The Reuse of Industrial By-Products for the Synthesis of Innovative Porous Materials, with the Aim to Improve Urban Air Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Materials Characterization
2.3. Samples Preparation
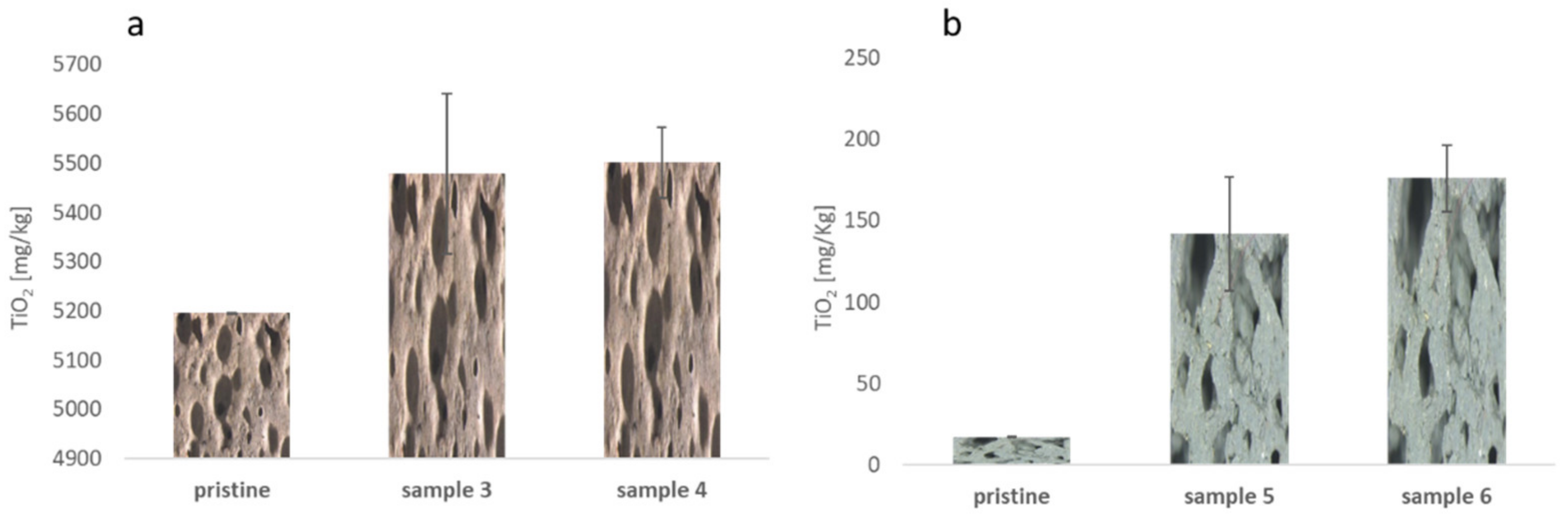
3. Results and Discussion
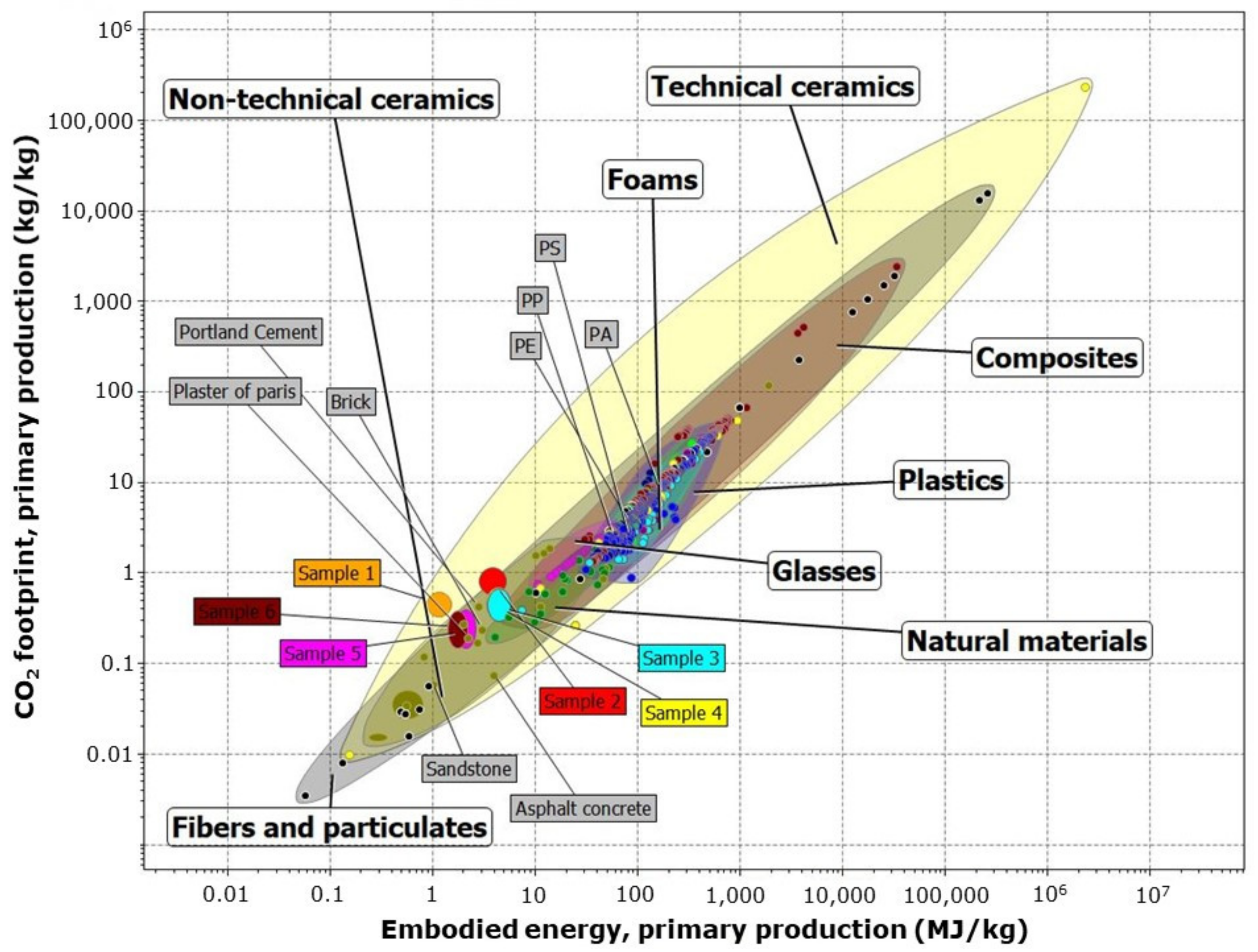
3.1. Sample Sustainability
3.2. Sample Colorimeter
3.3. Structural Analysis
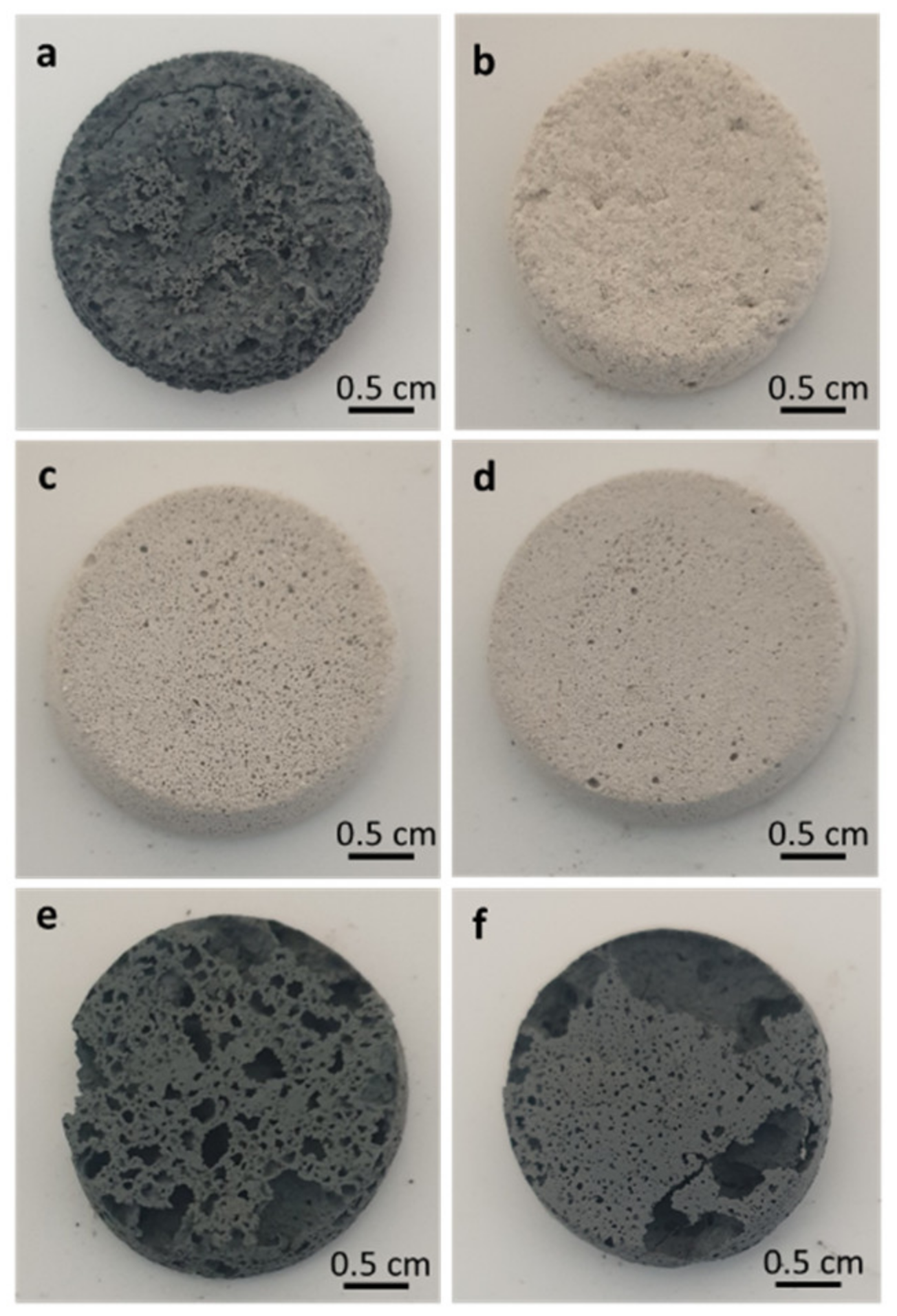
3.4. Morphological Analysis
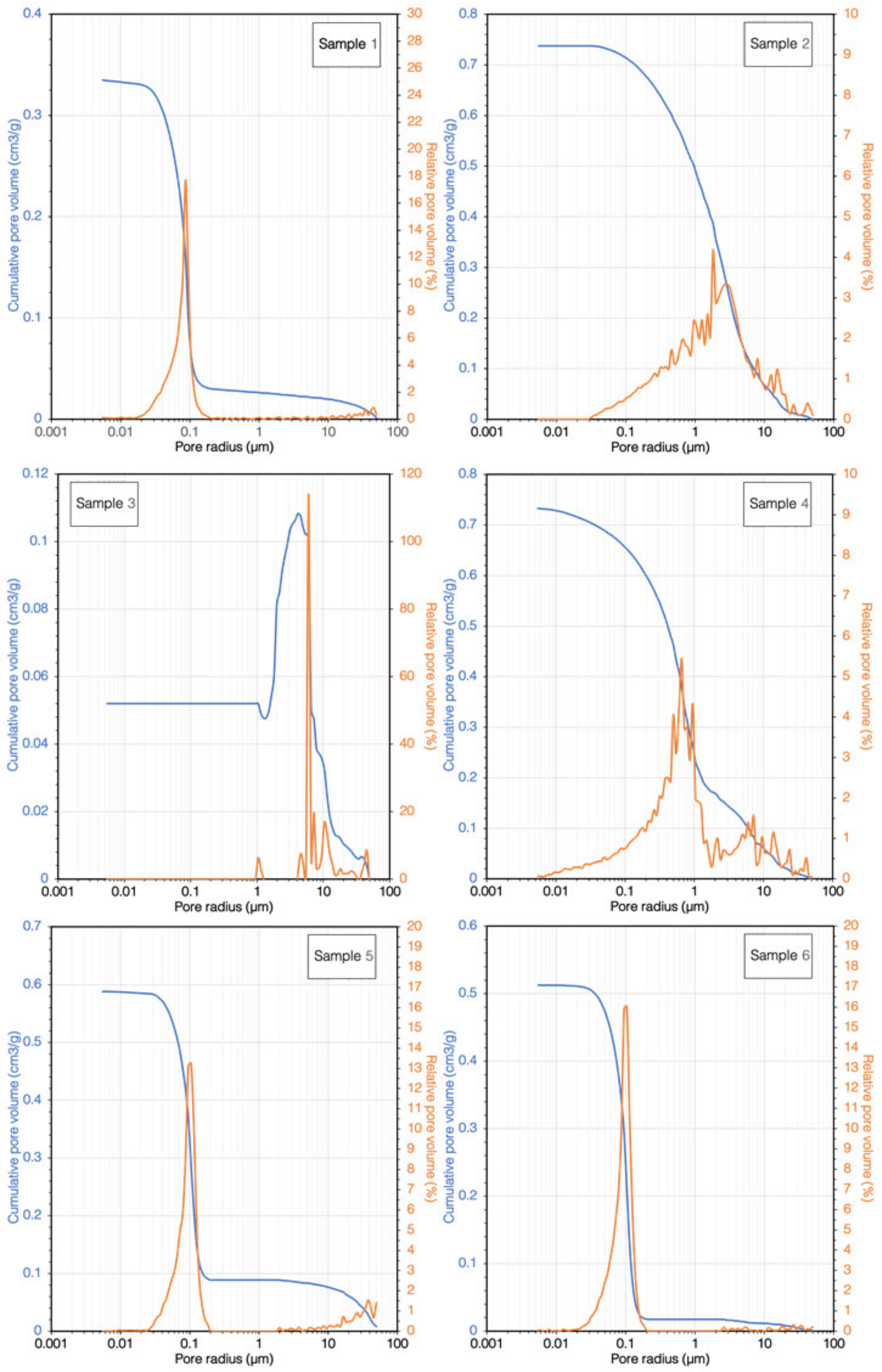
3.5. Porosimetric Analysis
3.6. Sample PM Capability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Su, Q.; Yang, J.; Zhang, G.; Long, S.; Wang, X. High-performance filter membrane composed of oxidized Poly (arylene sulfide sulfone) nanofibers for the high-efficiency air filtration. J. Hazard. Mater. 2021, 417, 126033. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Rovira, J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020, 187, 109650. [Google Scholar] [CrossRef]
- Przybysz, A.; Nersisyan, G.; Gawroński, S.W. Removal of particulate matter and trace elements from ambient air by urban greenery in the winter season. Environ. Sci. Pollut. Res. 2019, 26, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Gieré, R.; Querol, X. Solid particulate matter in the atmosphere. Elements 2010, 6, 215–222. