Amperometric Biosensors for L-Arginine Determination Based on L-Arginine Oxidase and Peroxidase-Like Nanozymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Enzyme Isolation and Purification
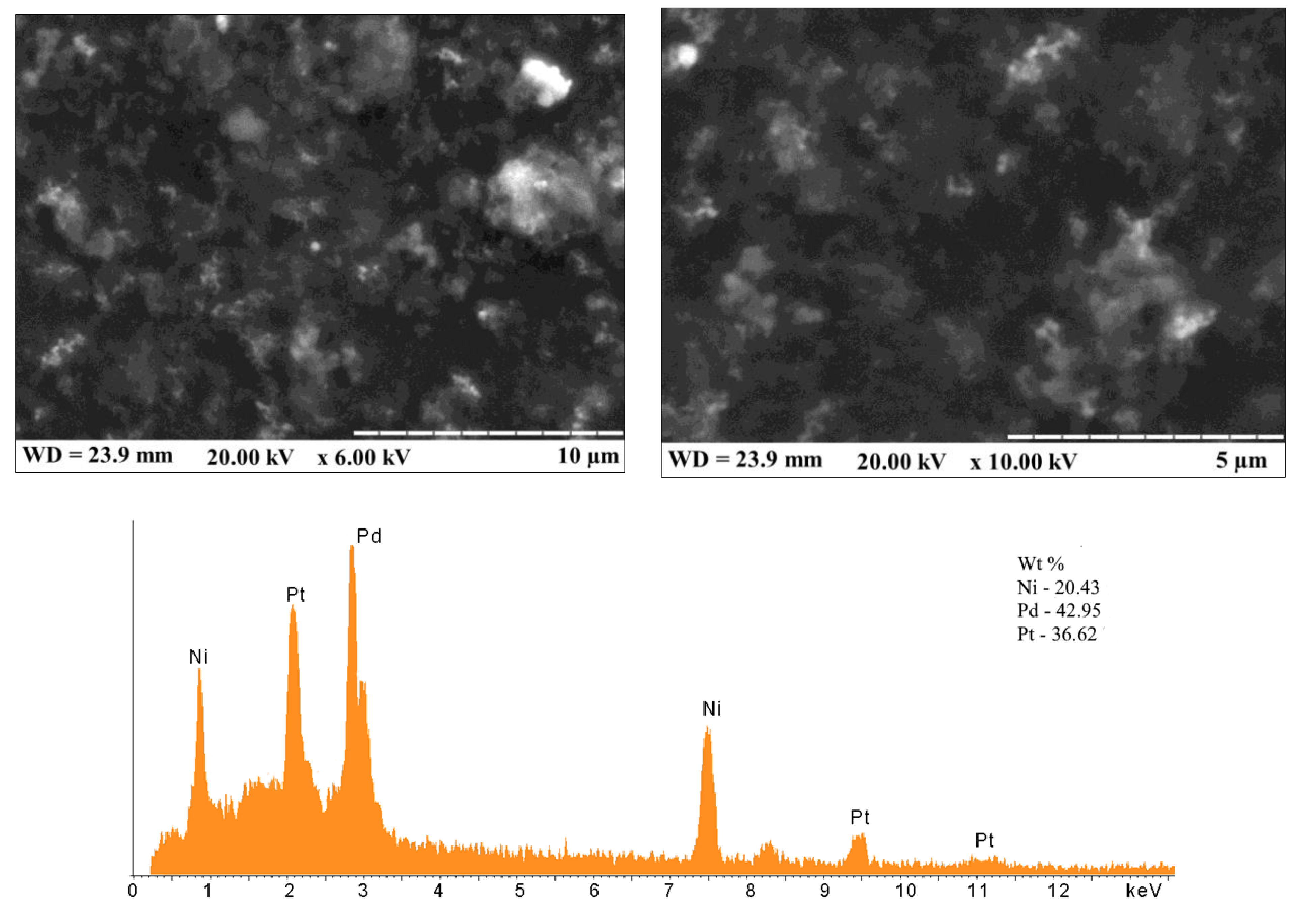
