Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends
Abstract
:1. Introduction
2. Commonly Used Pharmaceuticals: Their Classes, Chemical Structures, and Therapeutic Applications
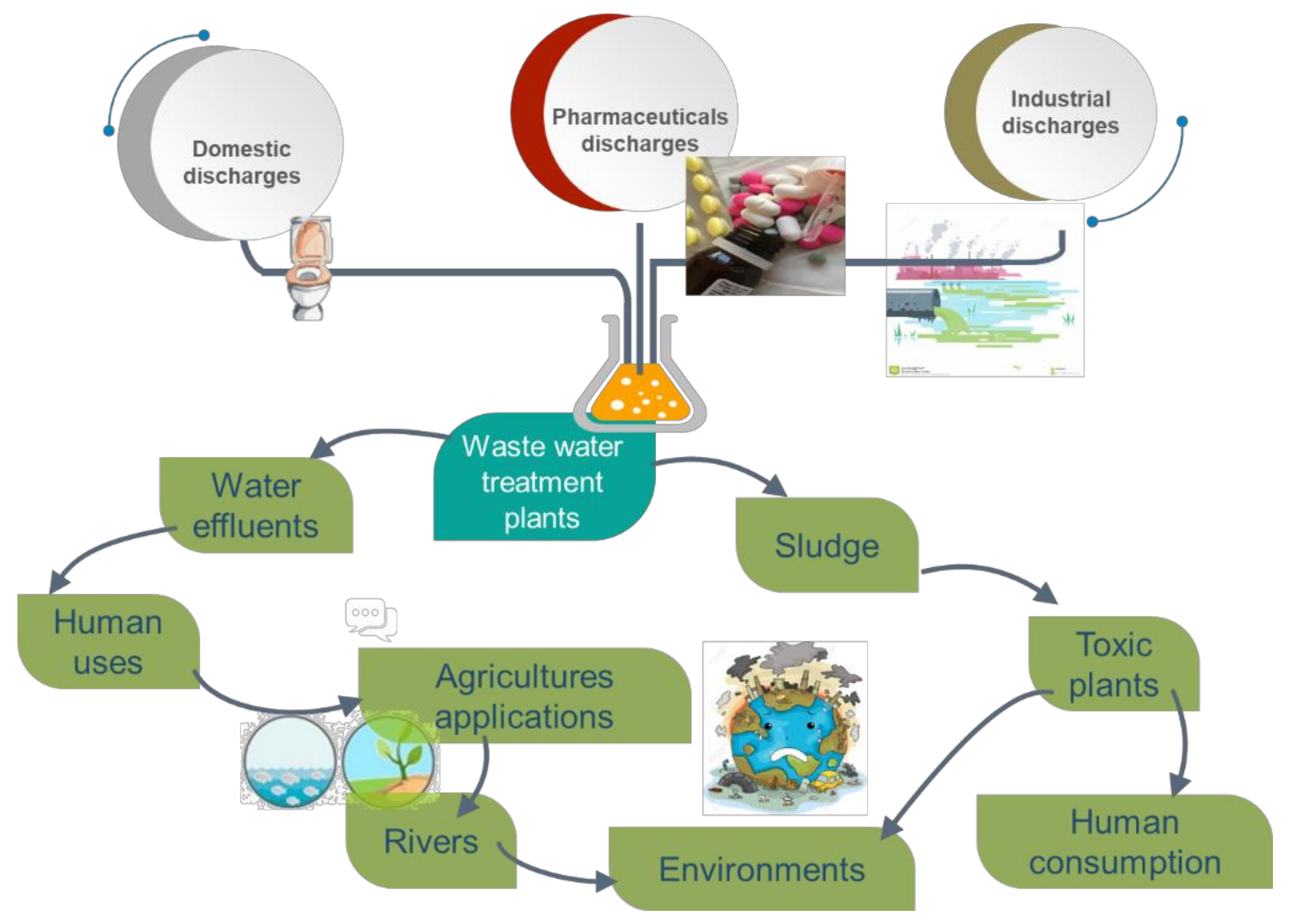
3. Concentration of Pharmaceuticals in Water Environment
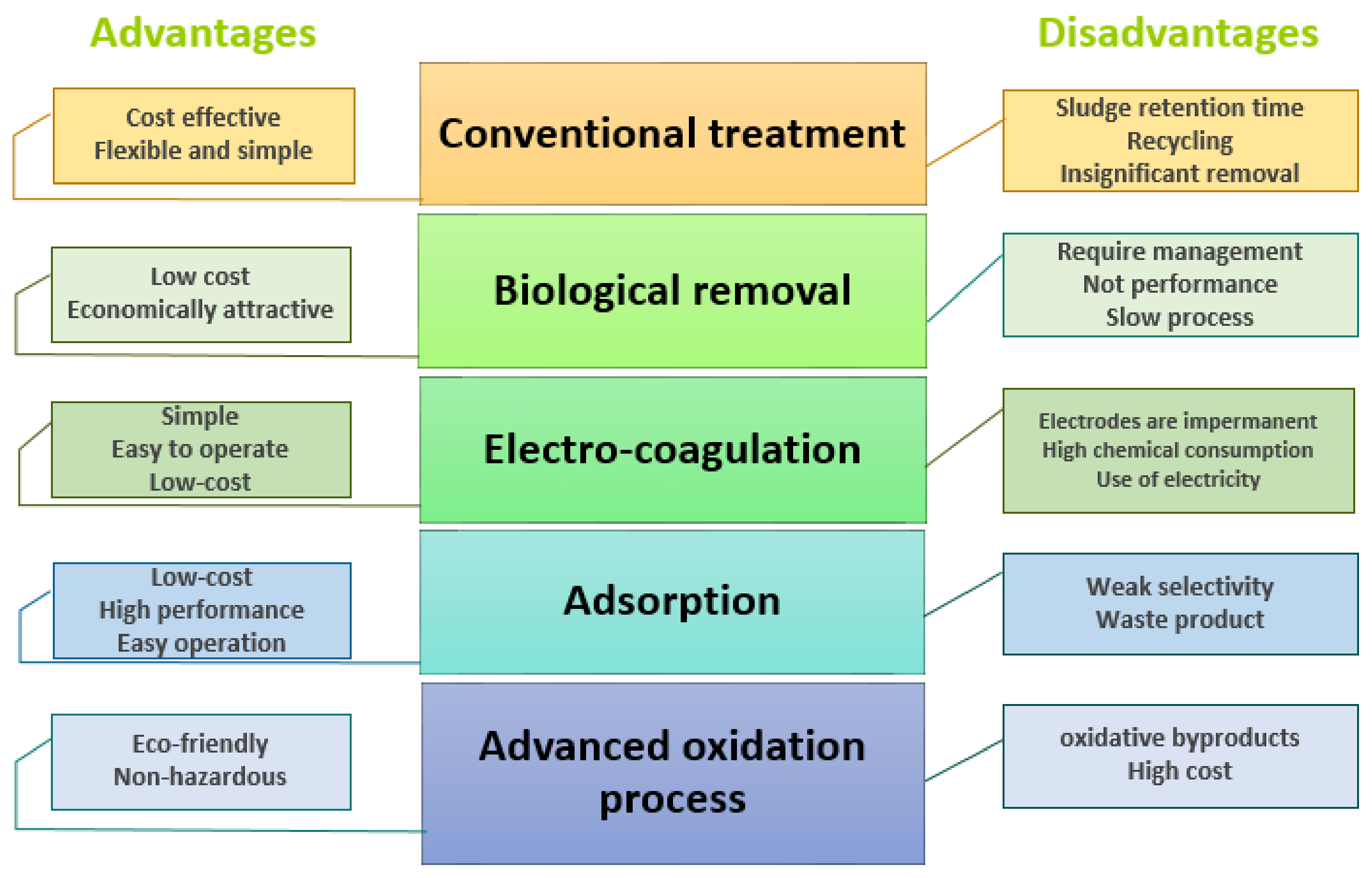
4. Removal of Pharmaceuticals during Wastewater Treatment
4.1. Conventional Treatments
4.2. Biological Removal of the Pharmaceuticals
4.3. Electrocoagulation Process
4.4. Sorption Process for the Removal of Pharmaceutical Products
4.4.1. Activated Carbon
4.4.2. Clays
4.4.3. Biochar
| Operation Process | Drugs | Adsorption Process | Scale | Matrix | Experience Time (min) | Reactor Volume (mL) | pH | T(°C) | BET (m2/g) | Capacity (mg/g) | Significant Findings | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch adsorption | Ibuprofen Naproxen Diclofenac | Green-synthesized copper nanoparticles (Cu NPs) | Lab-scale | Wastewater | 60 | 1000 | 4,5 | 24,85 | 33.9 33.9 36 | The percentage of removal of IBU for about 74.4%, for DIC about 91.4% and NPX about 86.9%. | [77] | |
| Naproxen | Ag-RGO Nano-composite film | Lab-scale | Aqueous solution | 3 | 50 | 4.5 | 24,85 | 229.25 | 92.62% of removal. | [76] | ||
| AC from gooseberry seed-shells | Lab-scale | Water treatment plants | 15 | 50 | 4.40 | 1328 | 154.98 | 61.99% of removal. | [87] | |||
| GB-GP-AgNs | 645 | |||||||||||
| Ibuprofen Amoxicillin | OP: Physical activation of the carbonized precursor using CO2 | Lab-scale | Aqueous solution | 60 | 100 | 9.5 | 25 | 1055 | 86.2 | IBU is more adsorbed than AMX. | [8] | |
| OC: Chemical activation of the raw precursor using phosphoric acid | 5.6 | 1106 | 78.8 | |||||||||
| OPox: Oxidation of OP in a saturated solution of ammonium persulfate in 4 N sulfuric acid | 3.4 | 903 | 75.4 | |||||||||
| Diclofenac | Cyclamen persicum tubers activated carbon (CTAC) | Lab-scale | Aqueous solution | 120 | 50 | 6 | 15 | Zinc chloride activated: 880.936 | Zinc chloride activated: 606.78 | Elimination of 81% when DIC concentration was 70 mg/L and 0.7 g CTAC. | [2] | |
| Physical activation: 799.028 | Physical activation: 522.07 | |||||||||||
| Gemfibrozil Mefenamic acid Naproxen | Exfoliated vermiculite | Lab-scale | Aqueous solution | 120 | 5000 | 7.47 | 20 | LECA have a higher absolute removal of drugs then exfoliated vermiculite. | [84] | |||
| LECA | 9.04 | |||||||||||
| Naproxen Clofidric acid | Aminomethanesulfonic acid (AMSA- MIL-101) | Lab-scale | Deionized water | 1.10 | 50 | <4 >6 | 25 | 2322 | 3764 | A higher rate constant for adsorption (k2) was found for ED-MIL-101 compared to the virgin and AMSA-MIL-101. | [81] | |
| ethylenediamine (ED- MIL-101) | 1.17 | 2555 | 4159 | |||||||||
| Salicylic acid Ibuprofen | Pine wood biochar | Lab-scale | Aqueous solution | 1000 | 2.5 3 | 45 35 | 1.35 | 10.74 | Methanol was able to achieve 93% and 88% desorption of salicylic acid and ibuprofen. | [88] | ||
| Naproxen | Waste apricot | Lab-scale | Aqueous solution | 60 | 1000 | 5.82 | 25 | 1060 | 106.38 | For the removal of naproxen, the Langmiur equation that best represents the equilibrium data was found. | [75] | |
| Column adsorption | Ibuprofen Diclofenac Naproxen Ketoprofen | Olive-waste cake | Lab-scale | Deionized water | 19.13 17.00 12.07 11.20 | 300 | 4.1 | 25 | 793 | 10.83 56.17 39.52 24.69 | 90.45% of removal for NPX 88.40% of removal for ketoprofen 70.07% of removal for IBU. | [79] |
| Ibuprofen | Activated carbon cloth | Lab-scale | Distilled water | 600 | 12.5 | 7 | 25 | 1910 | 491.9 | The adsorption kinetics of IBP on the raw carbon cloth were found to be accelerated by the decrease in pH. | [78] |
4.5. Removal of Different Pharmaceuticals by Advanced Oxidation Processes
5. Various Methods for the Removal of Pharmaceuticals from Waters
| Operation Process | Drugs | AOPs | Scale | Matrix | Experiment Duration (min) | Reactor Volume (mL) | Significant Findings | References |
|---|---|---|---|---|---|---|---|---|
| Photocatalysis | Acetaminophen ACE | Mixed Ti-Zr metal-organic-frameworks | Lab-scale | Aqueous solution | 180 | 150 | 90% of removal for ACE. | [96] |
| TiO2 nanotubes photo catalysts | Lab-scale | Aqueous solution | 120 | 12 | The maximum conversion value for elimination was reached at pH = 3 | [99] | ||
| Paracetamol | TiO2 nanoparticles and TiO2/ under UV and sunlight irradiation | Lab-scale | Ultrapure water | 150 | 1500 | More than 90% of degradation pH optimum = 9 | [98] | |
| Aspirin | TiO2-based nanosheets | Lab-scale | Aqueous solution | 210 | 30 | 100% of degradation 87.8% after 120 min | [125] | |
| Ibuprofen IBU | TiO2/ UV-LED | Lab-scale | Ultrapure water, municipal wastewater, pharmaceutical industry wastewater | 30 | 250 | 42% of removal for ultrapure water, 18% degradation for municipal wastewater and only 9% removal for PIWW | [100] | |
| Diclofenac | TiO2 suspensions | Lab-scale | Aqueous solution | 120 | 50 | 100% of degradation | [103] | |
| Azithromycin Trimethoprim Ofloxacin Sulfamethoxazole | TiO2/ UVA-LEDs | Lab-scale | Secondary urban wastewater | 60 | 150 | Total degradation | [126] | |
| Metronidiazole Amoxicillin | UV/TiO2 | Lab-scale | Aqueous solution | 120 | 1000 | More than 70% of degradation | [110] | |
| Metronidiazole | UV/TiO2 | Lab-scale | Water matrix | 30 | 1000 | Degradation of MNZ decreased in the presence of H2PO4−, Fe3+, and humic acid (HA) | [111] | |
| Ciptoflaxacin | TiO2/UVA ZnO/ UVA | Lab-scale | Ultra-pure water | 6 | 50 | Efficiency of degradation of CIP for two catalysts | [109] | |
| Amoxicillin | Carbon quantum dots modified K2Ti6O13 nanotubes/ UVA | Lab-scale | Deionized water | 90 | 50 | Completely removal of AMX. | [106] | |
| Amoxicillin Ampicillin Cloxacillin | UV/ZnO | Lab-scale | Aqueous solution | 180 | 500 | Total degradation for pH = 11. | [107] | |
| Penicillin G | Ti3+ self-doped TiO2 | Lab-scale | MQ-water | 120 | 50 | 98.3% elimination of penicillin G. | [108] | |
| Furosemide Ranitidine Ofloxacine Phenazone Naproxen Carbamazepine Clofibric acid | TiO2 | Lab-scale | Aqueous solution | 900 | 200 | Degradation of all pharmaceutical products. | [113] | |
| Carboplatin | TiO2/UV | Lab-scale | Aqueous solution | 30 | 250 | 100% of degradation | [127] | |
| Ifosfamide Cyclophosphamide | Pt-doped TiO2 TiO2 | Lab-scale | Aqueous solution | 240 | 50 | 92% removal for cyclophosphamide and 95% removal for ifosfamide. | [128] | |
| Cytarabine | TiO2/ solar light | Lab-scale | Aqueous solution | 360 | 100 | Cytarabine was degraded in all experimental conditions. | [123] | |
| Methotrexate Doxorubicin | TiO2 Degussa P25 | Lab-scale | Ultrapure water | 60 15 | 5 | 60% of removal for methotrexate 43% of removal for doxorubicin | [129] | |
