Dielectrophoretic Manipulation of Cell Transfection Efficiency during Electroporation Using a Center Needle Electrode
Abstract
:1. Introduction
2. Materials and Methods
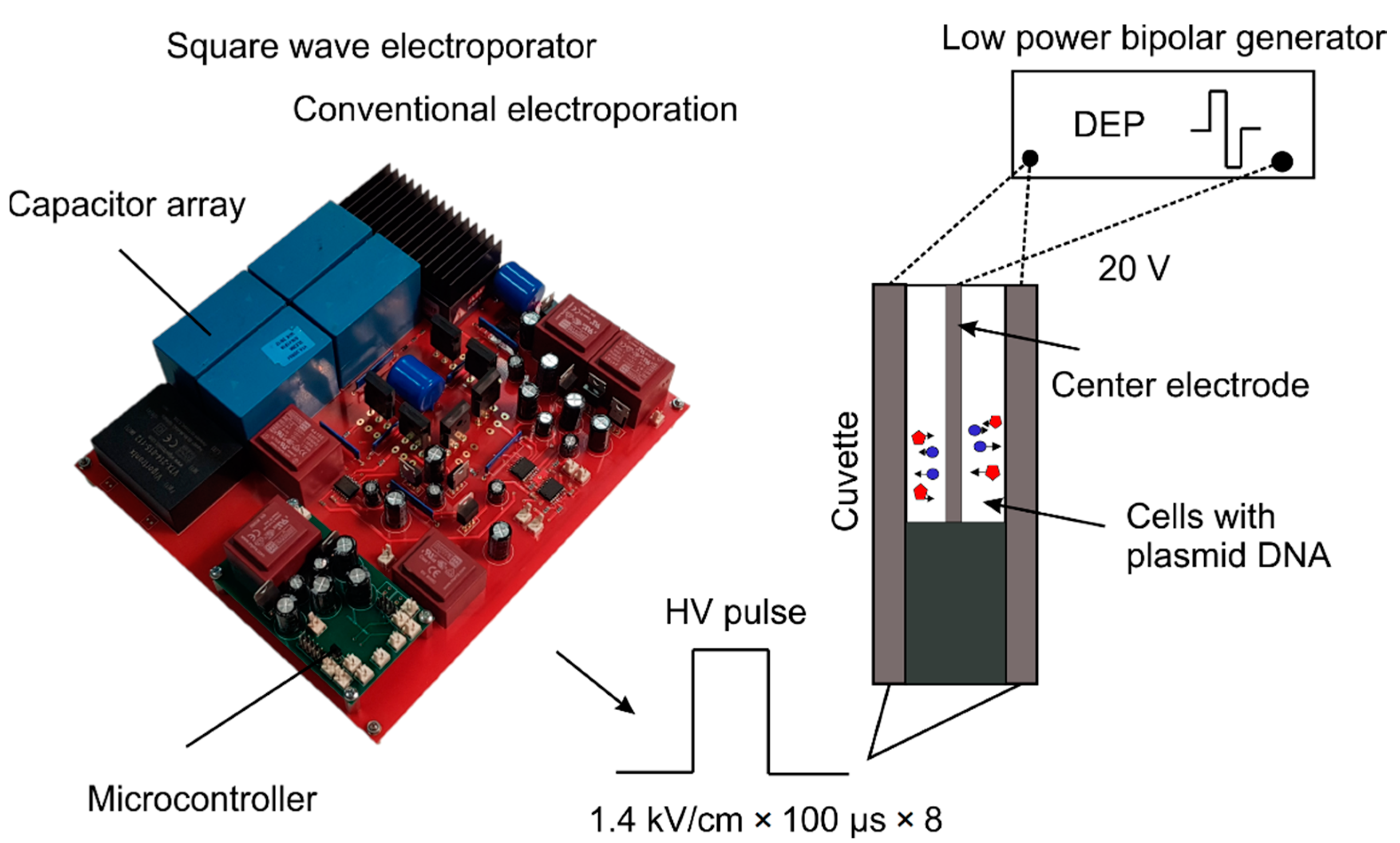
2.1. Dielectrophoresis-Assited Electroporation Setup
2.2. Preparation of Cells
2.3. Transfection of Cells
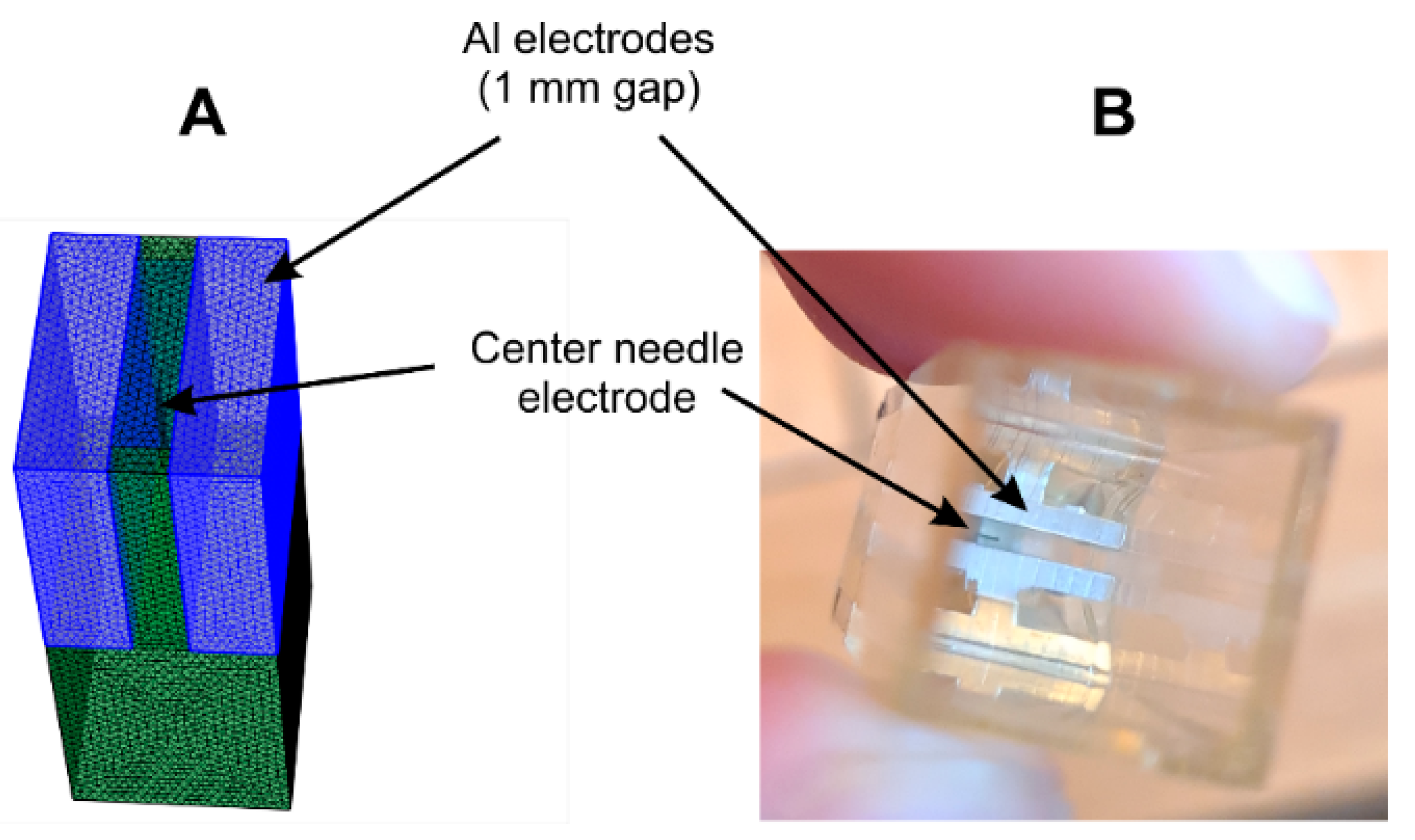
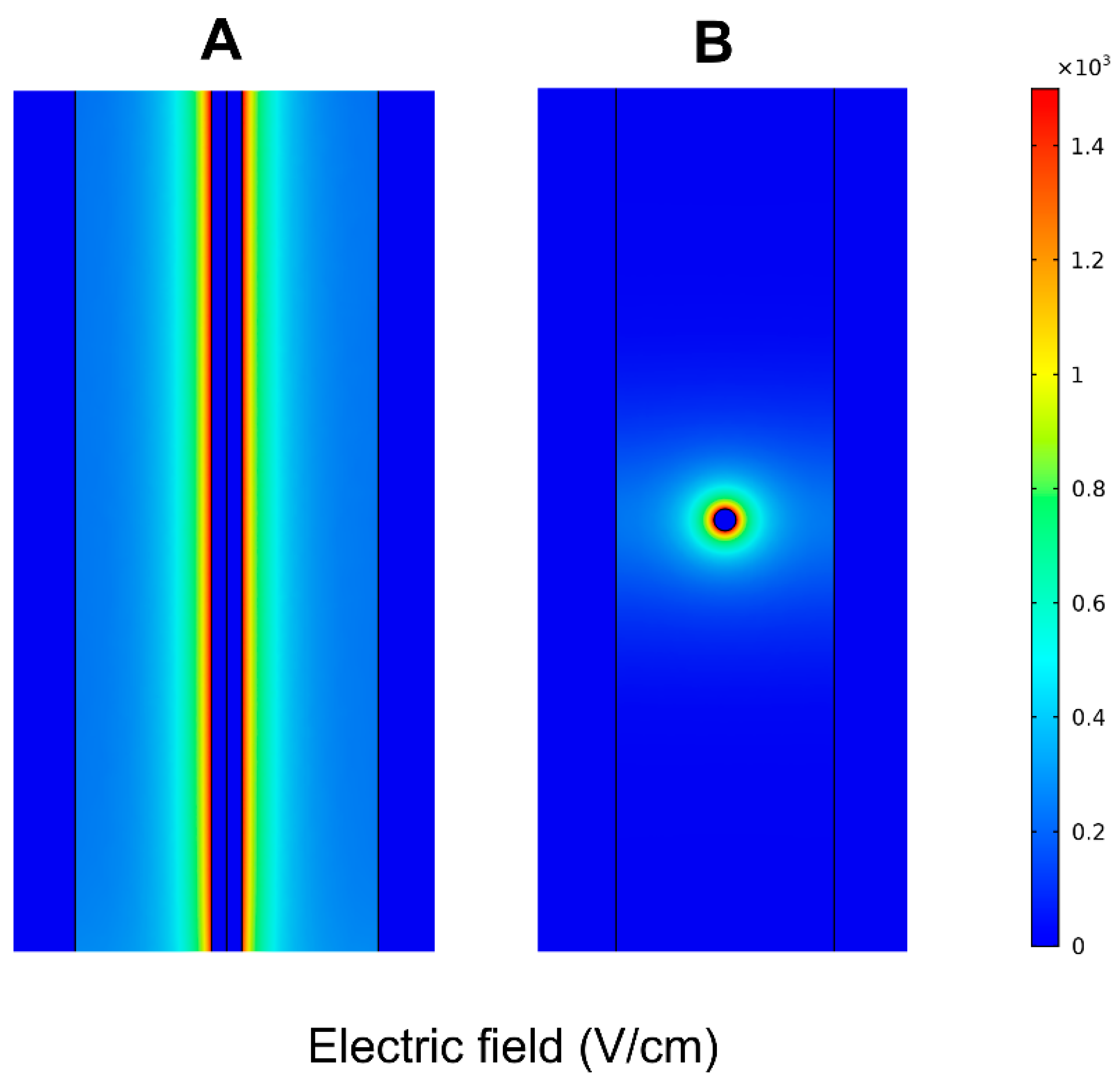
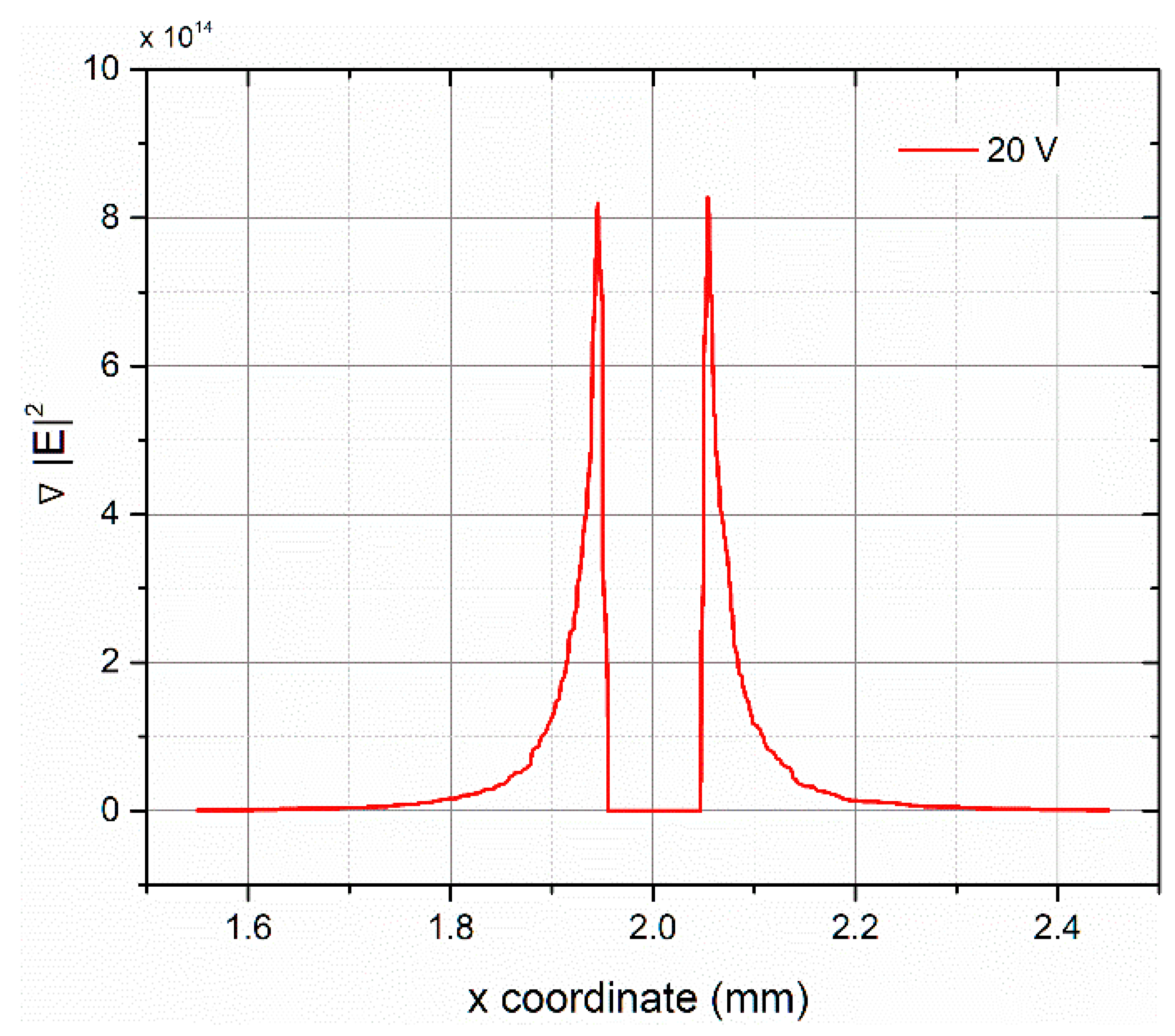
2.4. Finite Element Method Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 1–7. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in vitro gene delivery: It is now time to set the bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A review on electroporation-based intracellular delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef] [Green Version]
- Covello, G.; Siva, K.; Adami, V.; Denti, M.A. An electroporation protocol for efficient DNA transfection in PC12 cells. Cytotechnology 2014, 66, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubiniec, R.T.; Liang, H.; Hui, S.W. Effects of pulse length and pulse strength on transfection by electroporation. Biotechniques 1990, 8, 16–20. [Google Scholar]
- Šatkauskas, S.; André, F.; Bureau, M.F.; Scherman, D.; Miklavčič, D.; Mir, L.M. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum. Gene Ther. 2005, 16, 1194–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlin, M.; Flisar, K.; Kandušer, M. The role of electrophoresis in gene electrotransfer. J. Membr. Biol. 