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Federici, S.; Borgese, L.; Depero, L.E.; Ponti, J.; Valsesia, A.; La Spina, R.; Segata, M.; Montini, T.; et al. The first material made for air pollution control able to sequestrate fine and ultrafine air particulate matter. Sustain. Cities Soc. 2020, 53, 101961. [Google Scholar] [CrossRef]
- Juda-Rezler, K.; Reizer, M.; Oudinet, J.-P. Determination and analysis of PM10 source apportionment during episodes of air pollution in Central Eastern European urban areas: The case of wintertime. Atmos. Environ. 2011, 45, 6557–6566. [Google Scholar] [CrossRef]
- Majewski, G.; Kleniewska, M.; Brandyk, A. Seasonal variation of particulate matter mass concentration and content of metals. Pol. J. Environ. Stud. 2011, 20, 417–427. [Google Scholar]
- Wu, X.; Zhu, B.; Zhou, J.; Bi, Y.; Xu, S.; Zhou, B. The epidemiological trends in the burden of lung cancer attributable to PM2.5 exposure in China. BMC Public Health 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Y.; Lu, T.; Liu, K.; Huang, C. High performance, environmentally friendly and sustainable nanofiber membrane filter for removal of particulate matter 1.0. J. Colloid Interface Sci. 2021, 597, 48–55. [Google Scholar] [CrossRef]
- Heck, T.G.; Fiorin, P.B.G.; Frizzo, M.N.; Ludwig, M.S. Fine particulate matter (PM2.5) air pollution and type 2 diabetes mellitus (T2DM): When experimental data explains epidemiological facts. Diabetes Its Complicat. 2018. [Google Scholar] [CrossRef] [Green Version]