| Chlorhexidine digluconate | TiO2 | Technical scale | Aqueous solution | 60 | 100 | 99% of removal. | [130] | |
| Carbamazepine Gabapentin Lamotrigine Oxcarbazepine Venlafaxine Bisoprolol Celiprolol Talinolol Bezafibrate Tramadol Candesartan Eprosartan Irbesartan Valsartan | ZnO and TiO2 under UVA radiation | Lab-scale | Wastewater treatment plants | 30 | 100 | ZnO shows a higher degradation of pharmaceutical products than TiO2 | [131] | |
| Photolysis and photocatalysis | Propranolol Carbamazepine Diclofenac | TiO2/solar light | Lab-scale | Wastewater effluent | 5760 | 500 | 100% for propranolol, 100% for diclofenac; 76 ± 3% for carbamazepine | [102] |
| Naproxen | Direct photolysis and TiO2/UV | Lab-scale | Aqueous solution | 180 | 120 | 83% after photolysis 98% after photo catalysis | [104] | |
| Direct photolysis/ TiO2/UV | Lab-scale | Aqueous solution | 180 | 30 | 90% after photolysis 40% after photo catalysis | [23] | ||
| Propranolol | Direct photolysis | Technical- scale | Ultrapure water | 240 | 10000 | 71% of removal | [114] | |
| Lab-scale | 77% of removal | |||||||
| TiO2 | Technical-scale | 1000 | 81% of removal | |||||
| Lab-scale | 94% of removal | |||||||
| Oxolinic acid | TiO2 | Lab-scale | Ultrapure water | 30 | 100 | TiO2 photocatalysis can be an efficient methods and rapid way for the elimination of oxolinic acid. | [132] | |
| Photo-Fenton and photocatalysis | Ibuprofen Carbamazepine Ciprofloxacin | Photo-assisted H2O2/ UV AOP | Lab-scale | Real urban wastewater | 40 20 | 700 | 89.83 and 100% degradation for IBU 80.14 to 100% for carbamazepine 100% removal for CIP | [101] |
| Antipyrine | UV-A-LED-photo Fenton reaction | Lab-scale | Aqueous solution | 2.5 | 150 | 100% of degradation after 2.5 min. 93% of mineralization at 60 min. | [117] | |
| Cloxacillin | TiO2/ photo-Fenton process | Lab-scale | Synthetic pharmaceutical wastewater | 240 | 150 | Total degradation for antibiotic. | [116] | |
| Photo-Fenton | Tetracycline | Fe(NO3)3/ solar and black light irradiation | Lab-scale | STP effluents | 1.5 | 500 | Total degradation of TC. | [105] |
| Amoxicillin Bezafibrate Paracetamol | H2O2/ solar and black light irradiation | Lab-scale | Aqueous solution | 1 | 800 | With FeOx 84% removal for amoxicillin or 62% was observed using Fe(NO3)3; 98% of removal for bezafibrate and paracetamol in the presence of Fe(NO3)3. | [97] | |
| 5-fluorouracil | (Fe3+/H2O2)/ ([Fe(C2O4)3] 3− /H2O2, Fe3+/S2O8 2−) | Lab-scale | Ultrapure water | 20–60 | 100 | In this study, it was found that the ferrioxalate system was more efficient for the degradation and mineralization of 5- fluorouracil. | [133] | |
| Ozonation | Antibiotics, steroid hormone, lipid regulator, antineoplastic, non-steroidal anti-inflammatory drug, and psychostimulant | Dose of ozone | Lab-scale | Synthetic wastewater, surface water, and the effluent of wastewater treatment plant | 1 | 2000 | The optimum dose of ozone was found to be yielding of 118.1, 222.3, and 222.4 mg/h, respectively, for synthetic waste water, surface water, and effluent of wastewater treatment plants. Ozonation experiments were carried out at 20 °C and at a pH of 8. This experience eliminated >99.9% of removal for most of the studied pharmaceuticals. The increased toxicity for aqueous solutions of acidic pharmaceuticals at a specific ozone dose of 2.24 mg O3/mg DOC was due to formation of more toxic byproducts. | [118] |
| Indomethacin | Ozone and hydroxyl radicals | Lab-scale | Ultrapure water | 7 | 300 | This drug was eliminated within 7 min under the lowest ozone dose, but TOC removal was only 50% even under the highest ozone dose used in the experiments | [122] | |
| Amoxicillin | medium-high frequency ultrasonic irradiation and/or ozonation | Lab-scale | Aqueous solution | 90 | 250 | The highest removal was achieved at 575 kHz ultrasonic frequency (>99%) with the highest pseudo first order reaction rate constant 0.04 min at pH 10. | [134] | |
| Bezafibrate | Dose of ozone | Lab-scale | Aqueous solution | 10 | 800 | Total degradation after 10 min with a dose of ozone = 0.73 mmol/L | [119] | |
| Bisphenol A 17β-Estradiol, 17α-Ethynyl Estradiol | Ozone | Lab-scale | Aqueous solution | 10 | 40 | Total removal for drugs and a rapid transformation in 10 min. | [120] | |
| Pharmaceutical and personal care products (PPCPs) | Ozone | lab-scale | Aqueous solution | 120 | 680 | 99% of elimination for all pharmaceuticals products. | [121] |
6. AOPs/Adsorption as a Promising Alternative Treatment Technique
| Operation Process | Drugs | Combining Process | Scale | Matrix | Experience Time (Min) | Reactor Volume (mL) | Significant Findings | References |
|---|---|---|---|---|---|---|---|---|
| Ozone oxidation combined to adsorption | Acetaminophen Amoxicillin | Ozone gas diffusion | Technical scale | Aqueous solution | 25 | 2100 | 84.8% of removal 82.7% of removal | [137] |
| Acetaminophen Amoxicillin | Cross-linked chitosan/bentonite | 25 | 200 | Total removal of two drugs | ||||
| Sulfamethoxazole | PAC and Fe2O3/CeO2 loaded activated carbon MOPAC | Lab-scale | Aqueous solution | 30 | 100 | Sulfamethoxazole was highly removed by combining adsorption activated carbon MOPAC with ozonation at pH = 3.5. | [139] | |
| Ozone | 5 | 250 | ||||||
| Photocatalysis Combined to adsorption | Ciptoflaxacin | Graphitized mesoporous carbon | Lab-scale | Aqueous solution | 10 | 100 | Totally mineralized in 1.5 h. | [112] |
| TiO2 | 20 | 200 | ||||||
| UV/O3 process combined to adsorption | Bizafibrate, clofibric acid, carbamezapine, caffeine, chloramphenicol, N,Ndiethyl-meta-tolumide, diclofenac, gemfibrozil, mefenamic acid, metoprolol, propanolol, sulpiride, trimethoprim | UV and ozonation | Technical-scale | WWTPs | 40 | 6 | DF, TP, CP, and CBZ were removed about 80%. SP, MA, CA, PNN, and MTP are efficiently removed. | [140] |
| Photolysis combined to adsorption | Diclofenac, Etafenamete, Ibuprofen, ketoprofen, fluoxetine, clorazepate, hydroxyzine, indapamide, analapril, captopril, atenolol, cloridric-acid, ampicillin | Sludge adsorption | Technical-scale | Wastewater treatment plant | 2 weeks | 600 m3 | Incomplete removal of diclofenac. Removal of pharmaceuticals by adsorption was about 33% and UV was 22%. | [141] |
| UV radiation with hydrogen peroxide | benzene, toluene and chlorobenzene | UV/H2O2 with aeration | Technical-scale | Ultrapure water | 4 months | 1000 | High efficiency of both techniques for the removal of drugs. Removal efficiency ringing from 72% to 99%. | [149] |
| Electrooxidation-ozonation | Ciprofloxacin | Ti/PbO2 | Technical-scale | Aqueous solution | 90 | 280 | 90% of removal | [147] |
