Detection of Carrageenan in Cheese Using Lectin Histochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Choice of Fixative Solution
2.2. Production of Model Samples of Processed Cheese
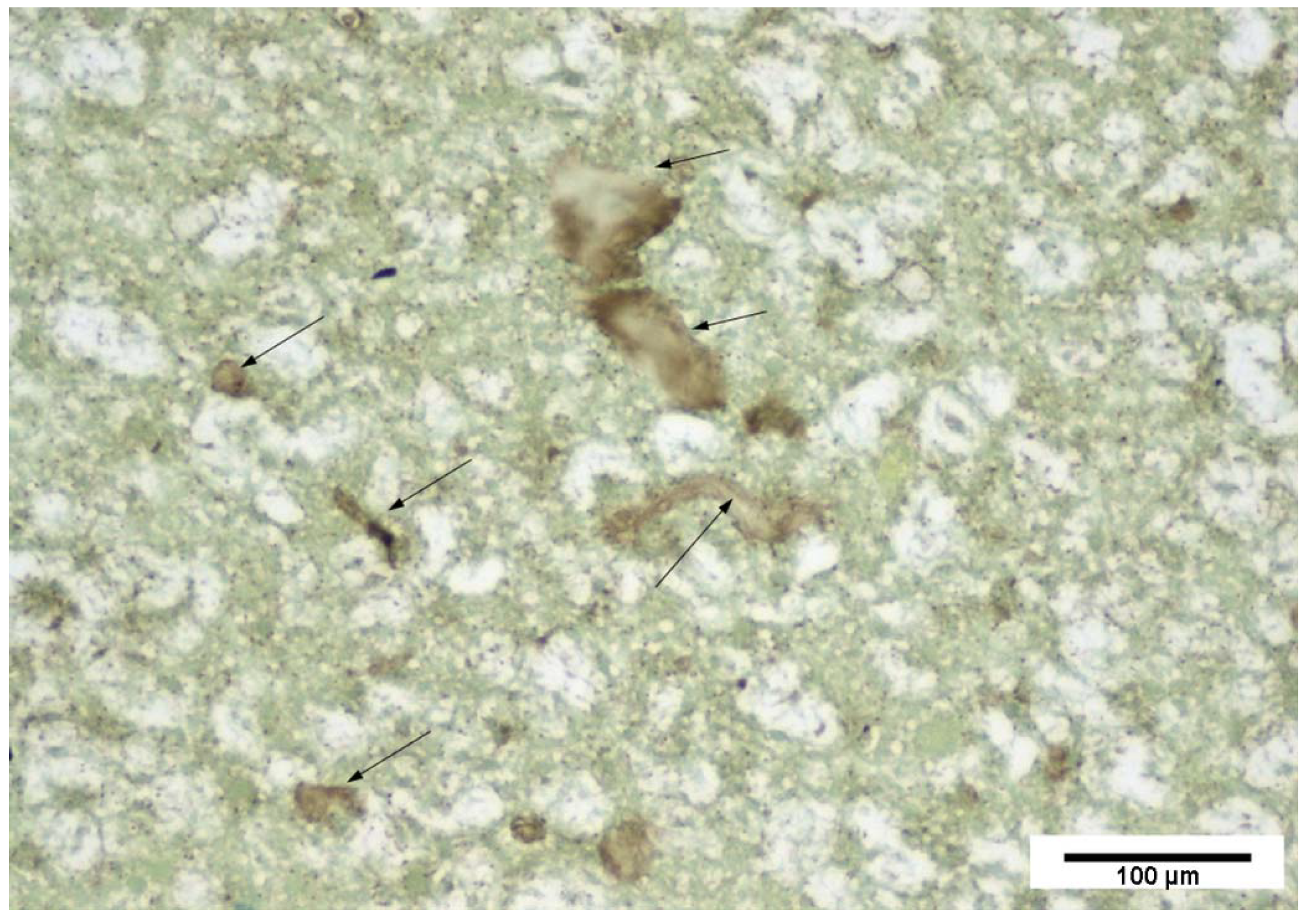
2.3. Lectin Histochemistry
Signal Intensity Evaluation
- Human Inspection
- Computer-Assisted Analysis of CIE L*a*b*
2.4. Statistical Processing
3. Results and Discussion
3.1. Choice of Fixative Solution
3.2. Lectin Histochemistry
3.2.1. Lectin Concentration
3.2.2. Limit of Detection
3.2.3. Validation of the Method on Samples from the Retail Market
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geonzon, L.C.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation mechanism and network structure in gels of carrageenans and their mixtures viewed at different length scales—A review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Ou, L.; Mai, Q. Flotation separation of chalcopyrite from talc using a new depressant carrageenan. Colloids Surf. Physicochem. Eng. Asp. 2020, 603, 125274. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Zhu, Y.; Bhandari, B.; Prakash, S. Tribo-rheometry behaviour and gel strength of κ-carrageenan and gelatin solutions at concentrations, pH and ionic conditions used in dairy products. Food Hydrocoll. 2018, 84, 292–302. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Feroz, A.; Khaki, P.S.S.; Bano, B. Binding of λ-carrageenan (a food additive) to almond cystatin: An insight involving spectroscopic and thermodynamic approach. Int. J. Biol. Macromol. 2017, 98, 684–690. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Tobacman, J.K.; Wallace, R.B.; Zimmerman, M.B. Consumption of carrageenan and other water-soluble polymers used as food additives and incidence of mammary carcinoma. Med. Hypotheses 2001, 56, 589–598. [Google Scholar] [CrossRef]
- Černíková, M.; Buňka, F.; Pospiech, M.; Tremlová, B.; Hladká, K.; Pavlínek, V.; Březina, P. Replacement of traditional emulsifying salts by selected hydrocolloids in processed cheese production. Int. Dairy J. 2010, 20, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Černíková, M.; Buňka, F.; Pavlínek, V.; Březina, P.; Hrabě, J.; Valášek, P. Effect of carrageenan type on viscoelastic properties of processed cheese. Food Hydrocoll. 2008, 22, 1054–1061. [Google Scholar] [CrossRef]
- Błaszak, B.; Gozdecka, G.; Shyichuk, A. Carrageenan as a functional additive in the production of cheese and cheese-like products [pdf]. Acta Sci. Pol. Technol. Aliment. 2018, 17, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.; Leathem, A.; Schumacher, U. Lectin Histochemistry: A Concise Practical Handbook, 1st ed.; Gerland Science: Oxford, UK, 1996; ISBN 1859961002. [Google Scholar]