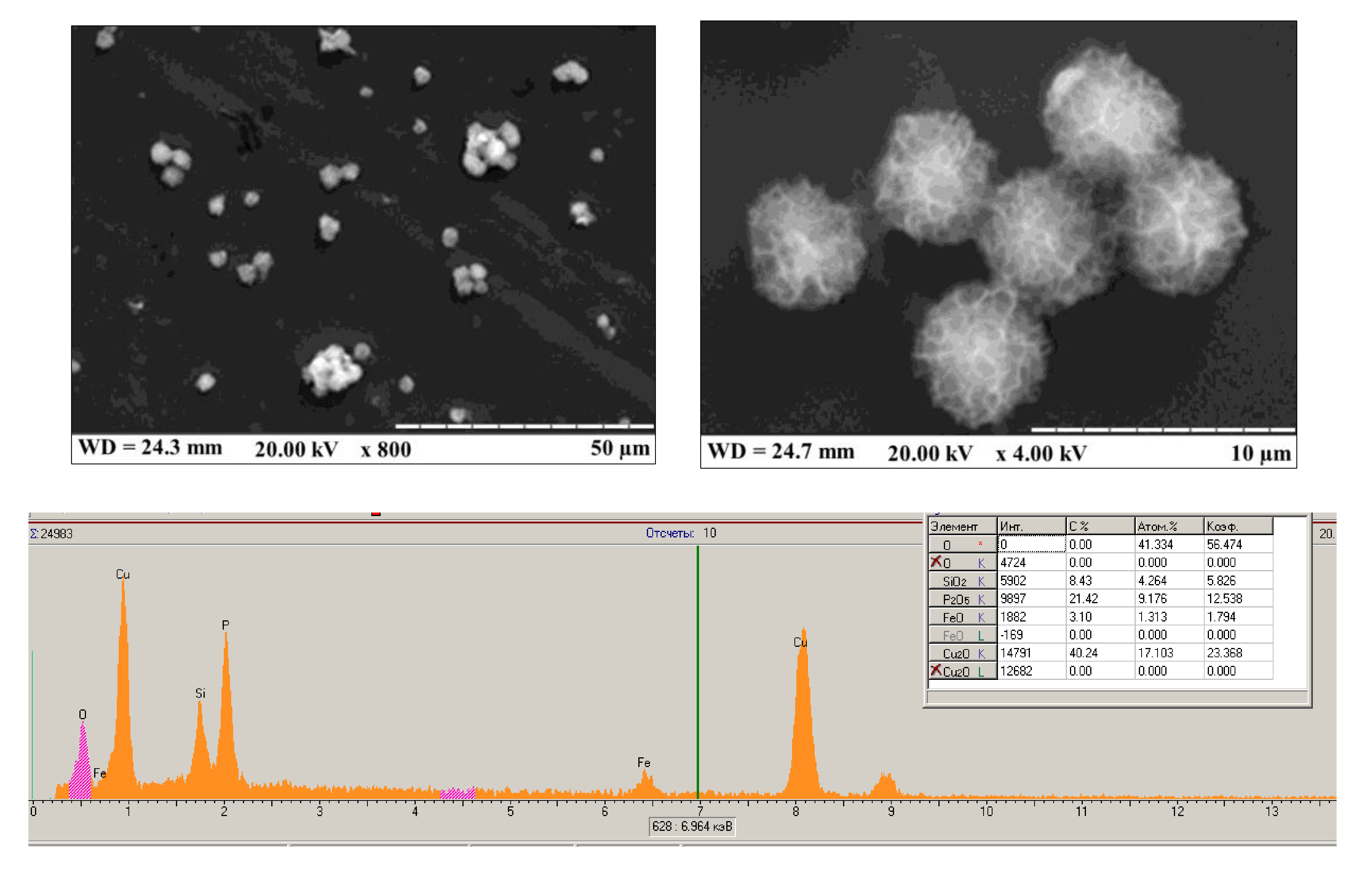
2.3. Synthesis of PO-Like NZs
2.4. Assay of Enzyme-Like Activities of the Synthesized PO-Like NZs in Solution
2.5. Sensor Evaluation
2.5.1. Apparatus and Measurements
2.5.2. Immobilization of NZs and Enzyme on the GE Surface