2010, 236, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Mahnič-Kalamiza, S.; Miklavčič, D. Scratching the electrode surface: Insights into a high-voltage pulsed-field application from in vitro & in silico studies in indifferent fluid. Electrochim. Acta 2020, 363, 137187. [Google Scholar] [CrossRef]
- Szlasa, W.; Kiełbik, A.; Szewczyk, A.; Rembiałkowska, N.; Novickij, V.; Tarek, M.; Saczko, J.; Kulbacka, J. Oxidative Effects during Irreversible Electroporation of Melanoma Cells—In Vitro Study. Molecules 2020, 26, 154. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Zhao, D.; Wei, Z.; Zhong, W.; Wang, X.; Liang, Z.; Li, Z. Electroporation on microchips: The harmful effects of pH changes and scaling down. Sci. Rep. 2015, 5, 17817. [Google Scholar] [CrossRef] [Green Version]
- Olaiz, N.; Signori, E.; Maglietti, F.H.; Soba, A.; Suárez, C.; Turjanski, P.; Michinski, S.D.; Marshall, G. Tissue damage modeling in gene electrotransfer: The role of pH. Bioelectrochemistry 2014, 100, 105–111. [Google Scholar] [CrossRef]
- Haberl, S.; Kandušer, M.; Flisar, K.; Hodžić, D.; Bregar, V.B.; Miklavcič, D.; Escoffre, J.M.; Rols, M.P.; Pavlin, M. Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J. Gene Med. 2013, 15, 169–181. [Google Scholar] [CrossRef]
- Satkauskas, S.; Bureau, M.F.; Puc, M.; Mahfoudi, A.; Scherman, D.; Miklavčič, D.; Mir, L.M. Mechanisms of in vivo DNA electrotransfer: Respective contributions of cell electropermeabilization and DNA electrophoresis. Mol. Ther. 2002, 5, 133–140. [Google Scholar] [CrossRef]
