Beetroot Microencapsulation with Pea Protein Using Spray Drying: Physicochemical, Structural and Functional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of the Feed Mixture and Spray-Drying (SD) Conditions
2.3. Product Yield, Drying Ratio, and Productivity
2.4. Analytical Determinations
2.4.1. Water Content (xw)
2.4.2. Soluble Solid Content (xs)
2.4.3. Crude Protein
2.4.4. Hygroscopicity
2.4.5. Bulk Density and Porosity
2.4.6. Water Solubility Index (WSI) and Water Absorption Index (WAI)
2.4.7. Color Measurement
2.4.8. Total Phenols (TP)
2.4.9. Betalains
2.4.10. Antioxidant Capacity (AC)
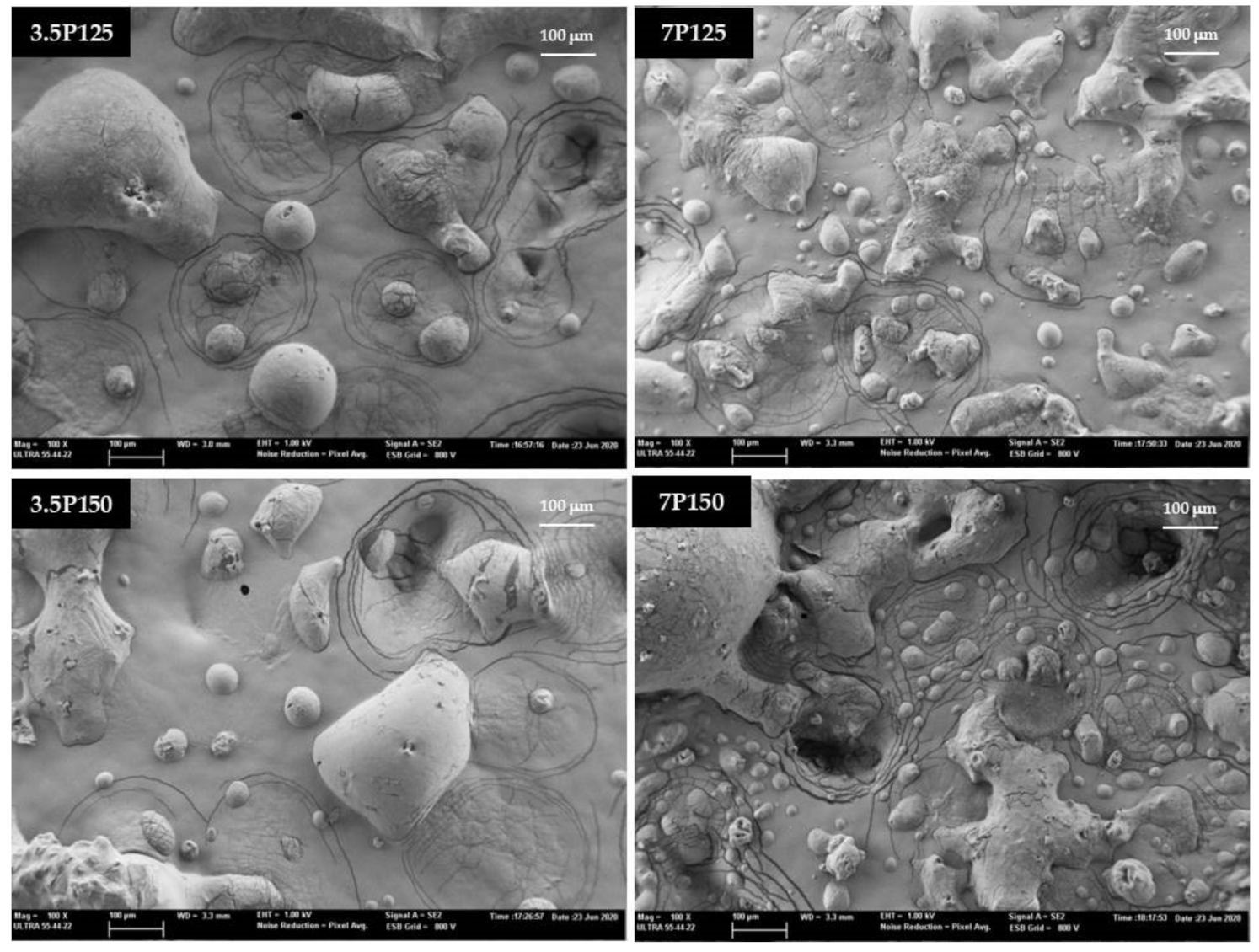
2.5. Powder Morphology
2.6. Encapsulation Efficiencies (EE)
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koul, V.K.; Jain, M.P.; Koul, S.; Sharma, V.K.; Tikoo, C.L.; Jain, S.M. Spray drying of beet root juice using different carriers. Indian J. Chem. Technol. 2002, 9, 442–445. [Google Scholar]
- Roy, K.; Gullapalli, S.; Chaudhuri, U.R.; Chakraborty, R. The use of a natural colorant based on betalain in the manufacture of sweet products in India. Int. J. Food Sci. Technol. 2004, 39, 1087–1091. [Google Scholar] [CrossRef]
- Panghal, A.; Virkar, K.; Kumar, V.; Dhull, S.B.; Gat, Y.; Chhikara, N. Development of Probiotic Beetroot Drink. Curr. Res. Nutr. Food Sci. 2017, 5. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Singh, B.; Hathan, B.S. Chemical composition, functional properties and processing of Beetroot. Int. J. Sci. Eng. Res. 2014, 5, 679–684. [Google Scholar]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Thies, C. A survey of microencapsulation processes. In Microencapsulation: Methods and Industrial Applications; Benita, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 133–154. [Google Scholar]
- Shahidi, F.; Han, X.-Q. Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, G.R.; González-García, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Abud-Archila, M. Spray-Drying of Cactus Pear Juice (Opuntia streptacantha): Effect on the Physicochemical Properties of Powder and Reconstituted Product. Dry. Technol. 2005, 23, 955–973. [Google Scholar] [CrossRef]
- Bogracheva, T.Y.; Davydova, N.I.; Bespalova, N.Y.; Kondrashina, S.A.; Bezrukov, M.G.; Braudo, E.E.; Tolstoguzov, V.B. A study of stability of O/W emulsions stabilized by soybean and pea globulins. Food/Nahrung 1994, 38, 121–127. [Google Scholar] [CrossRef]
- Ducel, V.; Richard, J.; Popineau, Y.; Boury, F. Adsorption Kinetics and Rheological Interfacial Properties of Plant Proteins at the Oil−Water Interface. Biomacromolecules 2004, 5, 2088–2093. [Google Scholar] [CrossRef]
- Franco, J.M.; Partal, P.; Conde, B.; Gallegos, C. Influence of pH and protei thermal treatment on the rheology of pea protein-stabilized oil-in-water emulsions. J. Am. Oil Chem. Soc. 2000, 77, 975–984. [Google Scholar] [CrossRef]
- Galazka, V.B.; Dickinson, E.; Ledward, D.A. Emulsifying behaviour of 11S globulin Vicia faba in mixtures with sulphated polysaccharides: Comparison of thermal and high-pressure treatments. Food Hydrocoll. 1999, 13, 425–435. [Google Scholar] [CrossRef]