- Apte, J.S.; Brauer, M.; Cohen, A.J.; Ezzati, M.; Pope, I.C.A. Ambient PM2.5 reduces global and regional life expectancy. Environ. Sci. Technol. Lett. 2018, 5, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Bahreini, R.; Lee, S.-B.; Bae, G.-N.; Jung, H. Correlations of PM metrics with human respiratory system deposited PM mass determined from ambient particle size distributions and effective densities. Aerosol Sci. Technol. 2019, 54, 262–276. [Google Scholar] [CrossRef]
- Bontempi, E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): The case of Lombardy (Italy). Environ. Res. 2020, 186, 109639. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, E. The Europe second wave of COVID-19 infection and the Italy “strange” situation. Environ. Res. 2021, 193, 110476. [Google Scholar] [CrossRef]
- Bontempi, E.; Vergalli, S.; Squazzoni, F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020, 188, 109814. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, E. Commercial exchanges instead of air pollution as possible origin of COVID-19 initial diffusion phase in Italy: More efforts are necessary to address interdisciplinary research. Environ. Res. 2020, 188, 109775. [Google Scholar] [CrossRef]
- Anand, U.; Cabreros, C.; Mal, J.; Ballesteros, F.; Sillanpää, M.; Tripathi, V.; Bontempi, E. Novel coronavirus disease 2019 (COVID-19) pandemic: From transmission to control with an interdisciplinary vision. Environ. Res. 2021, 197, 111126. [Google Scholar] [CrossRef] [PubMed]