- Kandušer, M.; Miklavčič, D.; Pavlin, M. Mechanisms involved in gene electrotransfer using high- and low-voltage pulses — An in vitro study. Bioelectrochemistry 2009, 74, 265–271. [Google Scholar] [CrossRef]
- Sadik, M.M.; Yu, M.; Zheng, M.; Zahn, J.D.; Shan, J.W.; Shreiber, D.I.; Lin, H. Scaling relationship and optimization of dou-ble-pulse electroporation. Biophys. J. 2014, 106, 801–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Jackson, D.L.; Burcus, N.I.; Chen, Y.J.; Xiao, S.; Heller, R. Gene electrotransfer enhanced by nanosecond pulsed elec-tric fields. Mol. Ther. Methods Clin. Dev. 2014, 1, 14043. [Google Scholar] [CrossRef] [PubMed]
- Chopinet, L.; Batista-Napotnik, T.; Montigny, A.; Rebersek, M.; Teissie, J.; Rols, M.-P.; Miklavčič, D. Nanosecond electric pulse effects on gene expression. J. Membr. Biol. 2013, 246, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Salomone, F.; Breton, M.; Leray, I.; Cardarelli, F.; Boccardi, C.; Bonhenry, D.; Tarek, M.; Mir, L.M.; Beltram, F. High-yield nontoxic gene transfer through conjugation of the CM 18-Tat11 chimeric peptide with nanosecond electric pulses. Mol. Pharm. 2014, 11, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Balevičiūtė, A.; Ruzgys, P.; Šatkauskas, S.; Novickij, J.; Zinkevičienė, A.; Girkontaitė, I. Sub-microsecond elec-trotransfection using new modality of high frequency electroporation. Bioelectrochemistry 2020, 136, 107594. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Yang, J.; Joo, S.W.; Banerjee, A.N.; Qian, S. Cell electrofusion in microfluidic devices: A review. Sens. Actuators B Chem. 2013, 178, 63–85. [Google Scholar] [CrossRef]
- Wang, C.H.; Lee, Y.H.; Kuo, H.T.; Liang, W.F.; Li, W.J.; Lee, G. Bin Dielectrophoretically-assisted electroporation using light-activated virtual microelectrodes for multiple DNA transfection. Lab Chip 2014, 14, 592–601. [Google Scholar] [CrossRef]
- Punjiya, M.; Nejad, H.R.; Mathews, J.; Levin, M.; Sonkusale, S. A flow through device for simultaneous dielectrophoretic cell trapping and AC electroporation. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.B.; Esveld, D.C.; Valero, A.; Lüttge, R.; Mastwijk, H.C.; Bartels, P.V.; Van Den Berg, A.; Boom, R.M. Electroporation of cells in microfluidic devices: A review. Anal. Bioanal. Chem. 2006, 385, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Pethig, R.; Lee, R.S.; Talary, M.S. Cell physiometry tools based on dielectrophoresis. JALA J. Assoc. Lab. Autom. 2004, 9, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.J.; Lin, Y.Y.; Lei, U.; Wu, M.S.; Yang, P.C. Measurement of the Clausius-Mossotti factor of generalized dielectrophoresis. Appl. Phys. Lett. 2014, 104, 083701. [Google Scholar] [CrossRef]