- Viroben, G.; Barbot, J.; Mouloungui, Z.; Guéguen, J. Preparation and characterization of films from pea protein. J. Agric. Food Chem. 2000, 48, 1064–1069. [Google Scholar] [CrossRef]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; Van Boekel, M.A.J.S. Characterization of Pea Vicilin. 2. Consequences of Compositional Heterogeneity on Heat-Induced Gelation Behavior. J. Agric. Food Chem. 2004, 52, 3149–3154. [Google Scholar] [CrossRef]
- Martins, V.B.; Netto, F.M. Physicochemical and functional properties of soy protein isolate as a function of water activity and storage. Food Res. Int. 2006, 39, 145–153. [Google Scholar] [CrossRef]
- Swanson, B.G. Pea and lentil protein extraction and functionality. J. Am. Oil Chem. Soc. 1990, 67, 276–280. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.Ł.; Jeżowski, P.; Singh, A.P. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef] [Green Version]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Vardin, H.; Yasar, M. Optimisation of pomegranate (Punica Granatum L.) juice spray-drying as affected by temperature and maltodextrin content. Int. J. Food Sci. Technol. 2012, 47, 167–176. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemist. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- AOAC. AOAC Official Method 990.03. Protein (Crude) in Animal Feed, Combustion Method, 18th ed.; Horwitz, W., Latimer, G.W., Jr., Eds.; AOAC: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Igual, M.; Cebadera, L.; Cámara, R.M.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel ingredients based on grapefruit freeze-dried formulations: Nutritional and bioactive value. Foods 2019, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Smith, A.C. A comparison of wheat starch, whole wheat meal and oat flour in the extrusion cooking process. J. Food Eng. 1997, 34, 15–32. [Google Scholar] [CrossRef]
- CIE. Colorimetry, 2nd ed.; Publication CIE No. 15.2; Commission Internationale de l’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Stability of micronutrients and phytochemicals of grapefruit jam as affected by the obtention process. Food Sci. Technol. Int. 2016, 22, 203–212. [Google Scholar] [CrossRef]
- Agudelo, C.; Igual, M.; Camacho, M.M.; Martínez-Navarrete, N. Effect of process technology on the nutritional, functional, and physical quality of grapefruit powder. Food Sci. Technol. Int. 2017, 23, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, T. Studies into the pigments in beetroot (Beta vulgaris L. ssp vulgaris var. rubra L.). Lantbrukshogskolans Annaler 1970, 36, 179–219. [Google Scholar]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa l. J. Food Process. Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Marmion, D.M. Handbook of US Colorants: Foods, Drugs, Cosmetics, and Medical Devices; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Resistant maltodextrin’s effect on the physicochemical and structure properties of spray dried orange juice powders. Eur. Food Res. Technol. 2021, 247, 1125–1132. [Google Scholar] [CrossRef]
