A Voxel-Based Assessment of Noise Properties in Computed Tomography Imaging with the ASiR-V and ASiR Iterative Reconstruction Algorithms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scanner and Phantom Acquisitions
2.2. Noise Maps
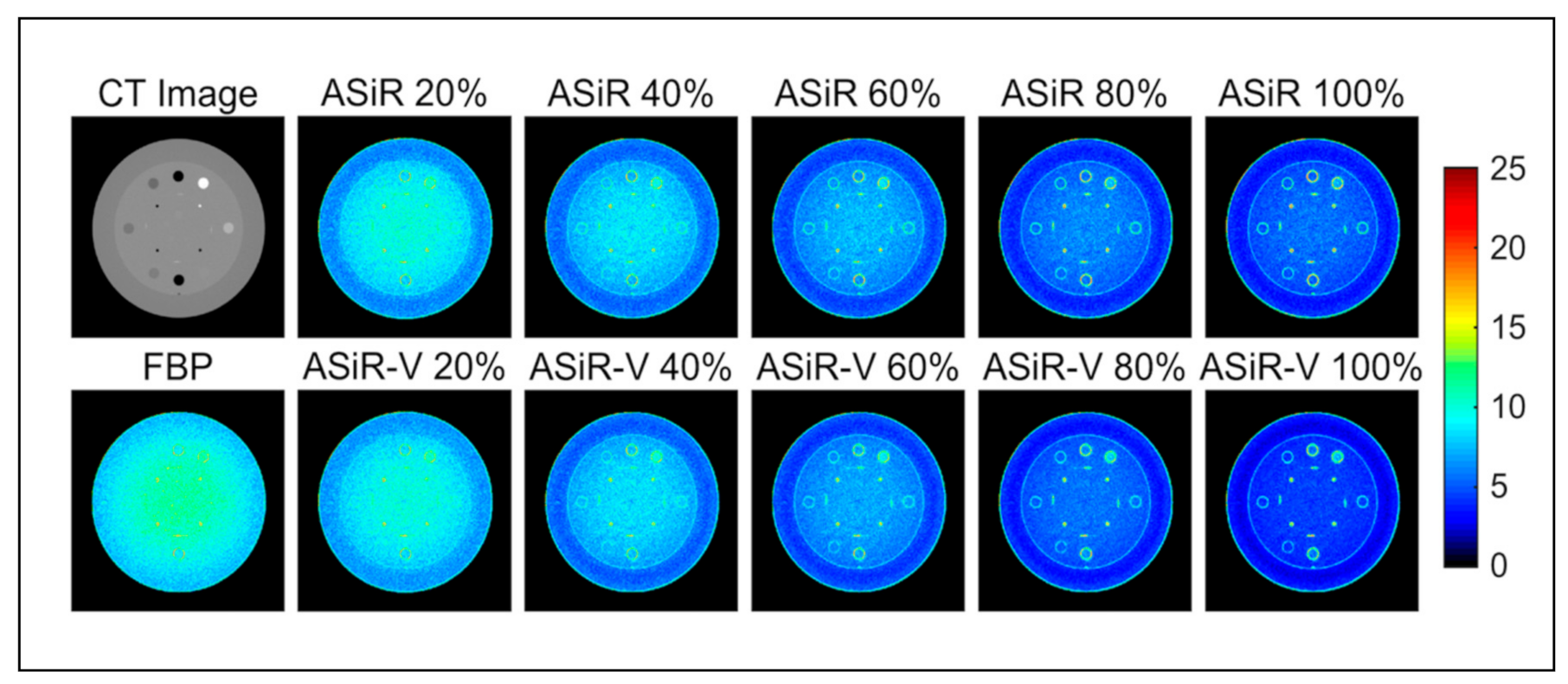
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boone, J.M.; Brink, J.A.; Edyvean, S.; Huda, W.; Leitz, W.; McCollough, C.H.; McNitt-Gray, M.F. Report 87. J. ICRU 2012, 12. [Google Scholar] [CrossRef]
- Thrall, J.H. Radiation Exposure in CT Scanning and Risk: Where Are We? Radiology 2012, 264, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith-Bindman, R.; Wang, Y.; Chu, P.; Chung, R.; Einstein, A.J.; Balcombe, J.; Cocker, M.; Das, M.; Delman, B.N.; Flynn, M.; et al. International Variation in Radiation Dose for Computed Tomography Examinations: Prospective Cohort Study. BMJ 2019, k4931. [Google Scholar] [CrossRef] [Green Version]
- Lumbreras, B.; Salinas, J.M.; Gonzalez-Alvarez, I. Cumulative Exposure to Ionising Radiation from Diagnostic Imaging Tests: A 12-Year Follow-up Population-Based Analysis in Spain. BMJ Open 2019, 9, e030905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, J.; Pavlicek, W.; Al-Senan, R.; Cody, D.; Dixon, R.; Columbo, P.; Dong, F.; Edyvean, S.; Jansen, J.; Kanal, K.; et al. Estimating Patient Organ Dose with Computed Tomography: A Review of Present Methodology and Required DICOM Information. A Joint Report of AAPM Task Group 246 and the European Federation of Organizations for Medical Physics (EFOMP); AAPM: Alexandria, VA, USA, 2019. [Google Scholar]
- Rehani, M.M.; Yang, K.; Melick, E.R.; Heil, J.; Šalát, D.; Sensakovic, W.F.; Liu, B. Patients Undergoing Recurrent CT Scans: Assessing the Magnitude. Eur. Radiol. 2020, 30, 1828–1836. [Google Scholar] [CrossRef]
- De Mattia, C.; Campanaro, F.; Rottoli, F.; Colombo, P.E.; Pola, A.; Vanzulli, A.; Torresin, A. Patient Organ and Effective Dose Estimation in CT: Comparison of Four Software Applications. Eur. Radiol. Exp. 2020, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- European Commission; Directorate-General for Energy. Medical Radiation Exposure of the European Population; Radiation Protection; Publications Office of the European Union: Luxembourg, 2015; ISBN 978-92-79-45374-8. [Google Scholar]
- National Council on Radiation Protection and Measurements (Ed.) Medical Radiation Exposure of Patients in the United States: Recommendations of the National Council on Radiation Protection and Measurements; NCRP Report; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 2019; ISBN 978-1-944888-16-9. [Google Scholar]