- Pesch, G.R.; Lorenz, M.; Sachdev, S.; Salameh, S.; Du, F.; Baune, M.; Boukany, P.E.; Thöming, J. Bridging the scales in high-throughput dielectrophoretic (bio-)particle separation in porous media. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Contreras Davila, G.; Gomez-Quinones, J.I.; Perez-Gonzalez, V.H.; Martinez-Duarte, R. Assessing the Advantages of Using Square Wave Signals for Particle Trapping in Carbon-Electrode Dielectrophoresis. ECS Trans. 2016, 72, 105. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, J.; Islam, M.; Duncan, J.; Natu, R.; Martinez-Duarte, R. Assessing the importance of the root mean square (RMS) value of different waveforms to determine the strength of a dielectrophoresis trapping force. Electrophoresis 2017, 38, 2561–2564. [Google Scholar] [CrossRef] [Green Version]
- Novickij, V.; Grainys, A.; Butkus, P.; Tolvaišienė, S.; Švedienė, J.; Paškevičius, A.; Novickij, J. High-frequency submicrosecond electroporator. Biotechnol. Biotechnol. Equip. 2016, 30, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Salimi, E.; Braasch, K.; Butler, M.; Thomson, D.J.; Bridges, G.E. Dielectric model for Chinese hamster ovary cells obtained by dielectrophoresis cytometry. Biomicrofluidics 2016, 10, 014111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethig, R.; Markx, G.H. Applications of dielectrophoresis in biotechnology. Trends Biotechnol. 1997, 15, 426–432. [Google Scholar] [CrossRef]