- Roth, J. Lectins for histochemical demonstration of glycans. Histochem. Cell Biol. 2011, 136, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134. [Google Scholar] [CrossRef]
- Sánchez-Pardo, M.E.; Ortiz-Moreno, A.; Mora-Escobedo, R.; Chanona-Pérez, J.J.; Necoechea-Mondragón, H. Comparison of crumb microstructure from pound cakes baked in a microwave or conventional oven. LWT Food Sci. Technol. 2008, 41, 620–627. [Google Scholar] [CrossRef]
- Saulacic, N.; Stajcic, Z.; Stajcic, L.S.; Piattelli, A.; Iizuka, T.; Lombardi, T. Effects of Carnoy’s solution on blood vessels of the axillary fossa of rats. Int. J. Oral Maxillofac. Surg. 2009, 38, 876–879. [Google Scholar] [CrossRef]
- Bartlová, M.; Tremlová, B.; Marcinčák, S.; Pospiech, M. Detection of carrageenan in meat products using lectin histochemistry. Foods 2021, 10, 764. [Google Scholar] [CrossRef]
- Coelho, L.C.B.B.; Silva, P.M.D.S.; Lima, V.L.D.M.; Pontual, E.V.; Paiva, P.M.G.; Napoleão, T.H.; Correia, M.T.D.S. Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–22. [Google Scholar] [CrossRef]
- Takaara, T.; Sasaki, S.; Fujii, M.; Ito, H.; Masago, Y.; Omura, T. Lectin-stimulated cellular iron uptake and toxin generation in the freshwater cyanobacterium Microcystis aeruginosa. Harmful Algae 2019, 83, 25–33. [Google Scholar] [CrossRef]
- Azevedo Tosta, T.A.; de Faria, P.R.; Neves, L.A.; do Nascimento, M.Z. Computational normalization of H&E-stained histological images: Progress, challenges and future potential. Artif. Intell. Med. 2019, 95, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Kim, H.-W.; Rhyu, I.J.; Uhm, C.-S. How to Get Well-Preserved Samples for Transmission Electron Microscopy. Appl. Microsc. 2016, 46, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Grillo, F.; Pigozzi, S.; Ceriolo, P.; Calamaro, P.; Fiocca, R.; Mastracci, L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem. Cell Biol. 2015, 144, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Webster, J.D.; DuSold, D.; Miller, M.A. Immunohistochemical Evaluation of the Effects of Paraffin Section Storage on Biomarker Stability. Vet. Pathol. 2014, 51, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllou, A.; Fletcher, D. Comparative histochemistry of posterior lingual salivary glands of mouse. Acta Histochem. 2017, 119, 57–63. [Google Scholar] [CrossRef]
- Wick, M.R. Histochemistry as a tool in morphological analysis: A historical review. Ann. Diagn. Pathol. 2012, 16, 71–78. [Google Scholar] [CrossRef]
- Betim, F.C.M.; Barbosa, V.; de Oliveira, C.F.; Miguel, O.G.; Raman, V.; Budel, J.M.; Miguel, M.D.; Dias, J.d.F.G. Microscopy and histochemistry of Ocotea nutans (Nees) Mez (Lauraceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 273. [Google Scholar] [CrossRef]