- McNitt-Gray, M.F. AAPM/RSNA Physics Tutorial for Residents. Topics in CT: Radiation Dose in CT. RadioGraphics 2002, 22, 1541–1553. [Google Scholar] [CrossRef]
- Gunn, M.L.D.; Kohr, J.R. State of the Art: Technologies for Computed Tomography Dose Reduction. Emerg. Radiol. 2010, 17, 209–218. [Google Scholar] [CrossRef]
- Silva, A.C.; Lawder, H.J.; Hara, A.; Kujak, J.; Pavlicek, W. Innovations in CT Dose Reduction Strategy: Application of the Adaptive Statistical Iterative Reconstruction Algorithm. Am. J. Roentgenol. 2010, 194, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.E.; Cecava, N.D.; Tucker, J.E.; Bau, J.L. CT Radiation Dose: Current Controversies and Dose Reduction Strategies. Am. J. Roentgenol. 2013, 201, 1283–1290. [Google Scholar] [CrossRef]
- Hara, A.K.; Paden, R.G.; Silva, A.C.; Kujak, J.L.; Lawder, H.J.; Pavlicek, W. Iterative Reconstruction Technique for Reducing Body Radiation Dose at CT: Feasibility Study. Am. J. Roentgenol. 2009, 193, 764–771. [Google Scholar] [CrossRef]
- Kalra, M.K.; Woisetschläger, M.; Dahlström, N.; Singh, S.; Lindblom, M.; Choy, G.; Quick, P.; Schmidt, B.; Sedlmair, M.; Blake, M.A.; et al. Radiation Dose Reduction with Sinogram Affirmed Iterative Reconstruction Technique for Abdominal Computed Tomography. J. Comput. Assist. Tomogr. 2012, 36, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, J.Y.; Jung, W.C.; Popescu, L.M.; Zeng, R.; Myers, K.J. Objective Assessment of Image Quality and Dose Reduction in CT Iterative Reconstruction: Validating Dose Reduction Claims for CT Iterative Reconstruction. Med. Phys. 2014, 41, 071904. [Google Scholar] [CrossRef]
- Padole, A.; Ali Khawaja, R.D.; Kalra, M.K.; Singh, S. CT Radiation Dose and Iterative Reconstruction Techniques. Am. J. Roentgenol. 2015, 204, W384–W392. [Google Scholar] [CrossRef]
- Beister, M.; Kolditz, D.; Kalender, W.A. Iterative Reconstruction Methods in X-Ray CT. Phys. Med. 2012, 28, 94–108. [Google Scholar] [CrossRef]
- Willemink, M.J.; de Jong, P.A.; Leiner, T.; de Heer, L.M.; Nievelstein, R.A.J.; Budde, R.P.J.; Schilham, A.M.R. Iterative Reconstruction Techniques for Computed Tomography Part 1: Technical Principles. Eur. Radiol. 2013, 23, 1623–1631. [Google Scholar] [CrossRef]
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef]
- Stiller, W. Basics of Iterative Reconstruction Methods in Computed Tomography: A Vendor-Independent Overview. Eur. J. Radiol. 2018, 109, 147–154. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Lubner, M.G.; Kim, D.H.; Tang, J.; Ruma, J.A.; del Rio, A.M.; Chen, G.-H. Abdominal CT with Model-Based Iterative Reconstruction (MBIR): Initial Results of a Prospective Trial Comparing Ultralow-Dose with Standard-Dose Imaging. Am. J. Roentgenol. 2012, 199, 1266–1274. [Google Scholar] [CrossRef] [Green Version]