- Shabnam, N.; Oh, J.; Park, S.; Kim, H. Impact of particulate matter on primary leaves of Vigna radiata (L.) R. Wilczek. Ecotoxicol. Environ. Saf. 2021, 212, 111965. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, A.; Bilo, F.; Borgese, L.; Depero, L.E.; Fahimi, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Montini, T.; Bontempi, E. SUNSPACE, A porous material to reduce air particulate matter (PM). Front. Chem. 2018, 6, 534. [Google Scholar] [CrossRef] [Green Version]
- Cornelio, A.; Zanoletti, A.; Federici, S.; Depero, L.E.; Bontempi, E. Porous materials derived from industrial by-products for titanium dioxide nanoparticles capture. Appl. Sci. 2020, 10, 8086. [Google Scholar] [CrossRef]
- Kasina, M.; Kajdas, B.; Michalik, M. The leaching potential of sewage sludge and municipal waste incineration ashes in terms of landfill safety and potential reuse. Sci. Total. Environ. 2021, 791, 148313. [Google Scholar] [CrossRef]
- Bontempi, E.; Sorrentino, G.; Zanoletti, A.; Alessandri, I.; Depero, L.; Caneschi, A. Sustainable materials and their contribution to the sustainable development goals (SDGs): A critical review based on an Italian example. Molecules 2021, 26, 1407. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Depero, L.E.; Zappa, D.; Bontempi, E. The first sustainable material designed for air particulate matter capture: An introduction to Azure Chemistry. J. Environ. Manag. 2018, 218, 355–362. [Google Scholar] [CrossRef]