- Čepurniene, K.; Ruzgys, P.; Treinys, R.; Šatkauskiene, I.; Šatkauskas, S. Influence of plasmid concentration on DNA electro-transfer in vitro using high-voltage and low-voltage pulses. J. Membr. Biol. 2010, 236, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.; Potier, E.; Logeart-Avramoglou, D.; Salomskaite-Davalgiene, S.; Mir, L.M.; Petite, H. Optimization of a gene electrotransfer method for mesenchymal stem cell transfection. Gene Ther. 2008, 15, 537–544. [Google Scholar] [CrossRef]
- Regtmeier, J.; Thanh, T.D.; Eichhorn, R.; Anselmetti, D.; Ros, A. Dielectrophoretic manipulation of DNA: Separation and polarizability. Anal. Chem. 2007, 79, 3925–3932. [Google Scholar] [CrossRef]
- Asbury, C.L.; Diercks, A.H.; Van Den Engh, G. Trapping of DNA by dielectrophoresis. Electrophoresis 2002, 23, 2658–2666. [Google Scholar] [CrossRef]
- Cervia, L.D.; Yuan, F. Current Progress in Electrotransfection as a Nonviral Method for Gene Delivery. Mol. Pharm. 2018, 15, 3617–3624. [Google Scholar] [CrossRef]
- Antov, Y.; Barbul, A.; Mantsur, H.; Korenstein, R. Electroendocytosis: Exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys. J. 2005, 88, 2206–2223. [Google Scholar] [CrossRef] [Green Version]
- Rosemberg, Y.; Korenstein, R. Incorporation of macromolecules into cells and vesicles by low electric fields: Induction of en-docytotic-like processes. Bioelectrochem. Bioenerg. 1997, 42, 275–281. [Google Scholar] [CrossRef]
- Abd-Elghany, A.A.; Mir, L.M. Endocytosis rate increase provoked by bipolar asymmetric electric pulses. Biotechnol. Biochem. Res. 2015, 3, 61–76. [Google Scholar]