- Miéville, F.A.; Gudinchet, F.; Brunelle, F.; Bochud, F.O.; Verdun, F.R. Iterative Reconstruction Methods in Two Different MDCT Scanners: Physical Metrics and 4-Alternative Forced-Choice Detectability Experiments—A Phantom Approach. Phys. Med. 2013, 29, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Willemink, M.J.; Leiner, T.; de Jong, P.A.; de Heer, L.M.; Nievelstein, R.A.J.; Schilham, A.M.R.; Budde, R.P.J. Iterative Reconstruction Techniques for Computed Tomography Part 2: Initial Results in Dose Reduction and Image Quality. Eur. Radiol. 2013, 23, 1632–1642. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jin, E.-H.; He, W.; Zhao, L.-Q. Combining Automatic Tube Current Modulation with Adaptive Statistical Iterative Reconstruction for Low-Dose Chest CT Screening. PLoS ONE 2014, 9, e92414. [Google Scholar] [CrossRef]
- Smith, E.A.; Dillman, J.R.; Goodsitt, M.M.; Christodoulou, E.G.; Keshavarzi, N.; Strouse, P.J. Model-Based Iterative Reconstruction: Effect on Patient Radiation Dose and Image Quality in Pediatric Body CT. Radiology 2014, 270, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Mangat, J.; Morgan, J.; Benson, E.; Båth, M.; Lewis, M.; Reilly, A. A Study of the Image Quality of Computed Tomography Adaptive Statistical Iterative Reconstructed Brain Images Using Subjective and Objective Methods. Radiat. Prot. Dosim. 2016, 169, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Samei, E.; Richard, S. Assessment of the Dose Reduction Potential of a Model-Based Iterative Reconstruction Algorithm Using a Task-Based Performance Metrology. Med. Phys. 2014, 42, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Dodge, C.T.; Tamm, E.P.; Cody, D.D.; Liu, X.; Jensen, C.T.; Wei, W.; Kundra, V.; Rong, X.J. Performance Evaluation of Iterative Reconstruction Algorithms for Achieving CT Radiation Dose Reduction—A Phantom Study. J. Appl. Clin. Med. Phys. 2016, 17, 511–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Liu, X.; Dodge, C.T.; Jensen, C.T.; Rong, X.J. A Noise Power Spectrum Study of a New Model-Based Iterative Reconstruction System: Veo 3.0. J. Appl. Clin. Med. Phys. 2016, 17, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.K.; Völgyes, D.; Martinsen, A.C.T. Image Quality with Iterative Reconstruction Techniques in CT of the Lungs—A Phantom Study. Eur. J. Radiol. Open 2018, 5, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Barca, P.; Giannelli, M.; Fantacci, M.E.; Caramella, D. Computed Tomography Imaging with the Adaptive Statistical Iterative Reconstruction (ASIR) Algorithm: Dependence of Image Quality on the Blending Level of Reconstruction. Australas. Phys. Eng. Sci. Med. 2018, 41, 463–473. [Google Scholar] [CrossRef]