| Photo-Fenton combined to adsorption | Sulfamethazine Caffeine, Tamoxifen Ketoprofen Sulfamethoxazole Mepanypirim Diclofenac Chlotianidin Amoxicillin Venlafaxine Fenofibric acid Carbamazepine Atenolol | GAC/photo-Fenton | Lab-scale Technical-scale | Biological domestic wastewater treatment plant | 120 | 6000 | 87.47% of removal 93.64% of removal 37.91% of removal 100% of removal 99.98% of removal 100% of removal 96.67% of removal 100% of removal 100% of removal 100% of removal 100% of removal 99.97% of removal 100% of removal | [145] |
| Photocatalysis combined to adsorption | Ibuprofen | TiO2/boron nitride (BN) nanocomposites | Lab-scale | Aqueous solution | 90 | 100 | Efficiency of the combination for the removal of ibuprofen | [148] |
| Acetaminophen and hydroxyphenylacetic acid (p-HPA) | BiOClxIy nanospheres | Lab-scale | Aqueous solution | 180 | 50 | 100% removal of p-HPA and 80% removal of acetaminophen. | [146] | |
| Persulfate oxidation process combined to adsorption | sulfadimethoxine (SDM), sulfamonomethoxine (SMM), and sulfachloropyridazine (SCP) | MIL-101(Cr) | Lab-scale | Deepwater | 90 | 1000 | The adsorption capacity of MIL-101(Cr) decreased with the increase of oxidation times. The maximum adsorption capacities to SDM, SMM, and SCP were 588, 196, and 196 mg/g, respectively, by Langmuir at 25 °C | [150] |
| Ozonation and sonolysis | Diclofenac, Sulfamethoxazole, Carbamazepine | Ozonation and ultrasound (O3/US) | Lab-scale | Wastewater | 120 | 8000 | The remove efficiency yielding from 80% to 84% | [151] |
7. Conclusions, Future Perspectives, and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AC: | activated carbon |
| ACE: | acetaminophen |
| AMX: | amoxicillin |
| AOPs: | advanced oxidation process |
| AUJ: | Aurajok |
| AZT: | azithromycin |
| B: | bentonite |
| BF: | Bezafibrate |
| CA: | Clofibric acid |
| CBZ: | carbamezapine |
| CF: | Caffeine |
| CIP: | Ciptoflaxacin |
| COD: | Chemical oxygen demand |
| CP: | Chloramphenicol |
| CTAC: | Cyclamen persicum tubers based activated carbon |
| CTC: | Chloitetracycline-HCI |
| CuNPs: | Copper nanoparticles |
| DEET: | N,N-diethyl-meta-tolumide |
| DIC: | Diclofenac |
| DMC: | Democlocycline-HCI |
| DXC: | Doxycycline-hyclate |
| EDCs: | Endocrine disruption coumpounds |
| GAC: | Granular activated carbon |
| Gf: | Gemfibrozil |
| IBU: | Ibuprofen |
| K: | kaolinite |
| Kyj: | kyronjoki |
| KOJ: | Kokemäenjoki |
| MA: | Mefenamic acid |
| MCC: | Meclocycline-sulfosalicylate |
| MDD: | major depressive disorder |
| MNC: | Minocycline-HCI |
| MNZ: | metronidiazole |
| MT: | Montmorillonite |
| MTP: | Metoprolol |
| MPs: | Microplastics |
| NPX: | Naproxen |
| NSAIDs: | Non-Steroidalanti-inflammatory drugs |
| OCD: | obsessive-compulsive disorder |
| OFX: | oflaxacin |
| OTC: | Oxytetracycline-HCI |
| PACI: | Poly-aluminuim chloride |
| PEC: | Photo-electrocatalytic |
| PMDD: | premenstrual dysphoric disorder |
| PPCPs: | Pharmaceuticals and personal care products |
| PPN: | Propanolol |
| SEJ: | Seinajok |
| SMX: | Sulfamethoxazole |
| SP: | Sulpiride |
| TAs: | tetracycline classes of antibiotics |
| TC: | tetracycline |
| TMP: | trimethoprim |
| UK: | united Kingdom |
| US: | united States |
| UVA: | ultraviolet A |
| VER: | vermiculite |
| WW: | waste water |
| WWT: | waste water treatment |
| WWTP: | waste water treatment plants |
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Jodeh, S.; Abdelwahab, F.; Jaradat, N.; Warad, I.; Jodeh, W. Adsorption of diclofenac from aqueous solution using Cyclamen persicum tubers based activated carbon (CTAC). J. Assoc. Arab. Univ. Basic Appl. Sci. 2016, 20, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Kalyva, M. Fate of Pharmaceuticals in the Environment—A Review; Dept. of Ecology and Environmental Science (EMG) S-901 87 Umeå: Vasterbotten County, Sweden, 2017; p. 30. [Google Scholar]
- Roberts, P.; Thomas, K. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total. Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total. Environ. 2020, 744, 140997. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Szabó, R.K.; Megyeri, C.; Illés, E.; Gajda-Schrantz, K.; Mazellier, P.; Dombi, A. Phototransformation of ibuprofen and ketoprofen in aqueous solutions. Chemosphere 2011, 84, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.; Carmona, R.J.; Gomis-Berenguer, A.; Souissi-Najar, S.; Ouederni, A.; Ania, C.O. Competitive adsorption of ibuprofen and amoxicillin mixtures from aqueous solution on activated carbons. J. Colloid Interface Sci. 2015, 449, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovic, M.; de Alda, M.J.L.; Diaz-Cruz, S.; Postigo, C.; Radjenovic, J.; Gros, M.; Barcelo, D. Fate and removal of pharmaceuticals and illicit drugs in conventional and membrane bioreactor wastewater treatment plants and by riverbank filtration. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2009, 367, 3979–4003. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef]
- Klein, E.Y.; van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total. Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Hapeshi, E.; Achilleos, A.; Meric, S.; Gros, M.; Petrovic, M.; Barcelo, D. Existence of Pharmaceutical Compounds in Tertiary Treated Urban Wastewater that is Utilized for Reuse Applications. Water Resour. Manag. 2011, 25, 1183–1193. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, R.; Yuesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Mesdaghinia, A. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci. Total Environ. 2018, 619–620, 446–459. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, M.; Lei, J.; Fu, C.; Gu, J.; Xu, G. The fate and risk assessment of psychiatric pharmaceuticals from psychiatric hospital effluent. Ecotoxicol. Environ. Saf. 2018, 150, 289–296. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Prata, L.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to Covid-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2020, 405, 126683. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, R.R.; Jamshidi, A.; Mofrad, M.M.G.; Jafari, A.J.; Heidari, N.; Fallahizadeh, S.; Arani, M.H.; Torkashvand, J. Effect of COVID-19 pandemic on medical waste management: A case study. J. Environ. Health Sci. Eng. 2021, 19, 831–836. [Google Scholar] [CrossRef]
- Haque, M.d.S.; Uddin, S.; Sayem, S.M.; Mohib, K.M. Coronavirus disease 2019 (COVID-19) induced waste scenario: A short overview. J. Environ. Chem. Eng. 2021, 9, 104660. [Google Scholar] [CrossRef]