- Rodella, N.; Bosio, A.; Dalipi, R.; Zacco, A.; Borgese, L.; Depero, L.E.; Bontempi, E. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab. J. Chem. 2017, 10, S3676–S3681. [Google Scholar] [CrossRef] [Green Version]
- Panjehpour, M.; Abdullah, A.; Ali, A.; Demirboga, R. A review for characterization of silica fume and its effects on concrete properties. Int. J. Sustain. Constr. Eng. Technol. 2011, 2, 1–7. [Google Scholar]
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Hazard. Mater. 2020, 393, 122433. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Federici, S.; Zacco, A.; Depero, L.E.; Bontempi, E. Bottom ash derived from municipal solid waste and sewage sludge co-incineration: First results about characterization and reuse. Waste Manag. 2020, 116, 147–156. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Bueno, E.T.; Tibbetts, C.M.; Townsend, T.G. Washed waste incineration bottom ash as a raw ingredient in cement production: Implications for lab-scale clinker behavior. Resour. Conserv. Recycl. 2021, 169, 105513. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2020, 245, 118779. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zheng, Y.; Hu, H.T.; Qi, J.Y. Use of municipal solid waste incineration bottom ash in adsorption of heavy metals. Key Eng. Mater. 2011, 474-476, 1099–1102. [Google Scholar] [CrossRef]
- Granta Design Cambridge Engineering Selector (CES) Software 2019; Granta Design Ltd.: Cambridge, UK, 2019.