- González, F.; Igual, M.; Camacho, M.M.; Martínez-Navarrete, N. Impact of Temperature, Gum Arabic and Carboxymethyl Cellulose on Some Physical Properties of Spray-Dried Grapefruit. Int. J. Food Eng. 2018, 14. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated with Spray Drying of Sugar-Rich Foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Papadakis, S.E.; Gardeli, C.; Tzia, C. Spray Drying of Raisin Juice Concentrate. Dry. Technol. 2006, 24, 173–180. [Google Scholar] [CrossRef]
- Roustapour, O.R.; Hosseinalipour, M.; Ghobadian, B. An Experimental Investigation of Lime Juice Drying in a Pilot Plant Spray Dryer. Dry. Technol. 2006, 24, 181–188. [Google Scholar] [CrossRef]
- Igual, M.; Ramires, S.; Mosquera, L.H.; Martínez-Navarrete, N. Optimization of spray drying conditions for lulo (Solanum quitoense L.) pulp. Powder Technol. 2014, 256, 233–238. [Google Scholar] [CrossRef]
- Mezreb, K.; Goullieux, A.; Ralainirina, R.; Queneudec, M. Application of image analysis to measure screw speed influence on physical properties of corn and wheat extrudates. J. Food Eng. 2003, 57, 145–152. [Google Scholar] [CrossRef]
- Augusto-Ruiz, W.; Bonato, S.; Arrieche, L.; Risso, F. Characterization of pregelatinized whole rice flour produced from broken rice grains. Rev. Ciênc. Exatas Eng. 2003, 13, 25–46. [Google Scholar]
- Rahman, M.S. Toward prediction of porosity in foods during drying: A brief review. Dry. Technol. 2001, 19, 1–13. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Ashtari, A.K.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Moghbeli, S.; Jafari, S.M.; Maghsoudlou, Y.; Dehnad, D. A Taguchi approach optimization of date powder production by spray drying with the aid of whey protein-pectin complexes. Powder Technol. 2020, 359, 85–93. [Google Scholar] [CrossRef]
- Bodart, M.; De Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Kingwatee, N.; Apichartsrangkoon, A.; Chaikham, P.; Worametrachanon, S.; Techarung, J.; Pankasemsuk, T. Spray drying Lactobacillus casei 01 in lychee juice varied carrier materials. LWT 2015, 62, 847–853. [Google Scholar] [CrossRef]
- Bazaria, B.; Kumar, P. Optimization of spray drying parameters for beetroot juice powder using response surface methodology (RSM). J. Saudi Soc. Agric. Sci. 2018, 17, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Tze, N.L.; Han, C.P.; Yusof, Y.A.; Ling, C.N.; Talib, R.A.; Taip, F.S.; Aziz, M.G. Physicochemical and nutritional properties of spray-dried pitaya fruit powder as natural colorant. Food Sci. Biotechnol. 2012, 21, 675–682. [Google Scholar] [CrossRef]
- Fang, Y.; Rogers, S.; Selomulya, C.; Chen, X.D. Functionality of milk protein concentrate: Effect of spray drying temperature. Biochem. Eng. J. 2012, 62, 101–105. [Google Scholar] [CrossRef]
- Čakarević, J.; Šeregelj, V.; Šaponjac, V.T.; Ćetković, G.; Brunet, J.Č.; Popović, S.; Kostić, M.H.; Popović, L. Encapsulation of beetroot juice: A study on the application of pumpkin oil cake protein as new carrier agent. J. Microencapsul. 2020, 37, 121–133. [Google Scholar] [CrossRef]



| Parameter | Beetroot Juice |
|---|---|
| xw (g/100 g) | 88.40 (0.03) |
| xs (g/100 g) | 12.2 (0.2) |
| Total phenols (mgGA/100 g) | 118 (3) |
| Betacyanins (mgBE/100 g) | 133 (2) |
| Betaxanthins (mgVE/100 g) | 64.7 (0.8) |