- El Majid, B.; El Hammoumi, A.; Motahhir, S.; Lebbadi, A.; El Ghzizal, A. Preliminary design of an innovative, simple, and easy-to-build portable ventilator for COVID-19 patients. Euro-Mediterr. J. Environ. Integr. 2020, 5, 23. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, X.; Geng, J.; Li, S.; Wu, G.; Ren, H. Degradation of three nonsteroidal anti-inflammatory drugs by UV/persulfate: Degradation mechanisms, efficiency in effluents disposal. Chem. Eng. J. 2019, 356, 1032–1041. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Gimenez, J.; Esplugas, S. Photolysis and TiO2 Photocatalytic Treatment of Naproxen: Degradation, Mineralization, Intermediates and Toxicity. J. Adv. Oxid. Technol. 2008, 11, 435–444. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Suwalsky, M.; Villena, F.; Garidel, P. Effects of the nonsteroidal anti-inflammatory drug naproxen on human erythrocytes and on cell membrane molecular models. Biophys. Chem. 2010, 147, 53–58. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Naproxen abatement by thermally activated persulfate in aqueous systems. Chem. Eng. J. 2015, 279, 861–873. [Google Scholar] [CrossRef]
- Lubet, R.A.; Scheiman, J.M.; Bode, A.; White, J.; Minasian, L.; Juliana, M.M.; Grubbs, C.J. Prevention of Chemically Induced Urinary Bladder Cancers by Naproxen: Protocols to Reduce Gastric Toxicity in Humans Do Not Alter Preventive Efficacy. Cancer Prev. Res. (Phila. Pa.) 2015, 8, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Moslah, B.; Hapeshi, E.; Jrad, A.; Fatta-Kassinos, D.; Hedhili, A. Pharmaceuticals and illicit drugs in wastewater samples in north-eastern Tunisia. Environ. Sci. Pollut. Res. 2018, 25, 18226–18241. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Rhee, M.-Y.; Lee, S.Y.; Park, S.W.; Jeon, D.; Kim, B.-W.; Kwan, J.; Choi, D. A prospective, randomized, open-label, active-controlled, clinical trial to assess central haemodynamic effects of bisoprolol and atenolol in hypertensive patients. J. Hypertens. 2013, 31, 813–819. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef]
- Radjenovic, J.; Petrovic, M.; Barceló, D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ. Int. 2007, 33, 596–601. [Google Scholar] [CrossRef]
- Terzić, S.; Senta, I.; Ahel, M.; Gros, M.; Petrovic, M.; Barcelo, D.; Muller, J.; Knepper, T.; Marti, I.; Ventura, F.; et al. Occurrence and fate of emerging wastewater contaminants in Western Balkan Region. Sci. Total Environ. 2008, 399, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalination Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Göbel, A.; McArdell, C.S.; Suter, M.J.-F.; Giger, W. Trace Determination of Macrolide and Sulfonamide Antimicrobials, a Human Sulfonamide Metabolite, and Trimethoprim in Wastewater Using Liquid Chromatography Coupled to Electrospray Tandem Mass Spectrometry. Anal. Chem. 2004, 76, 4756–4764. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Microbial degradation of sulfamethoxazole in the environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef]
- Puckowski, A.; Cwięk, W.; Mioduszewska, K.; Stepnowski, P.; Białk-Bielińska, A. Sorption of pharmaceuticals on the surface of microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef]
- Chouchene, K.; Rocha-Santos, T.; Ksibi, M. Types, occurrence, and distribution of microplastics and metals contamination in sediments from south west of Kerkennah archipelago, Tunisia. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Prinz, N.; Korez, Š. Understanding How Microplastics Affect Marine Biota on the Cellular Level Is Important for Assessing Ecosystem Function: A Review. In YOUMARES 9-The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 101–120. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Tang, S.; Wang, X.S.; Sun, X.; Han, Z.; Chen, Y. Accumulation mechanism of tetracycline hydrochloride from aqueous solutions by nylon microplastics. Environ. Technol. Innov. 2020, 18, 100750. [Google Scholar] [CrossRef]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: Concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.-E.; Fick, J. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities—A Study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar] [CrossRef]
- Kumar, N.N.; Aiyagari, N. Artificial Recharge of Groundwater. Groundwater Pollution Primer. Available online: https://leg.mt.gov/content/Committees/Interim/2005_2006/environmental_quality_council/eetings/minutes/eqc09112006_ex20.pdf (accessed on 7 June 1998).
- Skoczko, I.; Puzowski, P.; Szatyłowicz, E. Experience from the Implementation and Operation of the Biological Membrane Reactor (MBR) at the Modernized Wastewater Treatment Plant in Wydminy. Water 2020, 12, 3410. [Google Scholar] [CrossRef]
- Liao, B.; Bokhary, A.; Cui, L.; Lin, H. A Review of Membrane Technology for Integrated Forest Biorefinery. J. Membr. Sci. Res. 2017, 3, 120–141. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.J.; Scrimshaw, M.D.; Lester, J.N.; Cartmell, E. Fate of drugs during wastewater treatment. TrAC Trends Anal. Chem. 2013, 49, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Tauxe-Wuersch, A.; de Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Koenig, B.G.; Bennie, D.T.; Servos, M.; Ternes, T.A.; Hirsch, R. Occurrence Of Neutral And Acidic Drugs In The Effluents Of Canadian Sewage Treatment Plants. Environ. Toxicol. Chem. 2003, 22, 2872. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Strenn, B.; Clara, M.; Gans, O.; Kreuzinger, N. Carbamazepine, diclofenac, ibuprofen and bezafibrate--investigations on the behaviour of selected pharmaceuticals during wastewater treatment. Water Sci. Technol. 2004, 50, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.-J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of Pharmaceuticals during Drinking Water Treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef]
- Kreuzinger, N.; Clara, M.; Strenn, B.; Kroiss, H. Relevance of the sludge retention time (SRT) as design criteria for wastewater treatment plants for the removal of endocrine disruptors and pharmaceuticals from wastewater. Water Sci. Technol. 2004, 50, 149–156. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [Green Version]
- Kooijman, G.; de Kreuk, M.K.; Houtman, C.; van Lier, J.B. Perspectives of coagulation/flocculation for the removal of pharmaceuticals from domestic wastewater: A critical view at experimental procedures. J. Water Process Eng. 2020, 34, 101161. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total. Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-J.; Kim, S.-G.; Kim, S.-H. Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater. 2008, 151, 38–43. [Google Scholar] [CrossRef]