- de Melo, G.A.M.; de Araújo, M.G.P.; Stauffer, F. Fruit development and histochemistry of Attalea microcarpa Mart. (Arecaceae-Arecoideae). Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 235, 10–17. [Google Scholar] [CrossRef]
- Bednářová, M.; Pospiech, M.; Tremlová, B.; Řezáčová-Lukášková, Z.; Bednář, J. Antigen retrieval and fixation of sections on slides for immunohistochemical detection of soya protein in meat products. J. Food Nutr. Res. 2015, 54, 1–8. [Google Scholar]
- Lukášková, Z.Ř.; Tremlová, B.; Pospiech, M.; Renčová, E.; Randulová, Z.; Steinhauser, L.; Reichová, A.; Bednář, J. Comparison of immunohistochemical, histochemical and immunochemical methods for the detection of wheat protein allergens in meat samples and cooked, dry, raw and fermented sausage samples. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, A.; Yu, H.; Han, Y.; Zheng, S.; Li, H.; Yu, J. Effects of pre-emulsification with heat-treated whey protein on texture and microstructure of processed cheese. LWT 2020, 124, 109185. [Google Scholar] [CrossRef]
- Inoue, K.; Fu, W.; Nakamura, T. Explaining the different textures of commercial processed cheese from fractured structures. Int. Dairy J. 2019, 97, 40–48. [Google Scholar] [CrossRef]
- Howat, W.J.; Wilson, B.A. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods 2014, 70, 12–19. [Google Scholar] [CrossRef]
- Nietner, T.; Jarutat, T.; Mertens, A. Systematic comparison of tissue fixation with alternative fixatives to conventional tissue fixation with buffered formalin in a xenograft-based model. Virchows Arch. 2012, 461, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S. Heat-induced antigen retrieval: Mechanisms and application to histochemistry. Prog. Histochem. Cytochem. 2007, 41, 141–200. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.A. Formaldehyde, Formalin, Paraformaldehyde And Glutaraldehyde: What They Are And What They Do. Microsc. Today 2000, 8, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.S.; Furusato, B.; Wong, K.; Sesterhenn, I.A.; Mostofi, F.K.; Wei, M.Q.; Zhu, Z.; Abbondanzo, S.L.; Liang, Q. Ultrasound-accelerated formalin fixation of tissue improves morphology, antigen and mRNA preservation. Mod. Pathol. 2005, 18, 850–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Jamal, A.; Abd El-Aziz, G.S.; Hamdy, R.M.; Al-Hayani, A.; Al-Maghrabi, J. The innovative safe fixative for histology, histopathology, and immunohistochemistry techniques: “Pilot study using shellac alcoholic solution fixative”. Microsc. Res. Tech. 2014, 77, 385–393. [Google Scholar] [CrossRef]
- Singhal, P.; Singh, N.N.; Sreedhar, G.; Banerjee, S.; Batra, M.; Garg, A. Evaluation of histomorphometric changes in tissue architecture in relation to alteration in fixation protocol—An invitro study. J. Clin. Diagn. Res. 2016, 10, ZC28–ZC32. [Google Scholar] [CrossRef]