- Willemink, M.J.; Noël, P.B. The Evolution of Image Reconstruction for CT—From Filtered Back Projection to Artificial Intelligence. Eur. Radiol. 2019, 29, 2185–2195. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Hasegawa, A.; Luo, D.; Wu, C.C.; Vikram, R. Technical Note: Impact on Central Frequency and Noise Magnitude Ratios by Advanced CT Image Reconstruction Techniques. Med. Phys. 2020, 47, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J. Adaptive Statistical Iterative Reconstruction. White Papper GE Healthcare; 2008; pp. 1–4. [Google Scholar]
- Fan, J.; Yue, M.; Melnyk, R. Benefits of ASiR-V Reconstruction for Reducing Patient Radiation Dose and Preserving Diagnostic Quality in CT Exams. White Papper GE Healthcare; 2014; pp. 1–8. [Google Scholar]
- Thibault, J.-B. Veo CT Model-Based Iterative Reconstruction. White Papper GE Healthcare; 2010; pp. 1–12. [Google Scholar]
- Richard, S.; Husarik, D.B.; Yadava, G.; Murphy, S.N.; Samei, E. Towards Task-Based Assessment of CT Performance: System and Object MTF across Different Reconstruction Algorithms. Med. Phys. 2012, 39, 4115–4122. [Google Scholar] [CrossRef]
- McCollough, C.H.; Yu, L.; Kofler, J.M.; Leng, S.; Zhang, Y.; Li, Z.; Carter, R.E. Degradation of CT Low-Contrast Spatial Resolution Due to the Use of Iterative Reconstruction and Reduced Dose Levels. Radiology 2015, 276, 499–506. [Google Scholar] [CrossRef]
- Hsieh, J. Computed Tomography: Principles, Design, Artifacts, and Recent Advances; SPIE PRESS: Bellingham, WA, USA, 2015; ISBN 978-1-62841-825-5. [Google Scholar]
- Thibault, J.-B.; Sauer, K.D.; Bouman, C.A.; Hsieh, J. A Three-Dimensional Statistical Approach to Improved Image Quality for Multislice Helical CT. Med. Phys. 2007, 34, 4526–4544. [Google Scholar] [CrossRef]
- Hussain, F.A.; Mail, N.; Shamy, A.M.; Alghamdi, S.; Saoudi, A. A Qualitative and Quantitative Analysis of Radiation Dose and Image Quality of Computed Tomography Images Using Adaptive Statistical Iterative Reconstruction. J. Appl. Clin. Med. Phys. 2016, 17, 419–432. [Google Scholar] [CrossRef]
- Kim, H.G.; Chung, Y.E.; Lee, Y.H.; Choi, J.-Y.; Park, M.-S.; Kim, M.-J.; Kim, K.W. Quantitative Analysis of the Effect of Iterative Reconstruction Using a Phantom: Determining the Appropriate Blending Percentage. Yonsei Med. J. 2015, 56, 253. [Google Scholar] [CrossRef]