| Symbol | Parameters | ||
|---|---|---|---|
| Name | Value | Units | |
| σm | Medium conductivity | 0.1 | S/m |
| εm | Medium permittivity | 80 | - |
| g | Cuvette gap | 1 | mm |
| V | Applied voltage | 20 | V |
| d | Center electrode diameter | 0.1 | mm |
| Protocol | Viability | p-Value |
|---|---|---|
| PEF (1.4 kV/cm × 100 µs × 8) | 71 ± 15% | p < 0.05 * |
| PEF + DEP3, 1 min | 76 ± 8% | p > 0.05 ** |
| PEF + DEP3, 3 min | 72 ± 13% | p > 0.05 ** |
| PEF + DEP3, 5 min | 75 ± 7% | p > 0.05 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzevičiūtė, E.; Murauskas, A.; Ruzgys, P.; Šatkauskas, S.; Girkontaitė, I.; Novickij, J.; Novickij, V. Dielectrophoretic Manipulation of Cell Transfection Efficiency during Electroporation Using a Center Needle Electrode. Appl. Sci. 2021, 11, 7015. https://doi.org/10.3390/app11157015
Radzevičiūtė E, Murauskas A, Ruzgys P, Šatkauskas S, Girkontaitė I, Novickij J, Novickij V. Dielectrophoretic Manipulation of Cell Transfection Efficiency during Electroporation Using a Center Needle Electrode. Applied Sciences. 2021; 11(15):7015. https://doi.org/10.3390/app11157015
Chicago/Turabian StyleRadzevičiūtė, Eivina, Arūnas Murauskas, Paulius Ruzgys, Saulius Šatkauskas, Irutė Girkontaitė, Jurij Novickij, and Vitalij Novickij. 2021. "Dielectrophoretic Manipulation of Cell Transfection Efficiency during Electroporation Using a Center Needle Electrode" Applied Sciences 11, no. 15: 7015. https://doi.org/10.3390/app11157015