- Ahmad, S.; Butt, M.S.; Pasha, I.; Sameen, A. Quality of processed cheddar cheese as a function of emulsifying salt replaced by κ-Carrageenan. Int. J. Food Prop. 2015, 19, 1874–1883. [Google Scholar] [CrossRef]
- Cummings, R.D.; Etzler, M.E. Antibodies and Lectins in Glycan Analysis. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Trullols, E.; Ruisánchez, I.; Rius, F.X. Validation of qualitative analytical methods. TrAC Trends Anal. Chem. 2004, 23, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R. The impact of histochemistry—A historical perspective. Acta Histochem. 2000, 102, 5–14. [Google Scholar] [CrossRef]
- Zheng, H.; Gasbarrino, K.; Veinot, J.P.; Lai, C.; Daskalopoulou, S.S. New Quantitative Digital Image Analysis Method of Histological Features of Carotid Atherosclerotic Plaques. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 654–663. [Google Scholar] [CrossRef]
- Fu, R.; Ma, X.; Bian, Z.; Ma, J. Digital separation of diaminobenzidine-stained tissues via an automatic color-filtering for immunohistochemical quantification. Biomed. Opt. Express 2015, 6, 544. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.R.; Beck, M.; Hill, M.E. Replicating Red: Analysis of ceramic slip color with CIELAB color data. J. Archaeol. Sci. Rep. 2017, 14, 432–438. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Christiano, F.D.P.; Marczak, L.D.F.; Tessaro, I.C.; Thys, R.C.S. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT Food Sci. Technol. 2014, 58, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Quemener, B.; Marot, C.; Mouillet, L.; Da Riz, V.; Diris, J. Quantitative analysis of hydrocolloids in food systems by methanolysis coupled to reverse HPLC. Part Gelling carrageenans. Food Hydrocoll. 2000, 14, 9–17. [Google Scholar] [CrossRef]
- Hassan, R.A.; Heng, L.Y.; Tan, L.L. Novel DNA Biosensor for Direct Determination of Carrageenan. Sci. Rep. 2019, 9, 6379. [Google Scholar] [CrossRef]
- Bartlová, M.; Ziółkowska, D.; Pospiech, M.; Shyichuk, A.; Tremlová, B. Determination of carrageenan in jellies with new methylene blue dye using spectrophotometry, smartphone-based colorimetry and spectrophotometric titration. Food Sci. Technol. 2021, 41, 81–90. [Google Scholar] [CrossRef]
- Soedjak, H.S. Colorimetric micromethod for protein determination with erythrosin B. Anal. Biochem. 1994, 66, 4514–4518. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, D.; Kaniewska, A.; Lamkiewicz, J.; Shyichuk, A. Determination of carrageenan by means of photometric titration with Methylene Blue and Toluidine Blue dyes. Carbohydr. Polym. 2017, 165, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, J.; Rick, N.R.; Thomson, W.A.B. Quantitative determination of carrageenan in infant formulas. J. Food Sci. 1977, 42, 252–254. [Google Scholar] [CrossRef]