- Martínez-Espinosa, R.M. Microorganisms and Their Metabolic Capabilities in the Context of the Biogeochemical Nitrogen Cycle at Extreme Environments. Int. J. Mol. Sci. 2020, 21, 4228. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.; Bautista, L.F.; Catala, M.; de las Heras, M.R.; Martinez-Hidalgo, P.; San-Sebastian, J.; Gonzalez-Benitez, N. From Laboratory Tests to the Ecoremedial System: The Importance of Microorganisms in the Recovery of PPCPs-Disturbed Ecosystems. Appl. Sci. 2020, 10, 3391. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Sarvajith, M.; Mohan, T.V.K. Aerobic Granular Sludge:The Future of Wastewater Treatment. Curr. Sci. 2019, 117, 395. [Google Scholar] [CrossRef]
- Muter, O.; Perkons, I.; Selga, T.; Berzins, A.; Gudra, D.; Radovica-Spalvina, I.; Bartkevics, V. Removal of pharmaceuticals from municipal wastewaters at laboratory scale by treatment with activated sludge and biostimulation. Sci. Total Environ. 2017, 584–585, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Marchlewicz, A.; Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Bacillus thuringiensis B1(2015b) is a Gram-Positive Bacteria Able to Degrade Naproxen and Ibuprofen. Water Air Soil Pollut. 2016, 227, 197. [Google Scholar] [CrossRef] [Green Version]
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef]
- Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; -Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O.; et al. A Review on Recent Advances in Stabilizing Peptides/Proteins upon Fabrication in Hydrogels from Biodegradable Polymers. Pharmaceutics 2018, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Barnes, P.W.; Robson, T.M.; Neale, P.J.; Williamson, C.E.; Zepp, R.G.; Wilson, S.R.; Madronich, S.; Andrady, A.L.; Heikkilä, A.M.; et al. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020. Photochem. Photobiol. Sci. 2021, 20, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Yasri, N.; Hu, J.; Kibria, M.G.; Roberts, E.P.L. Electrocoagulation Separation Processes. In ACS Symposium Series; Chernyshova, I., Ponnurangam, S., Liu, Q., Eds.; American Chemical Society: Washington, DC, USA, 2020; Volume 1348, pp. 167–203. [Google Scholar] [CrossRef]
- Aitbara, A.; Khelalfa, A.; Bendaia, M.; Abrane, R.; Amrane, A.; Hazourli, S. Treatment of dairy wastewater by electrocoagulation using A-U4G (2017-Al) alloy and pure aluminum as electrode material. Euro-Mediterr. J. Environ. Integr. 2021, 6, 19. [Google Scholar] [CrossRef]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Ensano, B.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.; Ballesteros, F. Removal of Pharmaceuticals from Wastewater by Intermittent Electrocoagulation. Water 2017, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of pharmaceutical from water with an electrocoagulation process; effect of various parameters and studies of isotherm and kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Yoosefian, M.; Ahmadzadeh, S.; Aghasi, M.; Dolatabadi, M. Optimization of electrocoagulation process for efficient removal of ciprofloxacin antibiotic using iron electrode; kinetic and isotherm studies of adsorption. J. Mol. Liq. 2017, 225, 544–553. [Google Scholar] [CrossRef]
- Nageeb, M. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. In Organic Pollutants-Monitoring, Risk and Treatment; Rashed, M.N., Ed.; InTech: West Palm Beach, FL, USA, 2013. [Google Scholar] [CrossRef] [Green Version]
- Önal, Y.; Akmil-Başar, C.; Sarıcı-Özdemir, Ç. Elucidation of the naproxen sodium adsorption onto activated carbon prepared from waste apricot: Kinetic, equilibrium and thermodynamic characterization. J. Hazard. Mater. 2007, 148, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Patel, S.; Majumder, S.K. Bio-extract assisted in-situ green synthesis of Ag-RGO nanocomposite film for enhanced naproxen removal. Korean J. Chem. Eng. 2020, 37, 274–289. [Google Scholar] [CrossRef]
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M.F. Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 2019, 5, e02339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N.; Duclaux, L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017, 10, S3584–S3594. [Google Scholar] [CrossRef] [Green Version]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Jafari, K.; Heidari, M.; Rahmanian, O. Wastewater treatment for Amoxicillin removal using magnetic adsorbent synthesized by ultrasound process. Ultrason. Sonochemistry 2018, 45, 248–256. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.-J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Fougère, L.; Destandau, E.; Monnin, L.; Le Milbeau, C. Clay minerals for the removal of pharmaceuticals: Initial investigations of their adsorption properties in real wastewater effluents. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100266. [Google Scholar] [CrossRef]
- Styszko, K.; Nosek, K.; Motak, M.; Bester, K. Preliminary selection of clay minerals for the removal of pharmaceuticals, bisphenol A and triclosan in acidic and neutral aqueous solutions. Comptes Rendus Chim. 2015, 18, 1134–1142. [Google Scholar] [CrossRef]
- Dordio, A.V.; Miranda, S.; Ramalho, J.P.P.; Carvalho, A.J.P. Mechanisms of removal of three widespread pharmaceuticals by two clay materials. J. Hazard. Mater. 2017, 323, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Mondal, S.; Patel, S.; Majumder, S.K. Naproxen Removal Capacity Enhancement by Transforming the Activated Carbon into a Blended Composite Material. Water Air Soil. Pollut. 2020, 231, 37. [Google Scholar] [CrossRef]
- Essandoh, M.; Kunwar, B.; Pittman, C.U.; Mohan, D.; Mlsna, T. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total. Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Ikehata, K.; Naghashkar, N.J.; El-Din, M.G. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone: Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Jadda, A.; Chakraborty, S.; Amar, R.B. Characterization of an asymmetric ultrafiltration membrane prepared from TiO2-smectite nanocomposites doped with commercial TiO2 and its application to the treatment of textile wastewater. Euro-Mediterr. J. Environ. Integr. 2020, 5, 10. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Dionysiou, D.D.; Rodríguez, J.J.; Belver, C. Mixed Ti-Zr metal-organic-frameworks for the photodegradation of acetaminophen under solar irradiation. Appl. Catal. B Environ. 2019, 253, 253–262. [Google Scholar] [CrossRef]
- Trovó, A.G.; Melo, S.A.S.; Nogueira, R.F.P. Photodegradation of the pharmaceuticals amoxicillin, bezafibrate and paracetamol by the photo-Fenton process—Application to sewage treatment plant effluent. J. Photochem. Photobiol. A Chem. 2008, 198, 215–220. [Google Scholar] [CrossRef]
- Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M. Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem. 2017, 10, S3640–S3645. [Google Scholar] [CrossRef] [Green Version]
