences. 2021; 11(14):6553. https://doi.org/10.3390/app11146553
Chicago/Turabian StyleBrotman, Sarah, Mehdi Djafari Rouhani, Samuel Charlot, Alain Estève, and Carole Rossi. 2021. "A Benchmark Study of Burning Rate of Selected Thermites through an Original Gasless Theoretical Model" Applied Sciences 11, no. 14: 6553. https://doi.org/10.3390/app11146553
APA StyleBrotman, S., Djafari Rouhani, M., Charlot, S., Estève, A., & Rossi, C. (2021). A Benchmark Study of Burning Rate of Selected Thermites through an Original Gasless Theoretical Model. Applied Sciences, 11(14), 6553. https://doi.org/10.3390/app11146553