- Graham, H.D. Quantitative Determination of Carrageenan in Milk and Milk Products Using Papain and Cetyl Pyridinium Chloride. J. Food Sci. 1968, 33, 390–394. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Meyerhoff, M.E.; Badr, I.H.A.; Abd-Rabboh, H.S.M. Determination of carrageenan in food products using potentiometric polyion sensors. Electroanalysis 2002, 14, 439–444. [Google Scholar] [CrossRef]
- Bednářová, M.; Pospiech, M.; Jandásek, J.; Tremlová, B. Carrageenans in the meat industry: Detection using microscopic methods. Maso Int. 2014, 1, 11–14. [Google Scholar]
- Zhang, Y.; Jiang, H.; Wang, W. Feasibility of the Detection of Carrageenan Adulteration in Chicken Meat Using Visible/Near-Infrared (Vis/NIR) Hyperspectral Imaging. Appl. Sci. 2019, 9, 3926. [Google Scholar] [CrossRef] [Green Version]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Bartůňková, J.; Paulík, M. Examination Methods in Immunology, 2nd ed.; Grada: Prague, Czech Republic, 2011; ISBN 978-80-247-3533-7. [Google Scholar]
- Yang, X.; Vidunas, A.J.; Beniash, E. Optimizing immunostaining of enamel matrix: Application of sudan black B and minimization of false positives from normal sera and IgGs. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.-M. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur. J. Neurosci. 2008, 28, 2365–2370. [Google Scholar] [CrossRef]



| Lectin Concentration | Color Intensity | L* | a* | b* |
|---|---|---|---|---|
| 10 μg mL−1 | +++ | 81.54 ± 9.18 a | −2.63 ± 2.01 a | 18.17 ± 5.73 b |
| 2 μg mL−1 | +++ | 86.05 ± 5.68 ab | −5.08 ± 0.94 b | 17.91 ± 4.22 a |
| 1 μg mL−1 | +++ | 89.82 ± 3.73 b | −6.02 ± 0.94 b | 11.77 ± 3.53 a |
| Sample | Carrageenan Type | Carrageenan Concentration [mg kg−1] | Number of Positive/Negative Sections * | Correct Rate |
|---|---|---|---|---|
| LD1A | κ-carrageenan | 100 | 6/2 | 75% |
| LD1B | κ-carrageenan | 1000 | 8/0 | 100% |
| LD1C | κ-carrageenan | 10,000 | 8/0 | 100% |
| LD2A | ι-carrageenan | 100 | 8/0 | 100% |
| LD2B | ι-carrageenan | 1000 | 7/1 | 87.5% |
| LD2C | ι-carrageenan | 10,000 | 6/2 | 75% |
| LD3A | λ-carrageenan | 100 | 8/0 | 100% |
| LD3B | λ-carrageenan | 1000 | 3/5 | 60% |
| LD3C | λ-carrageenan | 10,000 | 8/0 | 100% |
| LD40 | Control sample (free from carrageenan) | 0 | 0/8 | 100% |
| Sample | Carrageenan Type | Carrageenan Concentration [mg kg−1] | L* | a* | b* |
|---|---|---|---|---|---|
| LD1A | κ | 100 | 81.31 ± 6.06 de | −12.66 ± 4.82 a | 38.12 ± 7.82 bc |
| LD1B | κ | 1000 | 86.62 ± 4.58 bc | −12.89 ± 2.33 a | 35.18 ± 5.76 cd |
| LD1C | κ | 10,000 | 88.28 ± 4.20 ab | −18.44 ± 1.59 f | 31.80 ± 6.18 ef |
| LD2A | ι | 100 | 26.2 ± 3.67 c | −13.30 ± 1.16 ab | 40.52 ± 4.15 b |
| LD2B | ι | 1000 | 81.51 ± 4.62 d | −15.89 ± 2.63 de | 36.63 ± 6.55 cd |
| LD2C | ι | 10,000 | 86.60 ± 3.77 bc | −16.59 ± 3.72 e | 35.42 ± 6.33 cd |