- Us-Epa Method 3052-Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- Kasaniya, M.; Thomas, M.D.; Moffatt, E.G. Pozzolanic reactivity of natural pozzolans, ground glasses and coal bottom ashes and implication of their incorporation on the chloride permeability of concrete. Cem. Concr. Res. 2021, 139, 106259. [Google Scholar] [CrossRef]
- Bontempi, E. A new approach for evaluating the sustainability of raw materials substitution based on embodied energy and the CO2 footprint. J. Clean. Prod. 2017, 162, 162–169. [Google Scholar] [CrossRef]
- Fahimi, A.; Federici, S.; Depero, L.E.; Valentim, B.; Vassura, I.; Ceruti, F.; Cutaia, L.; Bontempi, E. Evaluation of the sustainability of technologies to recover phosphorus from sewage sludge ash based on embodied energy and CO2 footprint. J. Clean. Prod. 2021, 289, 125762. [Google Scholar] [CrossRef]
- Ducoli, S.; Zacco, A.; Bontempi, E. Incineration of sewage sludge and recovery of residue ash as building material: A valuable option as a consequence of the COVID-19 pandemic. J. Environ. Manag. 2021, 282, 111966. [Google Scholar] [CrossRef]
- Bontempi, E. Raw Materials Substitution Sustainability; Springer Briefs in Applied Science and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Benassi, L.; Pasquali, M.; Zanoletti, A.; Dalipi, R.; Borgese, L.; Depero, L.E.; Vassura, I.; Quina, M.; Bontempi, E. Chemical stabilization of municipal solid waste incineration fly ash without any commercial chemicals: First pilot-plant scaling up. ACS Sustain. Chem. Eng. 2016, 4, 5561–5569. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ducoli, S.; Ramorino, G.; Gobetti, A.; Zacco, A.; Federici, S.; Depero, L.E.; Bontempi, E. A Circular Economy Virtuous Example—Use of a Stabilized Waste Material Instead of Calcite to Produce Sustainable Composites. Appl. Sci. 2020, 10, 754. [Google Scholar] [CrossRef] [Green Version]
- Bosio, A.; Rodella, N.; Gianoncelli, A.; Zacco, A.; Borgese, L.; Depero, L.E.; Bingham, P.; Bontempi, E. A new method to inertize incinerator toxic fly ash with silica from rice husk ash. Environ. Chem. Lett. 2013, 11, 329–333. [Google Scholar] [CrossRef]
- Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased sustainability of carbon dioxide mineral sequestration by a technology involving fly ash stabilization. Materials 2019, 12, 2714. [Google Scholar] [CrossRef] [Green Version]
- Zanoletti, A.; Vassura, I.; Venturini, E.; Monai, M.; Montini, T.; Federici, S.; Zacco, A.; Treccani, L.; Bontempi, E. A new porous hybrid material derived from silica fume and alginate for sustainable pollutants reduction. Front. Chem. 2018, 6, 60. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Depero, L.E.; Bontempi, E. Review of the reuse possibilities concerning ash residues from thermal process in a medium-sized urban system in northern Italy. Sustainability 2020, 12, 4193. [Google Scholar] [CrossRef]