- Borràs-Ferrís, J.; Sánchez-Tovar, R.; Blasco-Tamarit, E.; Muñoz-Portero, M.J.; Fernández-Domene, R.M.; García-Antón, J. TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Monteoliva-García, A.; Martín-Pascual, J.; Muñío, M.M.; Poyatos, J.M. Removal of carbamazepine, ciprofloxacin and ibuprofen in real urban wastewater by using light-driven advanced oxidation processes. Int. J. Environ. Sci. Technol. 2019, 16, 6005–6018. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 3–4. [Google Scholar] [CrossRef]
- Jallouli, N.; Elghniji, K.; Hentati, O.; Ribeiro, A.R.; Silva, A.M.T.; Ksibi, M. UV and solar photo-degradation of naproxen: TiO2 catalyst effect, reaction kinetics, products identification and toxicity assessment. J. Hazard. Mater. 2016, 304, 329–336. [Google Scholar] [CrossRef]
- Bautitz, I.R.; Nogueira, R.F.P. Degradation of tetracycline by photo-Fenton process—Solar irradiation and matrix effects. J. Photochem. Photobiol. A Chem. 2007, 187, 33–39. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.; Qi, J.; Tong, Y.; Lv, Y.; Xu, C.; Ni, J.; Liu, W. Photocatalytic degradation of amoxicillin by carbon quantum dots modified K2Ti6O13 nanotubes: Effect of light wavelength. Chin. Chem. Lett. 2019, 30, 1214–1218. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef]
- Li, J.; Deng, X.; Guo, R.; Li, B.; Cheng, Q.; Cheng, X. Visible light driven photocatalytic decomposition of penicillin G by Ti3+ self-doped TiO2 nano-catalyst through response surface methodology. J. Taiwan Inst. Chem. Eng. 2018, 87, 174–181. [Google Scholar] [CrossRef]
- Silva, A.R.; Martins, P.M.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Pereira, L.R. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.-C.; Juang, R.-S. Removal of metronidazole and amoxicillin mixtures by UV/TiO2 photocatalysis: An insight into degradation pathways and performance improvement. Environ. Sci Pollut Res. 2019, 26, 11846–11855. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Fu, C.-C.; Juang, R.-S. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Pirillo, F.; Loddo, V.; Palmisano, L. Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal. Today 2006, 118, 205–213. [Google Scholar] [CrossRef] [Green Version]
- De la Cruz, N.; Dantas, R.F.; Giménez, J.; Esplugas, S. Photolysis and TiO2 photocatalysis of the pharmaceutical propranolol: Solar and artificial light. Appl. Catal. B Environ. 2013, 130–131, 249–256. [Google Scholar] [CrossRef]
- Valari, M.; Antoniadis, A.; Mantzavinos, D.; Poulios, I. Photocatalytic reduction of Cr(VI) over titania suspensions. Catal. Today 2015, 252, 190–194. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Giraldo-Aguirre, A.L.; Silva-Agredo, J.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of antibiotic cloxacillin by means of electrochemical oxidation, TiO2 photocatalysis, and photo-Fenton processes: Analysis of degradation pathways and effect of the water matrix on the elimination of antimicrobial activity. Environ. Sci. Pollut. Res. 2017, 24, 6339–6352. [Google Scholar] [CrossRef]
- Davididou, K.; Monteagudo, J.M.; Chatzisymeon, E.; Durán, A.; Expósito, A.J. Degradation and mineralization of antipyrine by UV-A LED photo-Fenton reaction intensified by ferrioxalate with addition of persulfate. Sep. Purif. Technol. 2017, 172, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Almomani, F.A.; Shawaqfah, M.; Bhosale, R.R.; Kumar, A. Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environ. Prog. Sustain. Energy 2016, 35, 982–995. [Google Scholar] [CrossRef]
- Dantas, R.F.; Canterino, M.; Marotta, R.; Sans, C.; Esplugas, S.; Andreozzi, R. Bezafibrate removal by means of ozonation: Primary intermediates, kinetics, and toxicity assessment. Water Res. 2007, 41, 2525–2532. [Google Scholar] [CrossRef]
- Alum, A.; Yoon, Y.; Westerhoff, P.; Abbaszadegan, M. Oxidation of bisphenol A, 17?-estradiol, and 17?-ethynyl estradiol and byproduct estrogenicity. Environ. Toxicol. 2004, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Manterola, G.; Larrea, L.; Ternes, T.; Omil, F.; Lema, J.M. Influence of ozone pre-treatment on sludge anaerobic digestion: Removal of pharmaceutical and personal care products. Chemosphere 2007, 67, 1444–1452. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, J.; Zhang, S.; Li, X.; Wang, B.; Huang, J.; Deng, S.; Wang, Y.; Yu, G. Ozonation of indomethacin: Kinetics, mechanisms and toxicity. J. Hazard. Mater. 2017, 323, 460–470. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Evgenidou, E.; Konstantinou, I.; Lambropoulou, D.A. Cytarabine degradation by simulated solar assisted photocatalysis using TiO2. Chem. Eng. J. 2017, 316, 823–831. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cheng, X.; Yu, X.; Xing, Z. Preparation and characterization of TiO2-based nanosheets for photocatalytic degradation of acetylsalicylic acid: Influence of calcination temperature. Chem. Eng. J. 2015, 279, 994–1003. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Kitsiou, V.; Zachariadis, G.A.; Lambropoulou, D.A.; Tsiplakides, D.; Poulios, I. Mineralization of the antineoplastic drug carboplatin by heterogeneous photocatalysis with simultaneous synthesis of platinum-modified TiO2 catalysts. J. Environ. Chem. Eng. 2018, 6, 2409–2416. [Google Scholar] [CrossRef]
- Ofiarska, A.; Pieczyńska, A.; Borzyszkowska, A.F.; Stepnowski, P.; Siedlecka, E.M. Pt–TiO2-assisted photocatalytic degradation of the cytostatic drugs ifosfamide and cyclophosphamide under artificial sunlight. Chem. Eng. J. 2016, 285, 417–427. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Sarro, M.; Rosato, V.; Aigotti, R.; Baiocchi, C.; Minero, C. Photocatalytic degradation of selected anticancer drugs and identification of their transformation products in water by liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2014, 1362, 135–144. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S.; Bhattacharjee, C. Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photo reactor (PBPR). Ecotoxicol. Environ. Saf. 2015, 121, 263–270. [Google Scholar] [CrossRef]
- Teixeira, S.; Gurke, R.; Eckert, H.; Kühn, K.; Fauler, J.; Cuniberti, G. Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions. J. Environ. Chem. Eng. 2016, 4, 287–292. [Google Scholar] [CrossRef]
- Giraldo, A.L.; Peñuela, G.A.; Torres-Palma, R.A.; Pino, N.J.; Palominos, R.A.; Mansilla, H.D. Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension. Water Res. 2010, 44, 5158–5167. [Google Scholar] [CrossRef] [PubMed]
- Koltsakidou, A.; Antonopoulou, M.; Sykiotou, M.; Εvgenidou, Ε.; Konstantinou, I.; Lambropoulou, D.A. Photo-Fenton and Fenton-like processes for the treatment of the antineoplastic drug 5-fluorouracil under simulated solar radiation. Environ. Sci. Pollut. Res. 2017, 24, 4791–4800. [Google Scholar] [CrossRef]