- Kwon, H.; Cho, J.; Oh, J.; Kim, D.; Cho, J.; Kim, S.; Lee, S.; Lee, J. The Adaptive Statistical Iterative Reconstruction-V Technique for Radiation Dose Reduction in Abdominal CT: Comparison with the Adaptive Statistical Iterative Reconstruction Technique. Br. J. Radiol. 2015, 88, 20150463. [Google Scholar] [CrossRef] [Green Version]
- Benz, D.C.; Gräni, C.; Mikulicic, F.; Vontobel, J.; Fuchs, T.A.; Possner, M.; Clerc, O.F.; Stehli, J.; Gaemperli, O.; Pazhenkottil, A.P.; et al. Adaptive Statistical Iterative Reconstruction-V: Impact on Image Quality in Ultralow-Dose Coronary Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2016, 40, 958–963. [Google Scholar] [CrossRef] [Green Version]
- So, A.; Imai, Y.; Nett, B.; Jackson, J.; Nett, L.; Hsieh, J.; Wisenberg, G.; Teefy, P.; Yadegari, A.; Islam, A.; et al. Technical Note: Evaluation of a 160-Mm/256-Row CT Scanner for Whole-Heart Quantitative Myocardial Perfusion Imaging. Med. Phys. 2016, 43, 4821–4832. [Google Scholar] [CrossRef]
- Racine, D.; Ott, J.G.; Ba, A.; Ryckx, N.; Bochud, F.O.; Verdun, F.R. Objective Task-Based Assessment of Low-Contrast Detectability in Iterative Reconstruction. Radiat. Prot. Dosim. 2016, 169, 73–77. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, H.-J.; Lee, S.-K.; Kim, H.J.; Kim, M.-J. Head CT: Image Quality Improvement with ASIR-V Using a Reduced Radiation Dose Protocol for Children. Eur. Radiol. 2017, 27, 3609–3617. [Google Scholar] [CrossRef]
- Gatti, M.; Marchisio, F.; Fronda, M.; Rampado, O.; Faletti, R.; Bergamasco, L.; Ropolo, R.; Fonio, P. Adaptive Statistical Iterative Reconstruction–V versus Adaptive Statistical Iterative Reconstruction: Impact on Dose Reduction and Image Quality in Body Computed Tomography. J. Comput. Assist. Tomogr. 2018, 42, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Goodenberger, M.H.; Wagner-Bartak, N.A.; Gupta, S.; Liu, X.; Yap, R.Q.; Sun, J.; Tamm, E.P.; Jensen, C.T. Computed Tomography Image Quality Evaluation of a New Iterative Reconstruction Algorithm in the Abdomen (Adaptive Statistical Iterative Reconstruction–V) a Comparison with Model-Based Iterative Reconstruction, Adaptive Statistical Iterative Reconstruction, and Filtered Back Projection Reconstructions. J. Comput. Assist. Tomogr. 2018, 42, 184–190. [Google Scholar] [CrossRef]
- Lim, K.; Kwon, H.; Cho, J.; Oh, J.; Yoon, S.; Kang, M.; Ha, D.; Lee, J.; Kang, E. Initial Phantom Study Comparing Image Quality in Computed Tomography Using Adaptive Statistical Iterative Reconstruction and New Adaptive Statistical Iterative Reconstruction V. J. Comput. Assist. Tomogr. 2015, 39, 443–448. [Google Scholar] [CrossRef]
- Tang, H.; Yu, N.; Jia, Y.; Yu, Y.; Duan, H.; Han, D.; Ma, G.; Ren, C.; He, T. Assessment of Noise Reduction Potential and Image Quality Improvement of a New Generation Adaptive Statistical Iterative Reconstruction (ASIR-V) in Chest CT. Br. J. Radiol. 2018, 91, 20170521. [Google Scholar] [CrossRef]