- Obe, R.K.D.; de Brito, J.; Lynn, C.J.; Silva, R.V. Sustainable Construction Materials: Municipal Incinerated Bottom Ash; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cheriaf, M.; Cavalante Rocha, J.; Péra, J. Pozzolanic properties of pulverized coal combustion bottom ash. Cem. Concr. Res. 1999, 29, 1387–1391. [Google Scholar] [CrossRef]


| Silica Fume (SF) (g) | Bottom Ash (BA) (g) | Ca(OH)2 (g) | NaC6H7O6 (g) | Ca(IO3)2 (g) | NaHCO3 (g) | H2O2 (mL) | |
|---|---|---|---|---|---|---|---|
| Sample 1 | 17.88 | - | - | 0.6 | 1 | 5 | - |
| Sample 2 | - | 9 | 9 | 0.6 | 1 | 5 | - |
| Sample 3 | - | 9 | 9 | - | - | - | 7.2 |
| Sample 4 | - | 9 | 9 | - | - | - | 5.4 |
| Sample 5 | 17.88 | - | - | 0.6 | 1 | - | 7.2 |
| Sample 6 | 17.88 | - | - | 0.6 | 1 | - | 5.4 |
| Samples | SCI | SCE | ||||
|---|---|---|---|---|---|---|
| L | a | b | L | a | b | |
| Sample 1 | 38.83 ± 0.01 | −0.92 ± 0.01 | −2.33 ± 0.01 | 39.61 ± 0.01 | −0.89 ± 0.02 | −2.36 ± 0.01 |
| Sample 2 | 81.97 ± 0.01 | 0.77 ± 0.01 | 6.34 ± 0.01 | 81.49 ± 0.01 | 0.80 ± 0.01 | 6,22 ± 0.01 |
| Sample 3 | 86.32 ± 0.01 | 0.16 ± 0.01 | 3.13 ± 0.01 | 85.86 ± 0.01 | 0.21 ± 0.01 | 3.03 ± 0.02 |
| Sample 4 | 86.01 ± 0.01 | 0.21 ± 0.01 | 3.41 ± 0.01 | 85.82 ± 0.01 | 0.26 ± 0.10 | 3.31 ± 0.01 |
| Sample 5 | 46.69 ± 0.39 | 0.63 ± 0.02 | 0.76 ± 0.24 | 46.45 ± 0.39 | 0.59 ± 0.02 | 0.83 ± 0.24 |
| Sample 6 | 47.46 ± 0.52 | 0.81 ± 0.02 | 1.46 ± 0.06 | 47.21 ± 0.51 | 0.78 ± 0.01 | 1.53 ± 0.07 |
| Sample | Accessible Porosity (%) | Average Pore Radius (µm) | Median Pore Radius (µm) | Modal Pore Radius (µm) |
|---|---|---|---|---|
| 1 | 41.1 | 0.0356 | 0.0777 | 0.0849 |
| 2 | 63.2 | 0.0282 | 1.7808 | 1.7938 |
| 3 | 3.4 | 0.0141 | 9.6896 | 5.6504 |
| 4 | 58.1 | 0.0387 | 0.6153 | 0.6127 |
| 5 | 51.6 | 0.0418 | 0.0954 | 0.1058 |
| 6 | 49.1 | 0.0455 | 0.0911 | 0.1063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelio, A.; Zanoletti, A.; Braga, R.; Depero, L.E.; Bontempi, E. The Reuse of Industrial By-Products for the Synthesis of Innovative Porous Materials, with the Aim to Improve Urban Air Quality. Appl. Sci. 2021, 11, 6798. https://doi.org/10.3390/app11156798
Cornelio A, Zanoletti A, Braga R, Depero LE, Bontempi E. The Reuse of Industrial By-Products for the Synthesis of Innovative Porous Materials, with the Aim to Improve Urban Air Quality. Applied Sciences. 2021; 11(15):6798. https://doi.org/10.3390/app11156798
Chicago/Turabian StyleCornelio, Antonella, Alessandra Zanoletti, Roberto Braga, Laura Eleonora Depero, and Elza Bontempi. 2021. "The Reuse of Industrial By-Products for the Synthesis of Innovative Porous Materials, with the Aim to Improve Urban Air Quality" Applied Sciences 11, no. 15: 6798. https://doi.org/10.3390/app11156798
APA StyleCornelio, A., Zanoletti, A., Braga, R., Depero, L. E., & Bontempi, E. (2021). The Reuse of Industrial By-Products for the Synthesis of Innovative Porous Materials, with the Aim to Improve Urban Air Quality. Applied Sciences, 11(15), 6798. https://doi.org/10.3390/app11156798