- Kıdak, R.; Doğan, Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochemistry 2018, 40, 131–139. [Google Scholar] [CrossRef]
- Sundin, A.M.; Linderholm, L.; Hedlund, B.; Joyce, K.B.; Klingspor, K. Advanced Wastewater Treatment for Separation and Removal of Pharmaceutical Residues and Other Hazardous Substances: Needs, Technologies and Impacts: A Government-Commissioned Report; Swedish Environmental Protection Agency (Naturvårdsverket): Stockholm, Sweden, 2017; Report 6803; April 2017; Available online: https://www.naturvardsverket.se/Documents/publikationer6400/978-91-620-6803-5.pdf?pid=21820 (accessed on 15 July 2021).
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Mojiri, A.; Vakili, M.; Farraji, H.; Aziz, S.Q. Combined ozone oxidation process and adsorption methods for the removal of acetaminophen and amoxicillin from aqueous solution; kinetic and optimisation. Environ. Technol. Innov. 2019, 15, 100404. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Oller, I.; Gernjak, W.; Agüera, A.; Malato, S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res. 2009, 43, 661–668. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S.; Aris, A. Combined adsorption and catalytic ozonation for removal of sulfamethoxazole using Fe2O3/CeO2 loaded activated carbon. Chem. Eng. J. 2011, 170, 136–144. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Lu, S.; Deng, S.; Wang, B.; Zhao, W.; Qiu, Z.; Yu, G. Removal of pharmaceutical and personal care products by sequential ultraviolet and ozonation process in a full-scale wastewater treatment plant. Front. Environ. Sci. Eng. 2014, 8, 62–68. [Google Scholar] [CrossRef]
- Salgado, R.; Marques, R.; Noronha, J.P.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant. Environ. Sci. Pollut. Res. 2012, 19, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Szabó, E.; Takács, E.; Wojnárovits, L.; Dombi, A.; Gajda-Schrantz, K. Ketoprofen removal by O3 and O3/UV processes: Kinetics, transformation products and ecotoxicity. Sci. Total. Environ. 2014, 472, 178–184. [Google Scholar] [CrossRef]
- Knopp, G.; Prasse, C.; Ternes, T.A.; Cornel, P. Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Res. 2016, 100, 580–592. [Google Scholar] [CrossRef]
- Brienza, M.; Nir, S.; Plantard, G.; Goetz, V.; Chiron, S. Combining micelle-clay sorption to solar photo-Fenton processes for domestic wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 18971–18978. [Google Scholar] [CrossRef]
- Wang, X.; Bi, W.; Zhai, P.; Wang, X.; Li, H.; Mailhot, G.; Dong, W. Adsorption and photocatalytic degradation of pharmaceuticals by BiOCl x I y nanospheres in aqueous solution. Appl. Surf. Sci. 2016, 360, 240–251. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Nematollahi, D.; Samarghandi, M.R.; Samadi, M.T.; Azarian, G. A combined advanced oxidation process: Electrooxidation-ozonation for antibiotic ciprofloxacin removal from aqueous solution. J. Electroanal. Chem. 2018, 808, 82–89. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Bechalany, M.; Nasr, M.; Jarvis, J.; Schaub, T.; Sapkota, R.R.; Miele, P.; Wang, H.; Xu, P. Adsorption and photocatalytic oxidation of ibuprofen using nanocomposites of TiO2 nanofibers combined with BN nanosheets: Degradation products and mechanisms. Chemosphere 2019, 220, 921–929. [Google Scholar] [CrossRef]
- Lhotský, O.; Krákorová, E.; Mašín, P.; Žebrák, R.; Linhartová, L.; Křesinová, Z.; Kašlík, J.; Steinová, J.; Rodsand, T.; Filipová, A.; et al. Pharmaceuticals, benzene, toluene and chlorobenzene removal from contaminated groundwater by combined UV/H2O2 photo-oxidation and aeration. Water Res. 2017, 120, 245–255. [Google Scholar] [CrossRef]
- Peng, J.; Wu, E.; Wang, N.; Quan, X.; Sun, M.; Hu, Q. Removal of sulfonamide antibiotics from water by adsorption and persulfate oxidation process. J. Mol. Liq. 2019, 274, 632–638. [Google Scholar] [CrossRef]
- Prado, M.; Borea, L.; Cesaro, A.; Liu, H.; Naddeo, V.; Belgiorno, V.; Ballesteros, F., Jr. Removal of emerging contaminant and fouling control in membrane bioreactors by combined ozonation and sonolysis. Int. Biodeterior. Biodegrad. 2017, 119, 577–586. [Google Scholar] [CrossRef]
| Class | Pharmaceuticals | Chemical Formula | Molecular Weight g/mol | Uses | Structure |
|---|---|---|---|---|---|
| Analgesic/ antipyretic | Acetaminophen | C8H9NO2 | 151.16 | Used for mild-to-moderate pain and fever. | 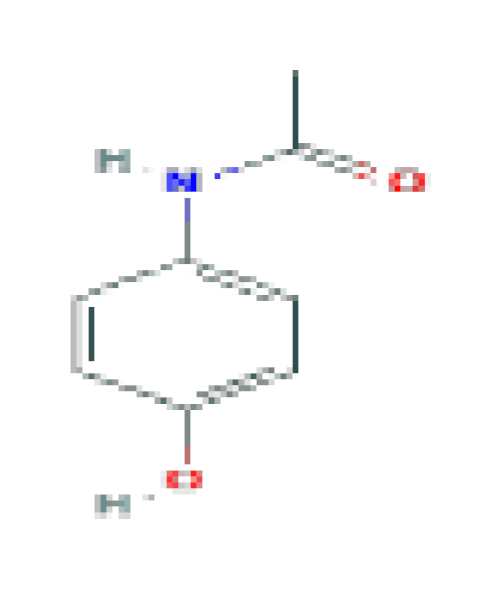 |
| Aspirin | C9H8O4 | 180.16 | Used in the prevention of arterial and venous thrombosis. | 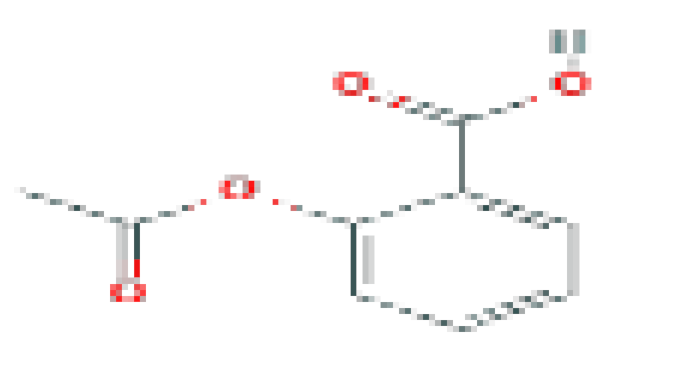 | |
| Lamotrigine | C9H7Cl2N5 | 256.09 | Used antiseizure medication that is a rare but well-known cause of idiosyncratic liver injury that can be severe and even fatal. | 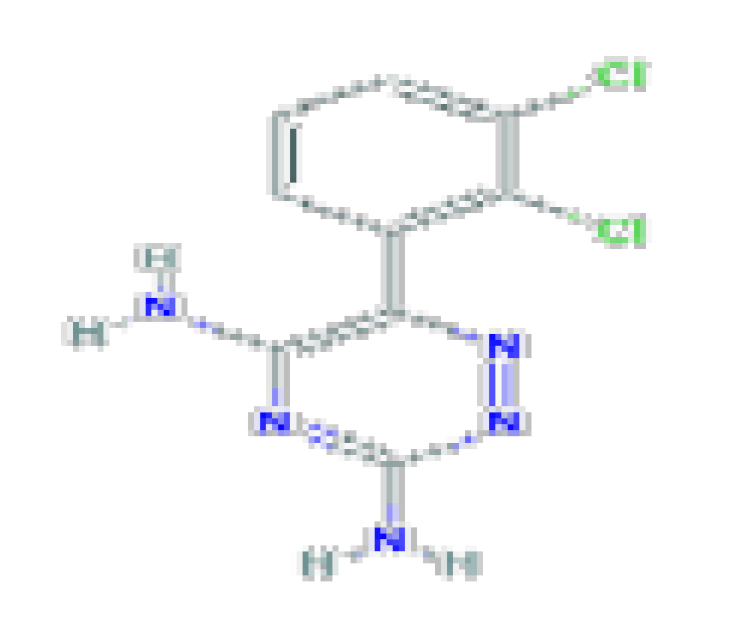 | |
| Tramadol | C16H25NO2 | 263.37 | Used as a narcotic analgesic for severe pain. | 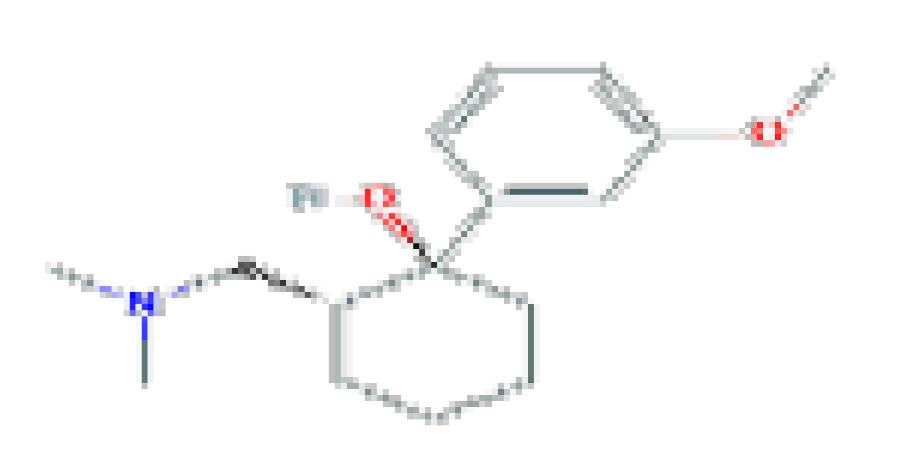 | |
| Antibiotic | Amoxicillin | C16H19N3O5S | 365.4 | Used in the treatment of mild-to-moderate bacterial infections such as sinusitis, bronchitis, otitis media, cellulitis, and community acquired pneumonia. |  |
| Azithromycin | C38H72N2O12 | 749 | Related for the rare instances of acute liver injury. | 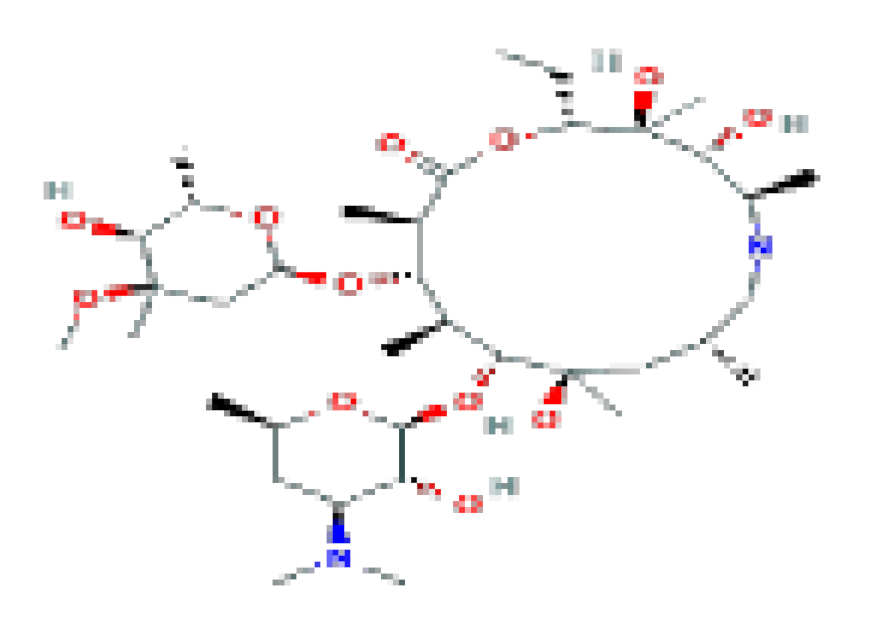 | |
| Doxorubicin | C27H29NO4 | 543.5 | Used in the therapy of several forms of lymphoma, leukemia, sarcoma, and solid organ cancers. | 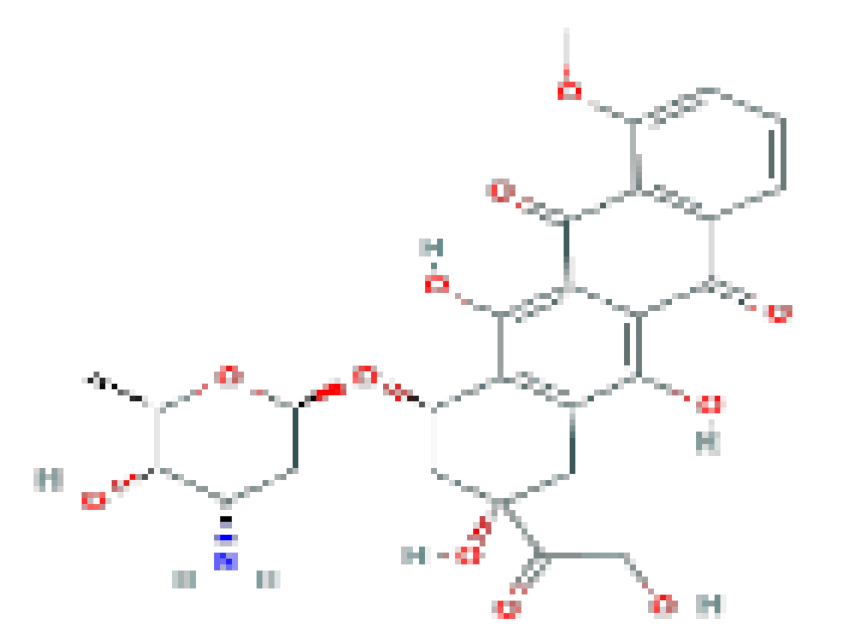 | |
| Ciprofloxacin | C17H18FN3O3 | 331.34 | Used in the therapy of mild-to-moderate urinary and respiratory tract infections caused by susceptible organisms. | 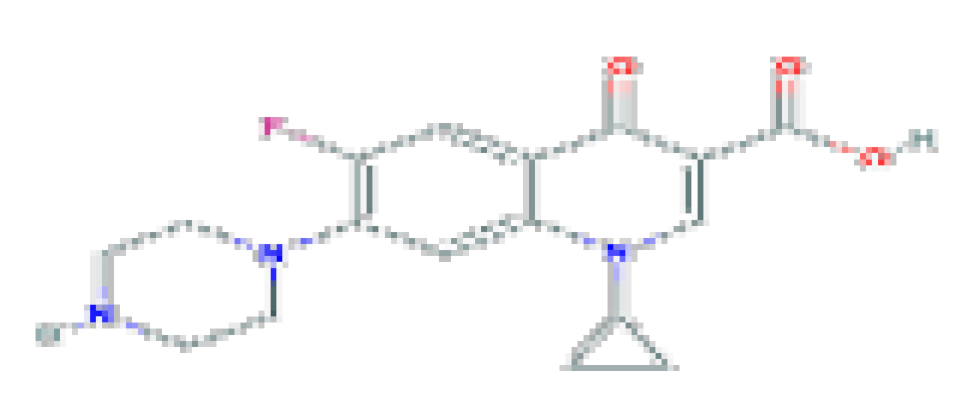 | |
| Metronidazole | C6H9N3O3 | 171.15 | Used in the treatment of many anaerobic and certain protozoan and parasitic infections. | 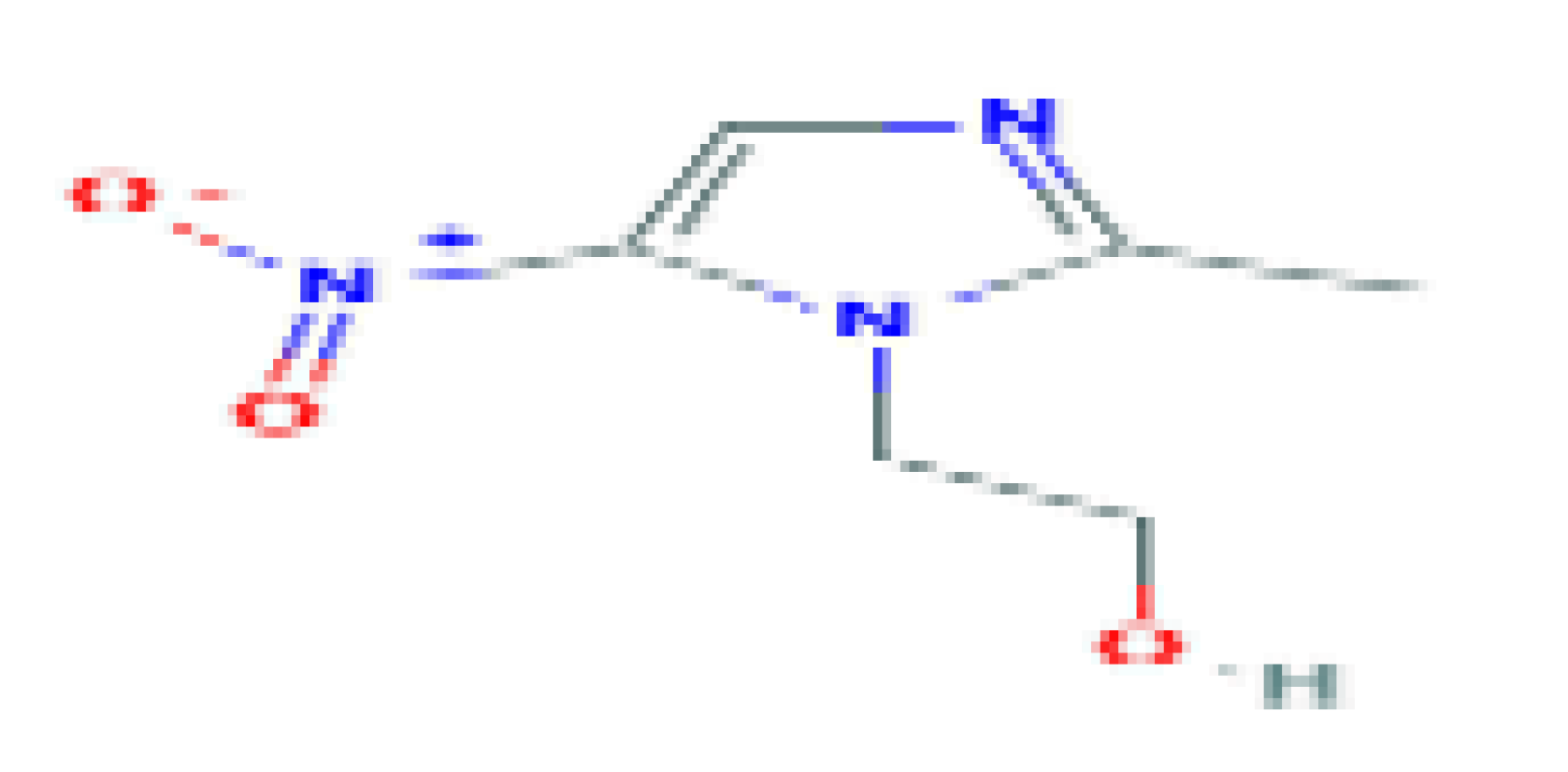 | |
| Ofloxacin | C18H20FN3O4 | 361.4 | Used in case of rare instances of acute hepatocellular injury |  | |
| Sulfamethoxazole | C10H11N3O3S | 253.28 | Used in combination with trimethoprim | 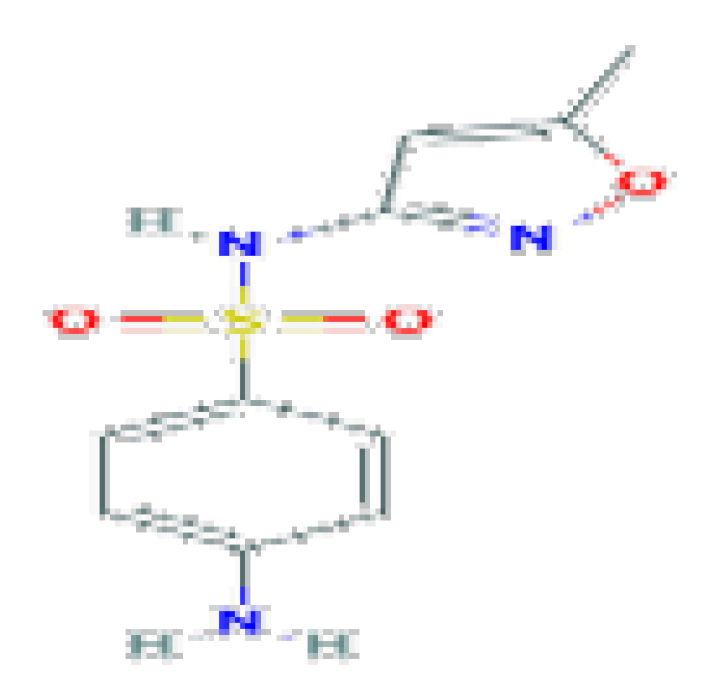 | |
| Trimethoprim | C14H18N4O3 | 290.32 | Used for mild-to-moderate bacterial infections and as prophylaxis against opportunistic infections |  | |
| Chloramphenicol | C11H12CI2N2O5 | 323.13 | Now used rarely and reserved for severe, life-threatening infections for which other antibiotics are not available. | 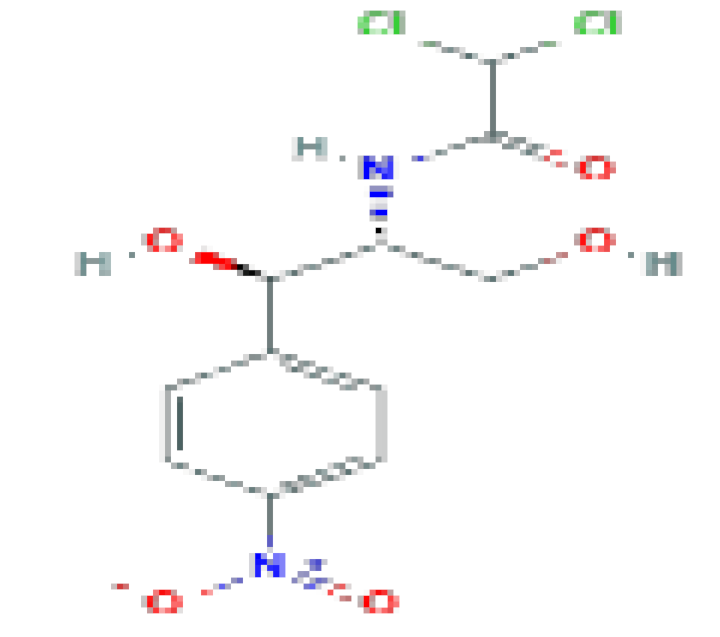 | |
| Oxolinic acid | C13H11NO5 | 261.23 | Used in urinary tract infections. |  | |
| Sulfamonomethoxine | C11H12N4O3S | 280.31 | It is a sulfonamide and a member of benzenes. |  | |
| NSAIDs | Ketoprofen | C16H14O3 | 254.28 | Used in the treatment of acute pain and chronic arthritis. |  |