- Euler, A.; Solomon, J.; Marin, D.; Nelson, R.C.; Samei, E. A Third-Generation Adaptive Statistical Iterative Reconstruction Technique: Phantom Study of Image Noise, Spatial Resolution, Lesion Detectability, and Dose Reduction Potential. Am. J. Roentgenol. 2018, 210, 1301–1308. [Google Scholar] [CrossRef]
- De Marco, P.; Origgi, D. New Adaptive Statistical Iterative Reconstruction ASiR-V: Assessment of Noise Performance in Comparison to ASiR. J. Appl. Clin. Med. Phys. 2018, 19, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Viry, A.; Aberle, C.; Racine, D.; Knebel, J.-F.; Schindera, S.T.; Schmidt, S.; Becce, F.; Verdun, F.R. Effects of Various Generations of Iterative CT Reconstruction Algorithms on Low-Contrast Detectability as a Function of the Effective Abdominal Diameter: A Quantitative Task-Based Phantom Study. Phys. Med. 2018, 48, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jin, C.; Li, J.; Wang, G.; Jia, Y.; Duan, H.; Pan, N.; Guo, J. Image Quality Comparison of Two Adaptive Statistical Iterative Reconstruction (ASiR, ASiR-V) Algorithms and Filtered Back Projection in Routine Liver CT. Br. J. Radiol. 2018, 91, 20170655. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Z.; Hu, Z.; He, T.; Li, D.; Yu, N.; Jia, Y.; Shi, H. Clinical Value of a New Generation Adaptive Statistical Iterative Reconstruction (ASIR-V) in the Diagnosis of Pulmonary Nodule in Low-Dose Chest CT. Br. J. Radiol. 2019, 92, 20180909. [Google Scholar] [CrossRef]
- Han, W.K.; Na, J.C.; Park, S.Y. Low-Dose CT Angiography Using ASiR-V for Potential Living Renal Donors: A Prospective Analysis of Image Quality and Diagnostic Accuracy. Eur. Radiol. 2020, 30, 798–805. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Quality Assurance Programme for Computed Tomography: Diagnostic and Therapy Applications; IAEA: Vienna, Austria, 2012; ISBN 978-92-0-128910-0. [Google Scholar]
- European Commission. Criteria for Acceptability of Medical Radiological Equipment Used in Diagnostic Radiology, Nuclear Medicine and Radiotherapy; Radiation Protection; Publications Office of the European Union: Luxembourg, 2013; ISBN 978-92-79-27747-4. [Google Scholar]
- Dance, D.R. (Ed.) Diagnostic Radiology Physics: A Handbook for Teachers and Students; STI/PUB; International Atomic Energy Agency: Vienna, Austria, 2014; ISBN 978-92-0-131010-1. [Google Scholar]
- Riederer, S.J.; Pelc, N.J.; Chesler, D.A. The Noise Power Spectrum in Computed X-Ray Tomography. Phys. Med. Biol. 1978, 23, 446–454. [Google Scholar] [CrossRef]