| Antioxidant Capacity (mgTE/100 g) | 38.4 (0.9) |
| Parameter | 3.5P125 | 3.5P150 | 7P125 | 7P150 |
|---|---|---|---|---|
| Outlet temperature (°C) | 74.2 (0.8) d | 86.5 (0.5) b | 77.8 (0.8) c | 92.8 (2.1) a |
| Product yield (g solutes in the powder/100 g solutes in the mixture) | 31.86 (0.02) c | 41.5 (1.8) a | 28.2 (0.7) d | 36.6 (0.8) b |
| Drying ratio | 8.8 (0.2) a | 8.95 (0.19) a | 6.48 (0.09) b | 6.56 (0.09) b |
| Productivity (g/h) | 67.1 (0.9) b | 66.5 (0.9) b | 84.1 (1.7) a | 83.7 (1.4) a |
| Parameter | 3.5P125 | 3.5P150 | 7P125 | 7P150 |
|---|---|---|---|---|
| xw (gw/g) | 0.0492 (0.0013) a | 0.0331 (0.0009) c | 0.0448 (0.0013) b | 0.0321 (0.0002) c |
| CP (gCP/g) | 20.380 (0.006) d | 21.79 (0.07) c | 26.70 (0.05) b | 27.65 (0.12) a |
| WAI | 0.49 (0.09) b | 0.53 (0.03) b | 0.70 (0.05) a | 0.824 (0.013) a |
| WSI (%) | 17.4 (0.9) a | 17.90 (0.03) a | 15.1 (0.2) b | 12.4 (0.7) c |
| ρb (g/cm3) | 0.5675 (0.0007) a | 0.44 (0.02) b | 0.417 (0.012) b | 0.363 (0.013) c |
| ε | 0.6143 (0.0006) d | 0.703 (0.008) c | 0.718 (0.006) b | 0.754 (0.006) a |
| 3.5P125 | 3.5P150 | 7P125 | 7P150 | |
|---|---|---|---|---|
| L* | 23.1 (0.3) d | 25.91 (0.13) c | 26.8 (0.6) b | 28.5 (1.3) a |
| a* | 34.66 (0.09) c | 36.80 (0.04) a | 36.1 (0.4) b | 35.7 (0.7) b |
| b* | 2.73 (0.12) a | 1.99 (0.03) b | 1.52 (0.04) c | 0.88 (0.12) d |
| C* | 34.765 (1.04) c | 36.85 (0.04) a | 36.2 (0.4) b | 35.8 (0.7) b |
| h* | 4.5 (0.2) a | 3.10 (0.04) b | 2.40 (0.05) c | 1.4 (0.2) d |
| ΔE1 | 3.61 (0.08) a | 2.5 (0.6) b | ||
| ΔE2 | 4.2 (0.6) a | 3.6 (0.5) a |
| Sample | Total Phenol (mgGA/100 gBS) | Betacyanin (mgBE/100 gBS) | Betaxanthin (mgVE/100 gBS) | Antioxidant Capacity (mgTE/100 gBS) | |
|---|---|---|---|---|---|
| Feed 3.5P | 935 (5) aA | 1015 (17) aA | 525 (7) aA | 314 (6) aA | |
| SD | 3.5P125 | 771 (25) bB | 941 (42) abA | 480 (21) abA | 235 (9) bB |
| 3.5P150 | 644 (40) cA | 845 (57) bA | 433 (26) bA | 213 (4) cA | |
| Feed 7P | 939 (4) aA | 1041 (14) aA | 525 (5) aA | 304 (11) aA | |
| SD | 7P125 | 896 (14) bA | 981 (54) abA | 506 (26) aA | 263.41 (0.08) bA |
| 7P150 | 737 (28) cA | 905 (42) bA | 474 (26) aA | 223 (4) cA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Beetroot Microencapsulation with Pea Protein Using Spray Drying: Physicochemical, Structural and Functional Properties. Appl. Sci. 2021, 11, 6658. https://doi.org/10.3390/app11146658
García-Segovia P, Igual M, Martínez-Monzó J. Beetroot Microencapsulation with Pea Protein Using Spray Drying: Physicochemical, Structural and Functional Properties. Applied Sciences. 2021; 11(14):6658. https://doi.org/10.3390/app11146658
Chicago/Turabian StyleGarcía-Segovia, Purificación, Marta Igual, and Javier Martínez-Monzó. 2021. "Beetroot Microencapsulation with Pea Protein Using Spray Drying: Physicochemical, Structural and Functional Properties" Applied Sciences 11, no. 14: 6658. https://doi.org/10.3390/app11146658
APA StyleGarcía-Segovia, P., Igual, M., & Martínez-Monzó, J. (2021). Beetroot Microencapsulation with Pea Protein Using Spray Drying: Physicochemical, Structural and Functional Properties. Applied Sciences, 11(14), 6658. https://doi.org/10.3390/app11146658