| Naproxen | C14H14O3 | 230.26 | Used for the reduction of pain, fever, inflammation, and stiffness caused by conditions such as osteoarthritis, kidney stones, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, menstrual cramps, tendinitis, bursitis, and for the treatment of primary dysmenorrhea. |  | |
| Ibuprofen | C13H18O2 | 206.28 | Treatment of rheumatism and arthritis. |  | |
| Diclofenac | C14H11CI2NO2 | 296.1 | Used for the therapy of chronic forms of arthritis and mild-to-moderate acute pain. |  | |
| Mefenamic acid | C15H15NO2 | 241.28 | Used in case of rare instances of clinically apparent, acute liver injury. |  | |
| Etofinamate | C18H18F3NO4 | 369.3 | Used to treat muscle and joint paint. |  | |
| Caffeine | C8H10N4O2 | 194.19 | Caffeine is the most widely consumed psychostimulant drug in the world and is mostly consumed in the form of coffee. |  | |
| Indomethacin | C19H16ClNO4 | 357.8 | Used for chronic inflammatory arthritis. |  | |
| β_blockers | Propranolol | C16H21NO2 | 259.34 | Used for the therapy of hypertension, cardiac arrhythmias, angina pectoris, and hyperthyroidism. |  |
| Bisprolol | C17H29NO4 | 311.4 | Used to counteract the effect of bradycardia through reduced reflection wave. (b) |  | |
| Celiprolol | C20H33N3O4 | 379.5 | Used for the management of mild to moderate hypertension and effort-induced angina pectoris. |  | |
| Metoprolol | C15H25NO3 | 267.36 | Used in the treatment of several diseases of the cardiovascular system. |  | |
| Talinolol | C20H33N3O3 | 363.5 | It has been investigated for the basic science of gastrointestinal motility disorder. |  | |
| Tricyclic antidepressants TCA | Carbamazepine | C15H12N2O | 236.27 | Used in therapy of epilepsy and trigeminal neuralgia. |  |
| Venlafaxine | C17H27NO2 | 277.4 | Can be associated with transient asymptomatic elevations in serum aminotransferase levels. |  | |
| Fluoxetine | C17H18F3NO | 309.33 | Used to treat major depressive disorder (MDD), moderate-to-severe bulimia nervosa, obsessive–compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD), panic disorder with or without agoraphobia, and, in combination with olanzapine, for treatment-resistant or bipolar I depression. | 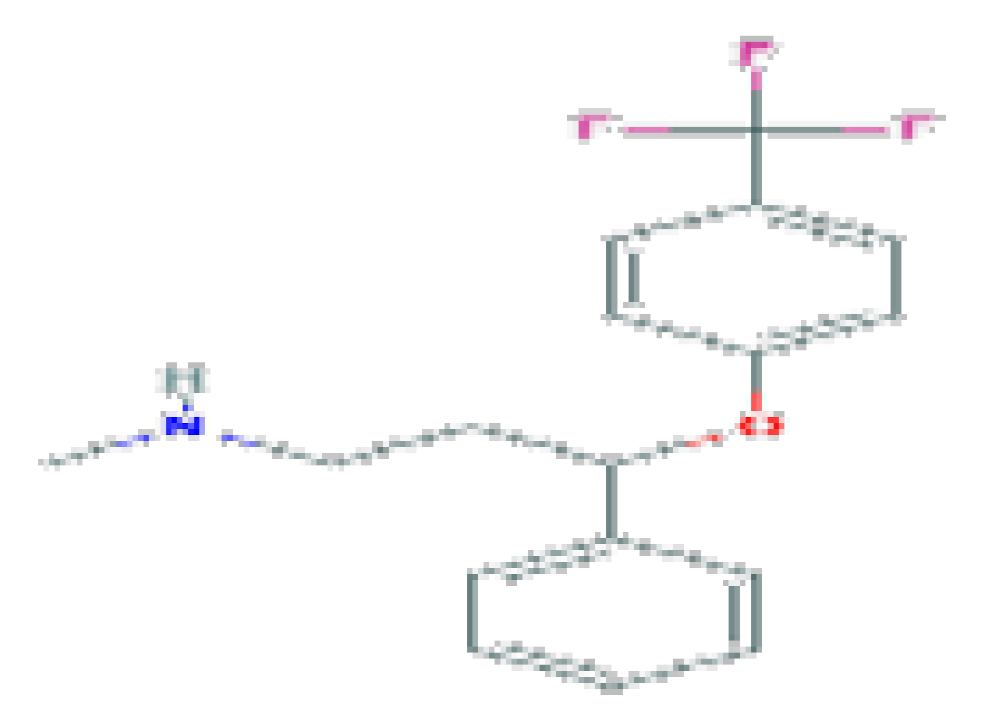 | |
| Sulpiride | C15H23N3O4S | 341.4 | Used therapeutically as an antidepressant, antipsychotic, and as a digestive aid. | 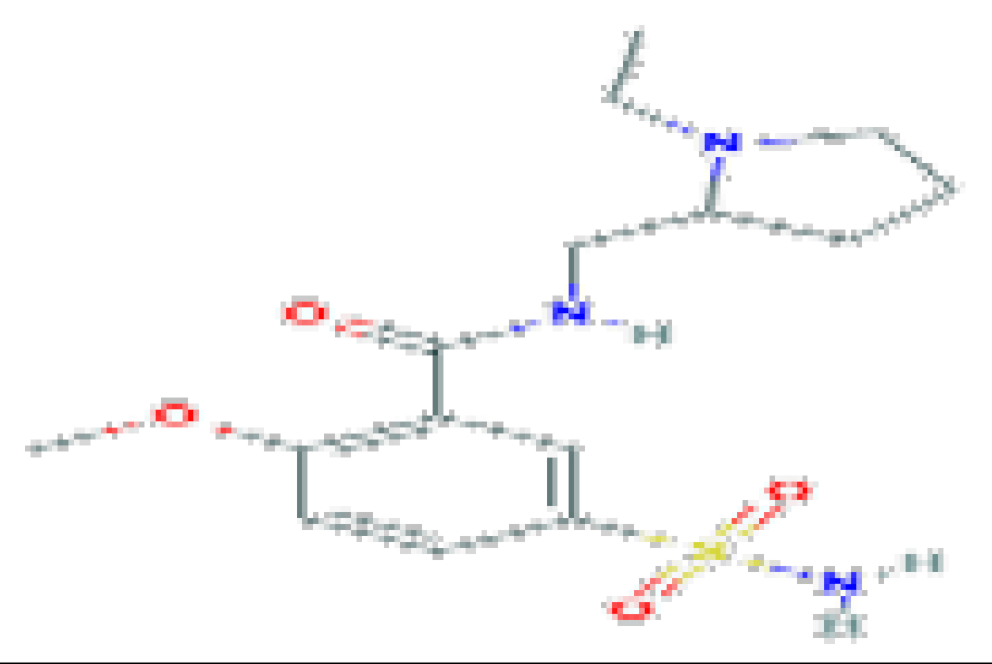 | |
| Antineoplastic | Carboplatin | C6H12O4Pt | 371.25 | Used as a chemotherapeutic agent for the treatment of various cancers, mainly ovarian, head and neck and lung cancers. | 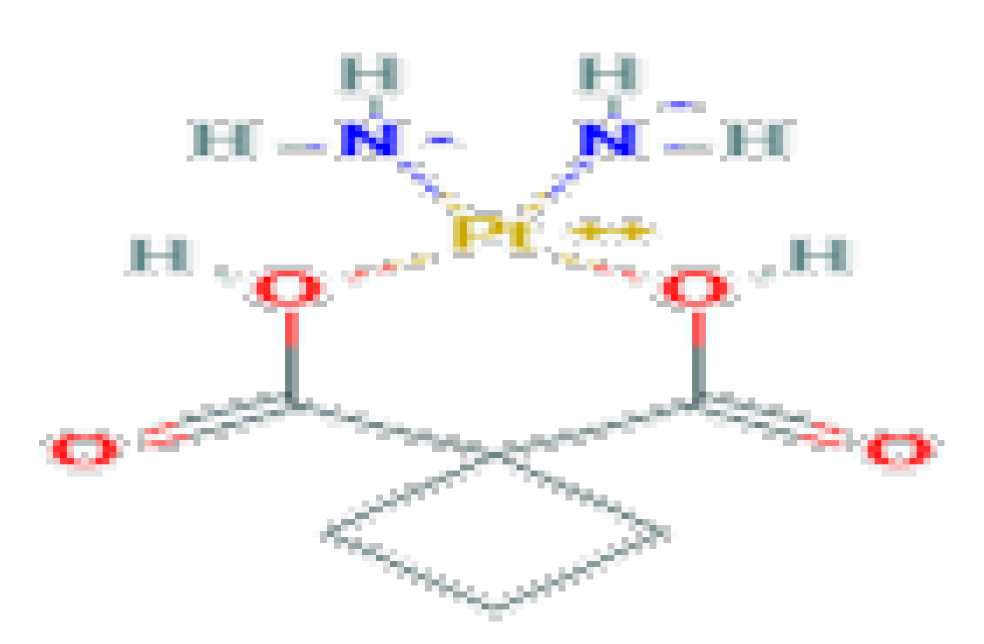 |
| Ifosfamide | C7H15Cl2N2O2P | 261.08 | It is associated with minor transient serum enzyme elevations and has been linked to cases of acute liver injury, including acute cholestatic hepatitis and veno-occlusive disease. | 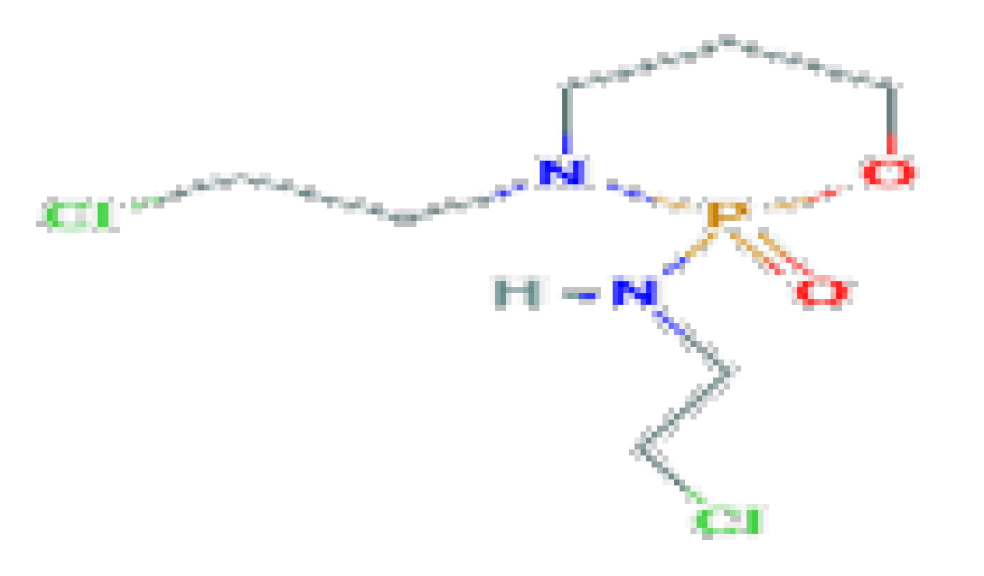 | |
| Cyclophosphamide | C7H15Cl2N2O2P | 261.08 | It is associated with minor transient serum enzyme elevations and has been linked to rare cases of acute liver injury. | 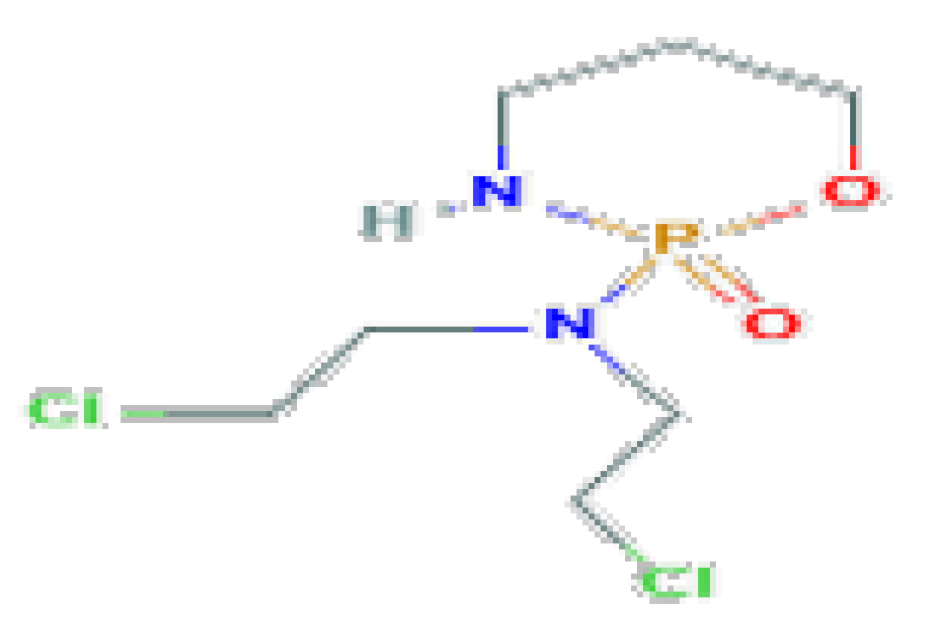 | |
| Cytarabine | C9H13N3O5 | 243.22 | Used largely in the therapy of acute leukemia. | 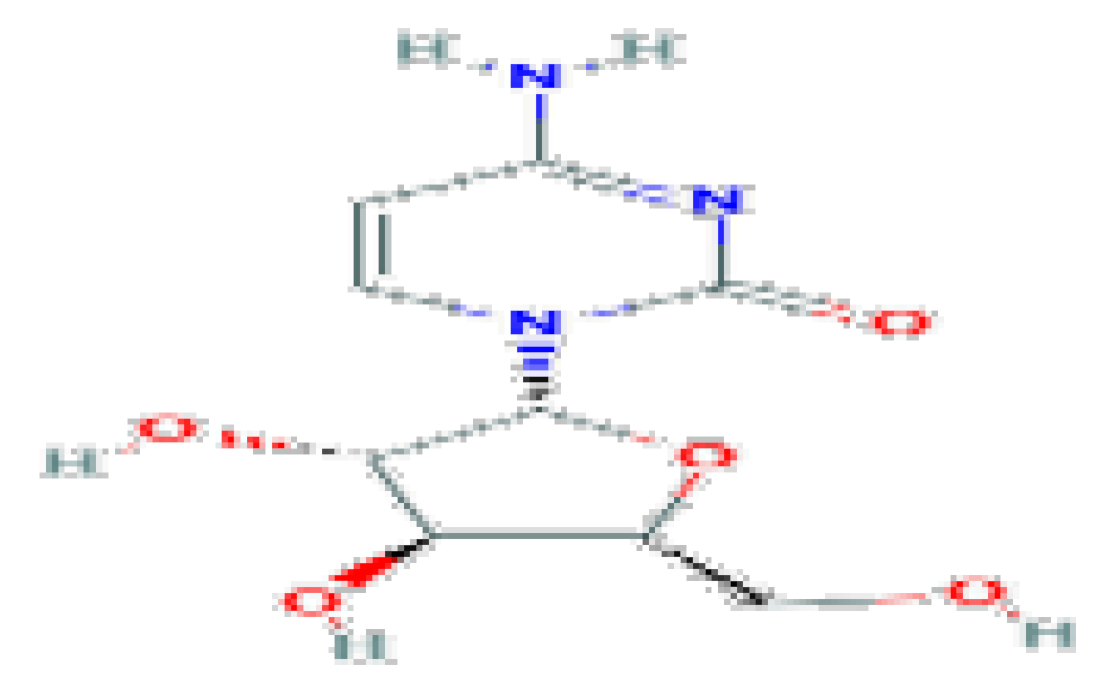 | |
| Methotrexate | C20H22N8O5 | 454.4 | It has been associated with frequent but mild elevations in serum liver enzymes and, more importantly, with development of chronic liver injury, progressive fibrosis, cirrhosis, and portal hypertension. | 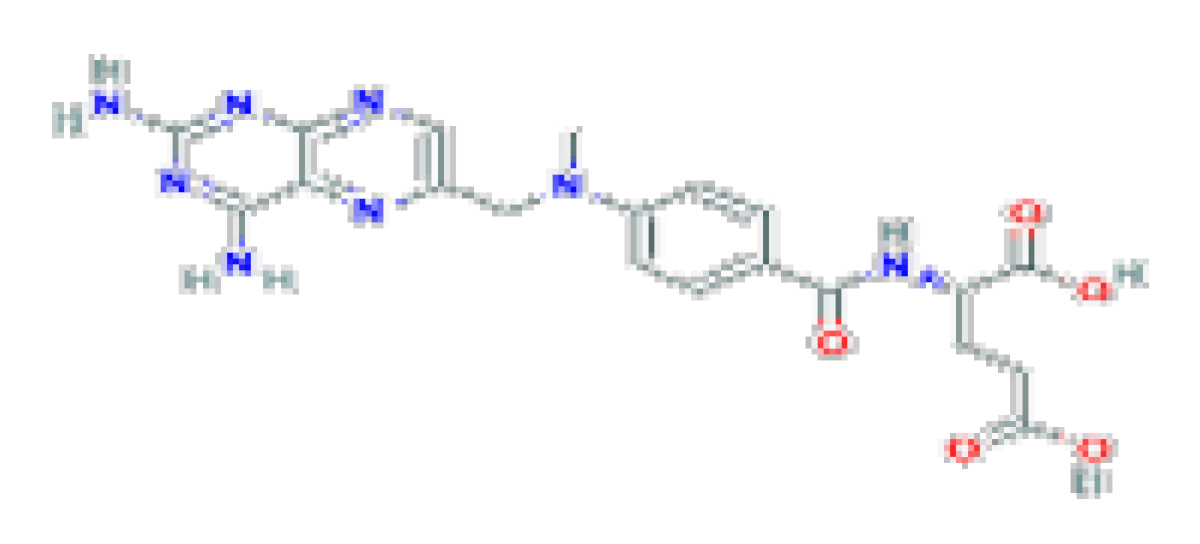 | |
| Digluconate (chlorhexidine) | C34H54Cl2N10O14 | 897.8 | It is used in various applications, including wound care, hand washing, preoperative body shower, oral hygiene, and general disinfection. | 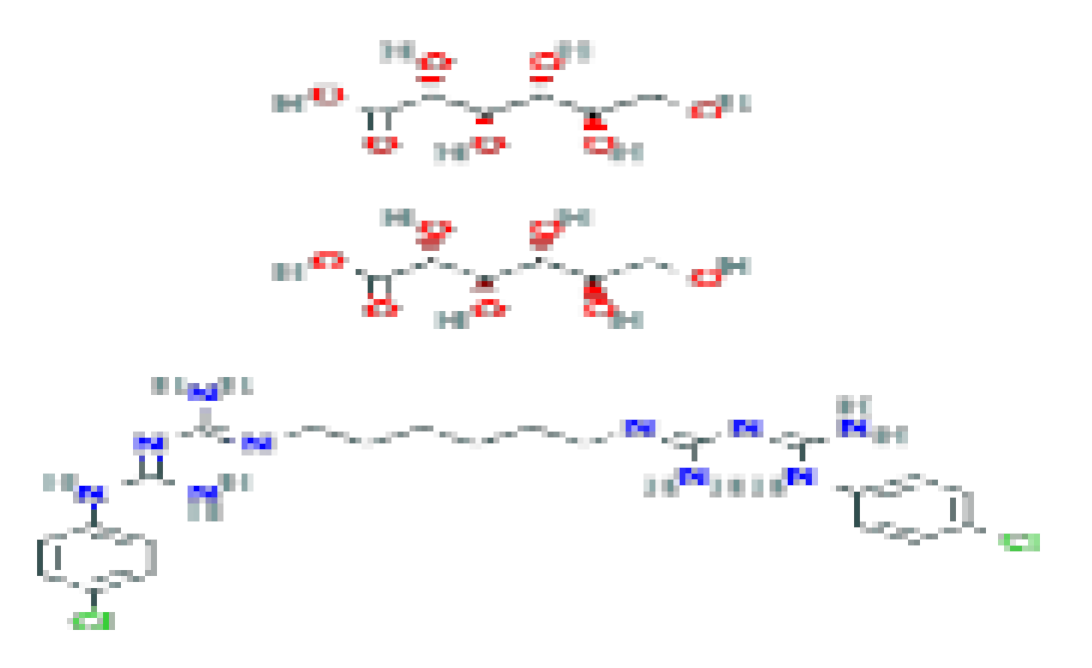 | |
| Antiseptic | Chlorhexidine | C22H30CI2N10 | 505.4 | Used as a topical antiseptic and in dental practice for the treatment of inflammatory dental conditions caused by microorganisms. | 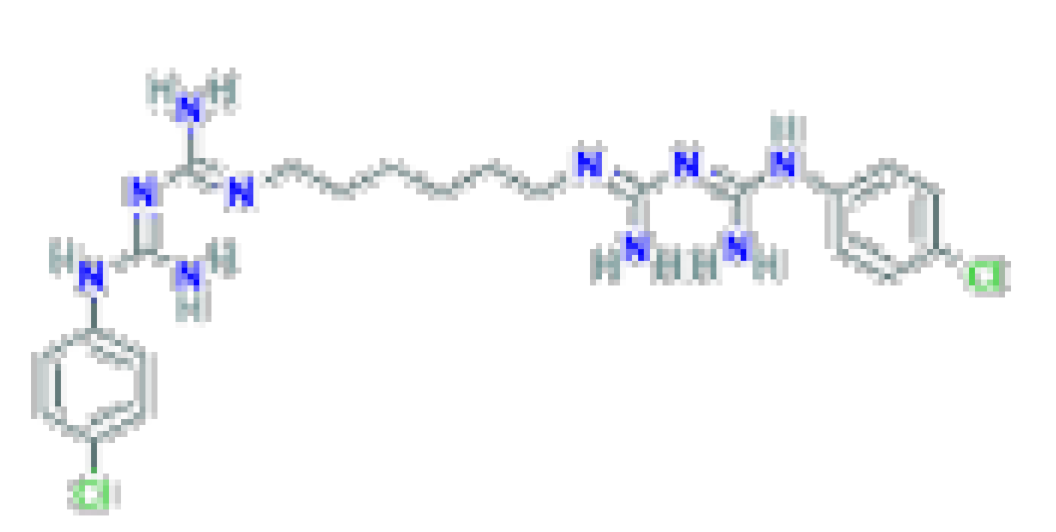 |
| Anti-convulsant | Clorazepate | C16H11CIN2O3 | 314.72 | Used as an anticonvulsant as adjunctive therapy in management of epilepsy and as an anxiolytic for therapy of anxiety and alcohol withdrawal. | 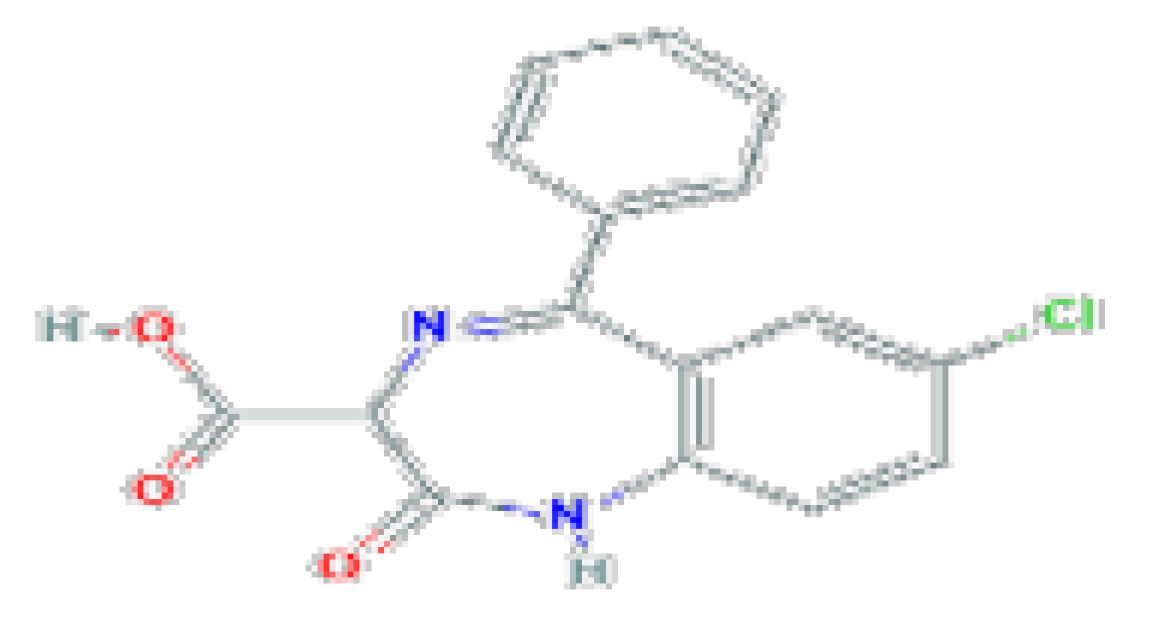 |
| Hydroxyzine | C21H27CIN2O2 | 374.9 | Used largely for symptoms of itching, nausea, anxiety, and tension. |  | |
| Indapamide | C16H16CINO3 | 365.8 | Antihypertensive agent and a diuretic. | 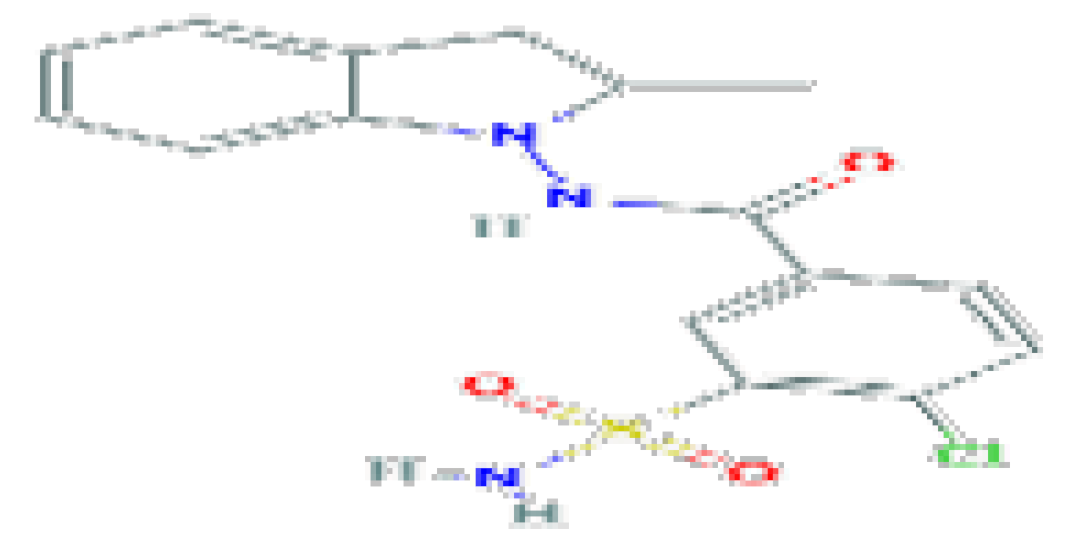 | |
| Enalapril | C20H28N2O5 | 376.4 | Used in the therapy of hypertension and heart failure. | 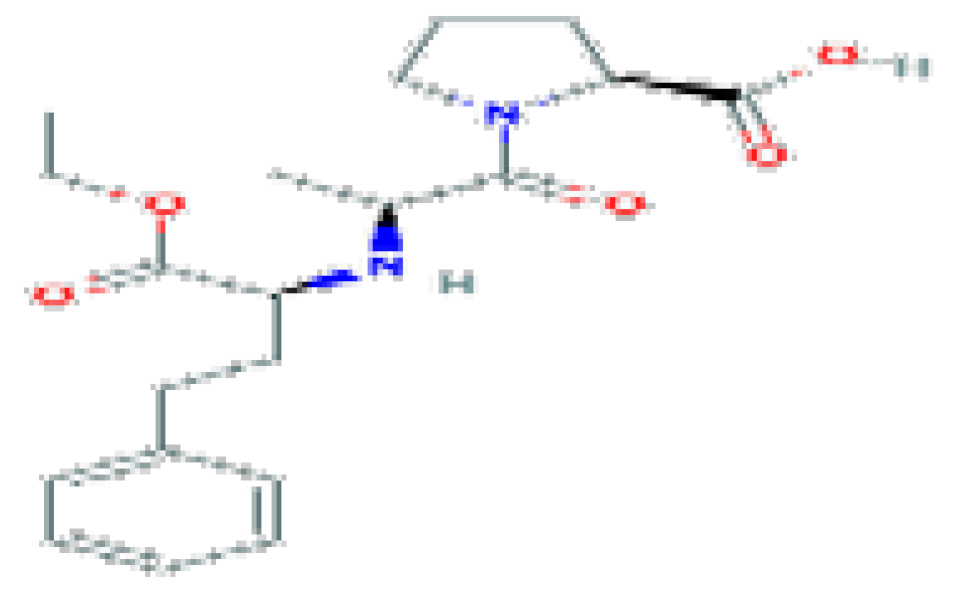 | |
| Captopril | C9H15NO3S | 217.29 | Used in the therapy of hypertension and heart failure. | 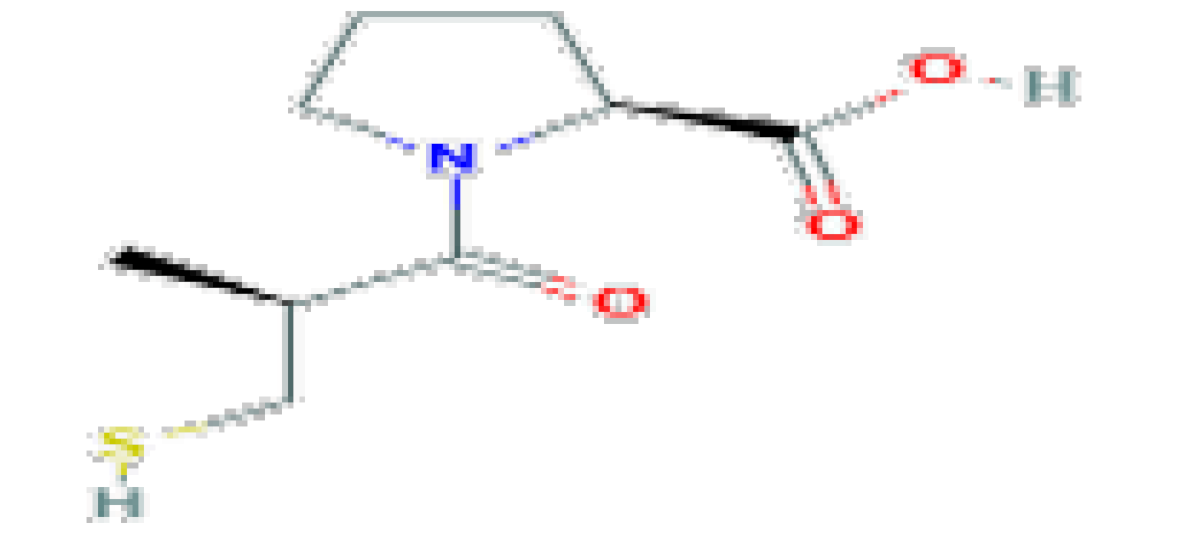 | |
| Atenolol | C14H22N2O3 | 266.34 | Used as an antihypertensive, hypotensive, and antiarrhythmic, Atenolol acts as a peripheral, cardioselective beta-blocker specific for beta-1 adrenergic receptors, without intrinsic sympathomimetic effects. | 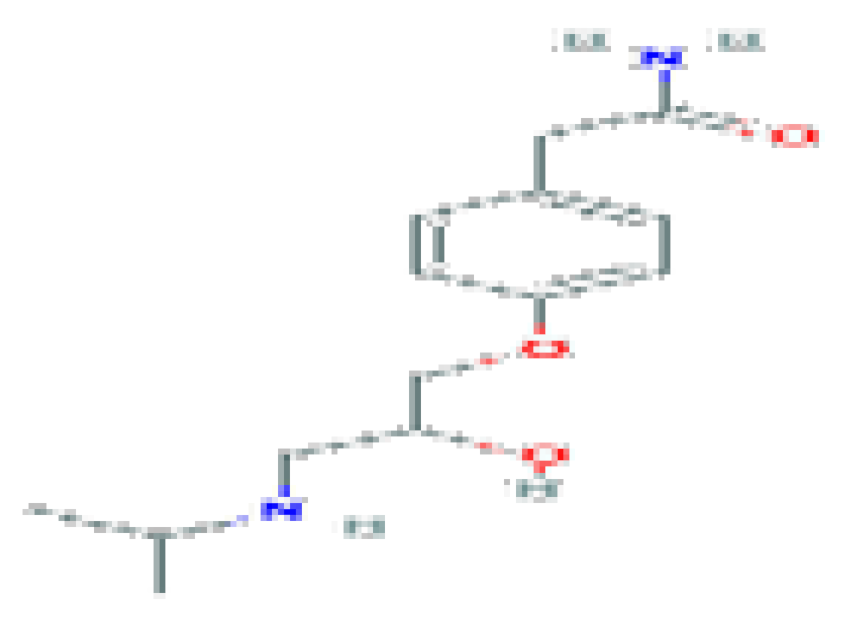 | |
| Bezafibrate | C19H20CINO4 | 361.8 | Used for the treatment of hyperlipidemia. | 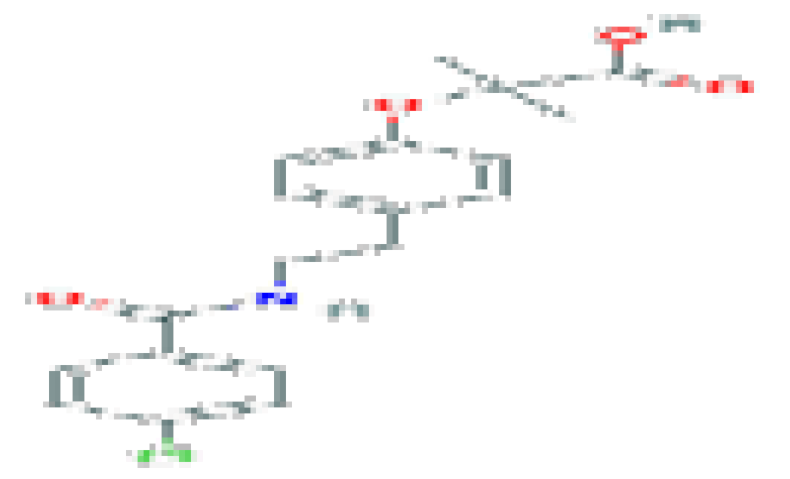 | |
| Gabapentin | C9H17NO2 | 171.24 | Used as adjunctive therapy in the management of epilepsy and for neuropathic pain syndromes. | 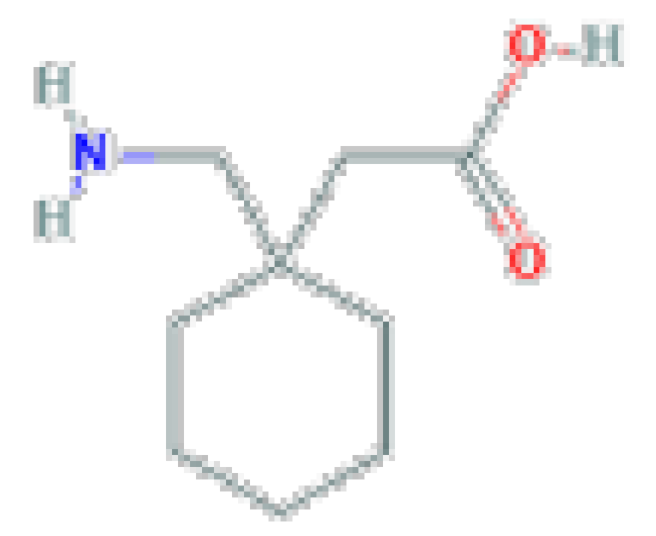 | |
| Oxcarbazepine | C15H12N2O2 | 252.27 | It is a potent anticonvulsant used alone or in combination with other agents in the therapy of partial seizures. | 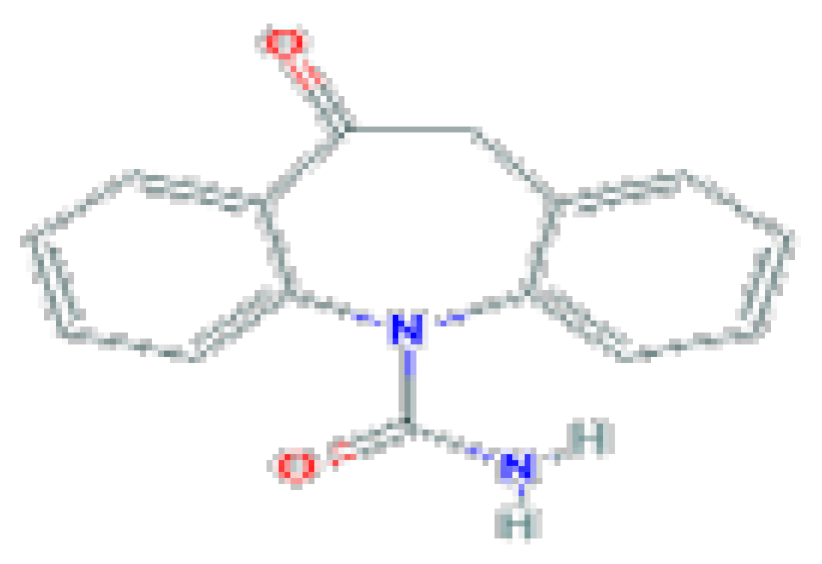 | |
| Antihypertensive | Candesartan | C24H20N6O3 | 440.5 | Used widely in the therapy of hypertension and heart failure. |  |
| Eprosartan | C23H24N2O4S | 424.5 | Used for the treatment of high blood pressure. | 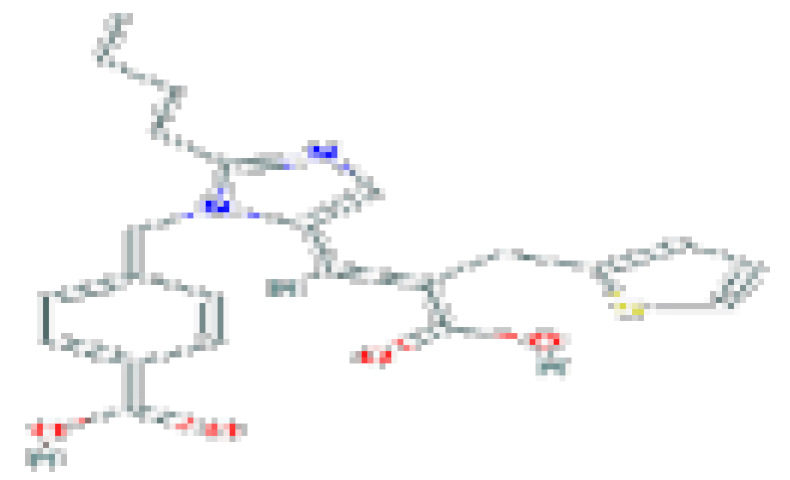 | |
| Irbesartan | C25H28N6O | 428.5 | It has been linked to rare instances of acute liver injury. |  | |
| Valsartan | C24H29N5O3 | 435.5 | Used alone or in combination with other agents to treat hypertension and reduce cardiovascular mortality after myocardial infarction. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. https://doi.org/10.3390/app11146659
Mansouri F, Chouchene K, Roche N, Ksibi M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Applied Sciences. 2021; 11(14):6659. https://doi.org/10.3390/app11146659
Chicago/Turabian StyleMansouri, Fatma, Khawla Chouchene, Nicolas Roche, and Mohamed Ksibi. 2021. "Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends" Applied Sciences 11, no. 14: 6659. https://doi.org/10.3390/app11146659
APA StyleMansouri, F., Chouchene, K., Roche, N., & Ksibi, M. (2021). Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Applied Sciences, 11(14), 6659. https://doi.org/10.3390/app11146659