- Friedman, S.N.; Fung, G.S.K.; Siewerdsen, J.H.; Tsui, B.M.W. A Simple Approach to Measure Computed Tomography (CT) Modulation Transfer Function (MTF) and Noise-Power Spectrum (NPS) Using the American College of Radiology (ACR) Accreditation Phantom. Med. Phys. 2013, 40, 051907. [Google Scholar] [CrossRef] [Green Version]
- Samei, E.; Bakalyar, D.; Boedeker, K.; Brady, S.; Fan, J.; Leng, S.; Myers, K.; Popescu, L.; Ramirez-Giraldo, J.C.; Ranallo, F.; et al. Performance Evaluation of Computed Tomography Systems—The Report of AAPM Task Group 233; AAPM: Alexandria, VA, USA, 2019. [Google Scholar]
- The Phantom Laboratory. Catphan ® 504 Manual; The Phantom Laboratory: Salem, NY, USA, 2013. [Google Scholar]
- Li, K.; Tang, J.; Chen, G.-H. Statistical Model Based Iterative Reconstruction (MBIR) in Clinical CT Systems: Experimental Assessment of Noise Performance. Med. Phys. 2014, 41, 041906. [Google Scholar] [CrossRef] [Green Version]
- Solomon, J.; Samei, E. Quantum Noise Properties of CT Images with Anatomical Textured Backgrounds across Reconstruction Algorithms: FBP and SAFIRE. Med. Phys. 2014, 41, 091908. [Google Scholar] [CrossRef]
- Solomon, J.; Wilson, J.; Samei, E. Characteristic Image Quality of a Third Generation Dual-Source MDCT Scanner: Noise, Resolution, and Detectability. Med. Phys. 2015, 42, 4941–4953. [Google Scholar] [CrossRef] [PubMed]
- Funama, Y.; Taguchi, K.; Utsunomiya, D.; Oda, S.; Katahira, K.; Tokuyasu, S.; Yamashita, Y. Image Quality Assessment of an Iterative Reconstruction Algorithm Applied to Abdominal CT Imaging. Phys. Med. 2014, 30, 527–534. [Google Scholar] [CrossRef]
- Geyer, L.L.; Körner, M.; Harrieder, A.; Mueck, F.G.; Deak, Z.; Wirth, S.; Linsenmaier, U. Dose Reduction in 64-Row Whole-Body CT in Multiple Trauma: An Optimized CT Protocol with Iterative Image Reconstruction on a Gemstone-Based Scintillator. Br. J. Radiol. 2016, 89, 20160003. [Google Scholar] [CrossRef] [Green Version]
- Brady, S.L.; Yee, B.S.; Kaufman, R.A. Characterization of Adaptive Statistical Iterative Reconstruction Algorithm for Dose Reduction in CT: A Pediatric Oncology Perspective. Med. Phys. 2012, 39, 5520–5531. [Google Scholar] [CrossRef]







| Material | CT Numbers (HU) |
|---|---|
| Air | −1046: −986 |
| PMP a | −220: −172 |
| LDPE b | −121: −87 |
| Polystyrene | −65: −29 |
| Acrylic | 92: 137 |
| Delrin | 344: 387 |
| Teflon | 941: 1060 |
| Blending Level | FBP | ASiR | ASiR-V |
|---|---|---|---|
| 0% | 9.5 (1.3) | ||
| 20% | 7.9 (1.1) | 8.0 (1.2) | |
| 40% | 6.9 (1.0) | 6.5 (1.0) | |
| 60% | 5.8 (0.8) | 5.4 (0.8) | |
| 80% | 4.9 (0.7) | 4.4 (0.6) | |
| 100% | 4.5 (0.7) | 3.4 (0.5) |