| LD3A | λ | 100 | 89.71 ± 2.57 a | −14.49 ± 1.19 bc | 36.32 ± 5.64 cd |
| LD3B | λ | 1000 | 76.57 ± 3.18 f | −14.88 ± 1.86 cd | 34.16 ± 4.88 de |
| LD3C | λ | 10,000 | 87.99 ± 4.64 ab | −13.83 ± 2.08 abc | 29.48 ± 5.55 f |
| LD40 | Control | 0 | 78.95 ± 5.76 e | −14.15 ± 3.67 abc | 43.64 ± 4.11 a |
| Sample No. | Result | Declared Ingredient | Human Inspection LH | CIE b* | |
|---|---|---|---|---|---|
| Threshold Comparison | ANOVA Comparison | ||||
| Control | 0/2 0/2 0/2 0/2 | N | N | N | 43.64 ± 4.10 a |
| 65-19 | 1/1 0/2 1/1 0/2 | N | N | N | 41.18 ± 9.74 ab |
| 66-19 | 0/2 0/2 0/2 0/2 | N | N | P | 17.73 ± 2.63 hij |
| 67-19 | 2/0 2/0 2/0 2/0 | N | P | P * | 25.54 ± 8.63 de |
| 68-19 | 2/0 2/0 2/0 2/0 | N | P | N | 35.61 ± 4.45 c |
| 69-19 | 0/2 0/2 0/2 0/2 | N | N | N | 39.39 ± 4.30 b |
| 70-19 | 2/0 2/0 2/0 2/0 | N | P | P | 22.89 ± 5.64 ef |
| 71-19 | 2/0 2/0 2/0 2/0 | N | P | P | 17.57 ± 4.62 hij |
| 72-19 | 2/0 2/0 2/0 2/0 | P | P | P * | 23.11 ± 7.74 ef |
| 73-19 | 2/0 2/0 2/0 2/0 | P | P | P | 19.33 ± 4.73 ghi |
| 74-19 | 2/0 2/0 2/0 2/0 | P | P | P | 20.95 ± 4.97 fg |
| 75-19 | 2/0 0/2 2/0 2/0 | P | P | P | 15.89 ± 2.94 jk |
| 76-19 | 2/0 2/0 2/0 2/0 | P | P | P | 20.64 ± 5.63 fgh |
| 77-19 | 2/0 2/0 2/0 2/0 | P | P | P | 13.87 ± 2.47 k |
| 78-19 | 2/0 2/0 2/0 2/0 | P | P | P | 20.50 ± 6.30 fgh |
| 79-19 | 2/0 2/0 2/0 2/0 | P | P | P | 15.54 ± 2.88 jk |
| 80-19 | 1/1 2/0 1/1 1/1 | P | P | P | 20.01 ± 7.03 fgh |
| 81-19 | 2/0 2/0 2/0 2/0 | P | P | P | 17.78 ± 5.50 hij |
| 82-19 | 2/0 1/1 2/0 0/2 | P | P | P | 16.05 ± 3.12 jk |
| 83-19 | 1/1 0/2 2/0 2/0 | P | P | P | 15.12 ± 2.10 jk |
| 146-19 | 2/0 2/0 2/0 2/0 | N | P | P | 15.13 ± 5.19 jk |
| 147-19 | 2/0 2/0 2/0 2/0 | N | P | P | 13.84 ± 3.69 k |
| 148-19 | 2/0 2/0 2/0 2/0 | N | P | P | 20.36 ± 4.25 fgh |
| 149-19 | 2/0 2/0 2/0 2/0 | P | P | P | 20.96 ± 4.99 fg |
| 151-19 | 2/0 2/0 2/0 2/0 | P | P | P | 18.14 ± 5.91 ghij |
| 152-19 | 2/0 2/0 2/0 2/0 | P | P | P | 13.92 ± 2.79 k |
| 153-19 | 2/0 1/1 2/0 2/0 | N | P | P * | 27.13 ± 7.92 d |
| 154-19 | 2/0 2/0 2/0 2/0 | N | P | P | 16.53 ± 3.26 ijk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlová, M.; Pospiech, M.; Javůrková, Z.; Tremlová, B. Detection of Carrageenan in Cheese Using Lectin Histochemistry. Appl. Sci. 2021, 11, 6903. https://doi.org/10.3390/app11156903
Bartlová M, Pospiech M, Javůrková Z, Tremlová B. Detection of Carrageenan in Cheese Using Lectin Histochemistry. Applied Sciences. 2021; 11(15):6903. https://doi.org/10.3390/app11156903
Chicago/Turabian StyleBartlová, Marie, Matej Pospiech, Zdeňka Javůrková, and Bohuslava Tremlová. 2021. "Detection of Carrageenan in Cheese Using Lectin Histochemistry" Applied Sciences 11, no. 15: 6903. https://doi.org/10.3390/app11156903