| Air | PMP | LDPE | Polystyrene | Acrylic | Delrin | Teflon | ||
|---|---|---|---|---|---|---|---|---|
| FBP | 9.0 (1.0) | 9.7 (1.1) | 9.8 (1.1) | 9.6 (1.0) | 9.7 (1.2) | 10.4 (1.1) | 11.3 (1.3) | |
| ASiR | 20% | 8.1 (0.9) | 8.6 (0.9) | 8.6 (0.9) | 8.4 (0.9) | 8.5 (1.0) | 9.2 (0.9) | 10.2 (1.2) |
| 40% | 7.3 (0.8) | 7.5 (0.8) | 7.6 (0.8) | 7.4 (0.8) | 7.5 (0.9) | 8.1 (0.8) | 9.4 (1.1) | |
| 60% | 6.6 (0.8) | 6.5 (0.7) | 6.5 (0.7) | 6.4 (0.7) | 6.4 (0.8) | 7.0 (0.7) | 8.5 (1.1) | |
| 80% | 5.8 (0.7) | 5.5 (0.7) | 5.6 (0.6) | 5.4 (0.6) | 5.5 (0.6) | 6.0 (0.6) | 7.7 (1.0) | |
| 100% | 5.4 (0.7) | 5.1 (0.7) | 5.0 (0.6) | 4.9 (0.6) | 5.0 (0.7) | 5.5 (0.7) | 7.3 (1.0) | |
| ASiR-V | 20% | 8.4 (0.9) | 8.5 (0.9) | 8.6 (0.9) | 8.5 (0.9) | 8.5 (1.0) | 9.1 (0.9) | 10.5 (1.2) |
| 40% | 7.7 (0.9) | 7.4 (0.8) | 7.4 (0.8) | 7.3 (0.8) | 7.4 (0.9) | 7.9 (0.8) | 9.7 (1.2) | |
| 60% | 7.1 (0.9) | 6.3 (0.7) | 6.3 (0.7) | 6.2 (0.7) | 6.2 (0.7) | 6.7 (0.7) | 9.0 (1.2) | |
| 80% | 6.5 (0.9) | 5.3 (0.6) | 5.2 (0.6) | 5.2 (0.6) | 5.1 (0.6) | 5.6 (0.6) | 8.3 (1.2) | |
| 100% | 6.0 (1.0) | 4.3 (0.5) | 4.2 (0.5) | 4.2 (0.5) | 4.1 (0.5) | 4.6 (0.5) | 7.7 (1.3) |
| Median | Interquartile Range | Kurtosis | Skewness | ||
|---|---|---|---|---|---|
| FBP | 9.16 | 1.62 | 3.00 | 0.23 | |
| ASiR | 20% | 7.81 | 1.39 | 3.07 | 0.27 |
| 40% | 6.86 | 1.17 | 3.10 | 0.26 | |
| 60% | 5.88 | 0.99 | 3.13 | 0.25 | |
| 80% | 5.00 | 0.83 | 3.14 | 0.22 | |
| 100% | 4.51 | 0.80 | 3.57 | 0.41 | |
| ASiR-V | 20% | 7.91 | 1.37 | 3.03 | 0.24 |
| 40% | 6.57 | 1.15 | 3.10 | 0.27 | |
| 60% | 5.51 | 0.92 | 3.11 | 0.25 | |
| 80% | 4.52 | 0.74 | 3.14 | 0.23 | |
| 100% | 3.60 | 0.57 | 3.35 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barca, P.; Marfisi, D.; Marzi, C.; Cozza, S.; Diciotti, S.; Traino, A.C.; Giannelli, M. A Voxel-Based Assessment of Noise Properties in Computed Tomography Imaging with the ASiR-V and ASiR Iterative Reconstruction Algorithms. Appl. Sci. 2021, 11, 6561. https://doi.org/10.3390/app11146561
Barca P, Marfisi D, Marzi C, Cozza S, Diciotti S, Traino AC, Giannelli M. A Voxel-Based Assessment of Noise Properties in Computed Tomography Imaging with the ASiR-V and ASiR Iterative Reconstruction Algorithms. Applied Sciences. 2021; 11(14):6561. https://doi.org/10.3390/app11146561
Chicago/Turabian StyleBarca, Patrizio, Daniela Marfisi, Chiara Marzi, Sabino Cozza, Stefano Diciotti, Antonio Claudio Traino, and Marco Giannelli. 2021. "A Voxel-Based Assessment of Noise Properties in Computed Tomography Imaging with the ASiR-V and ASiR Iterative Reconstruction Algorithms" Applied Sciences 11, no. 14: 6561. https://doi.org/10.3390/app11146561