2.6. Preparation of the Real Samples for Biosensor Analysis
3. Results and Discussion
3.1. Amperometric Characteristics of the PO-Like NZs/GE
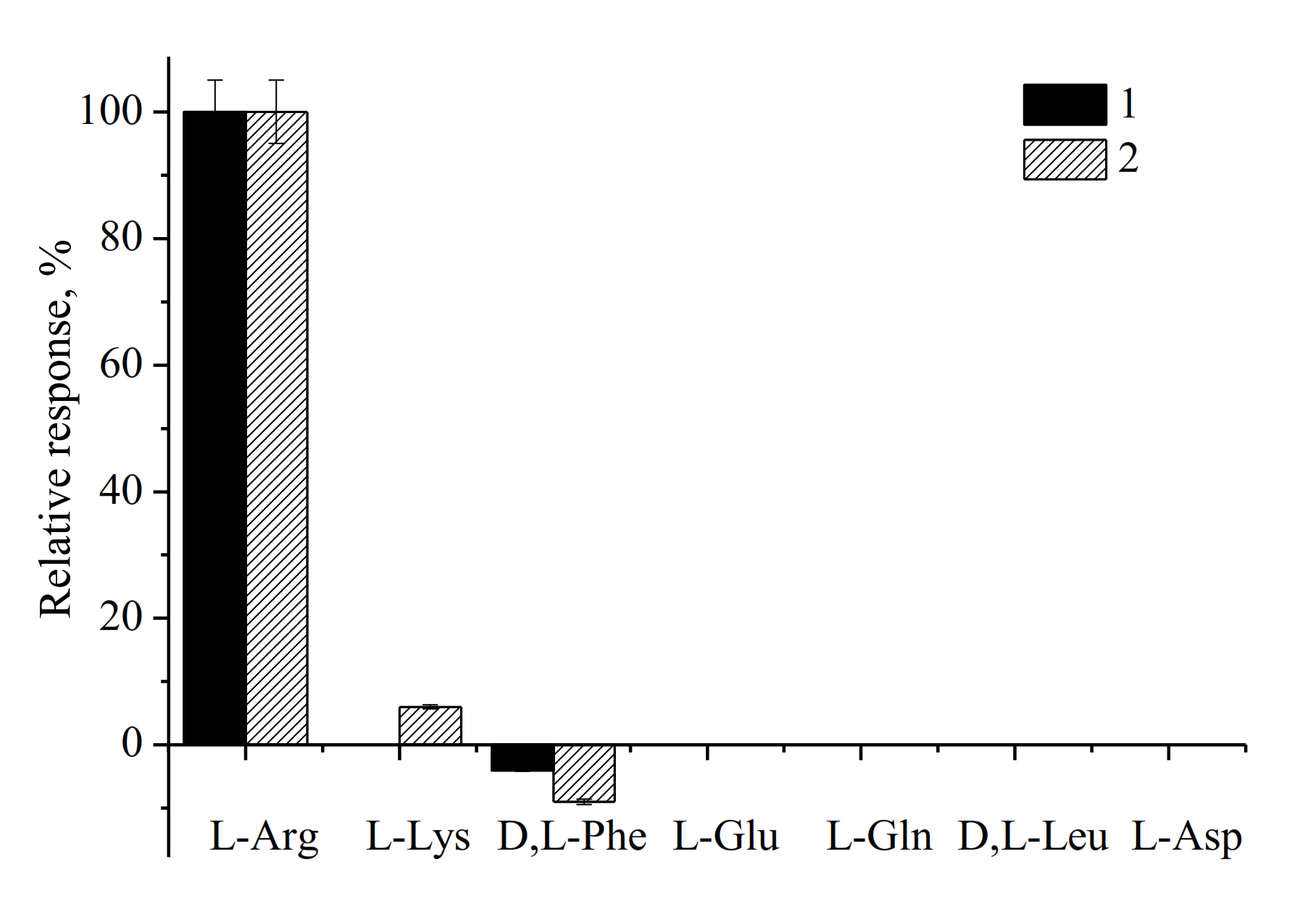
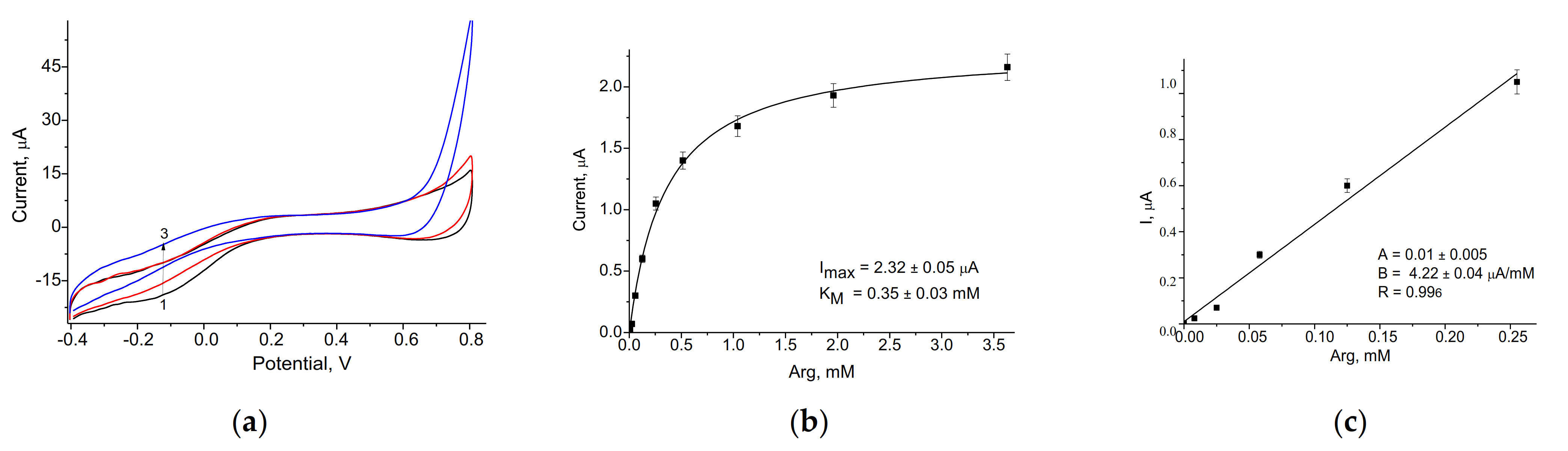
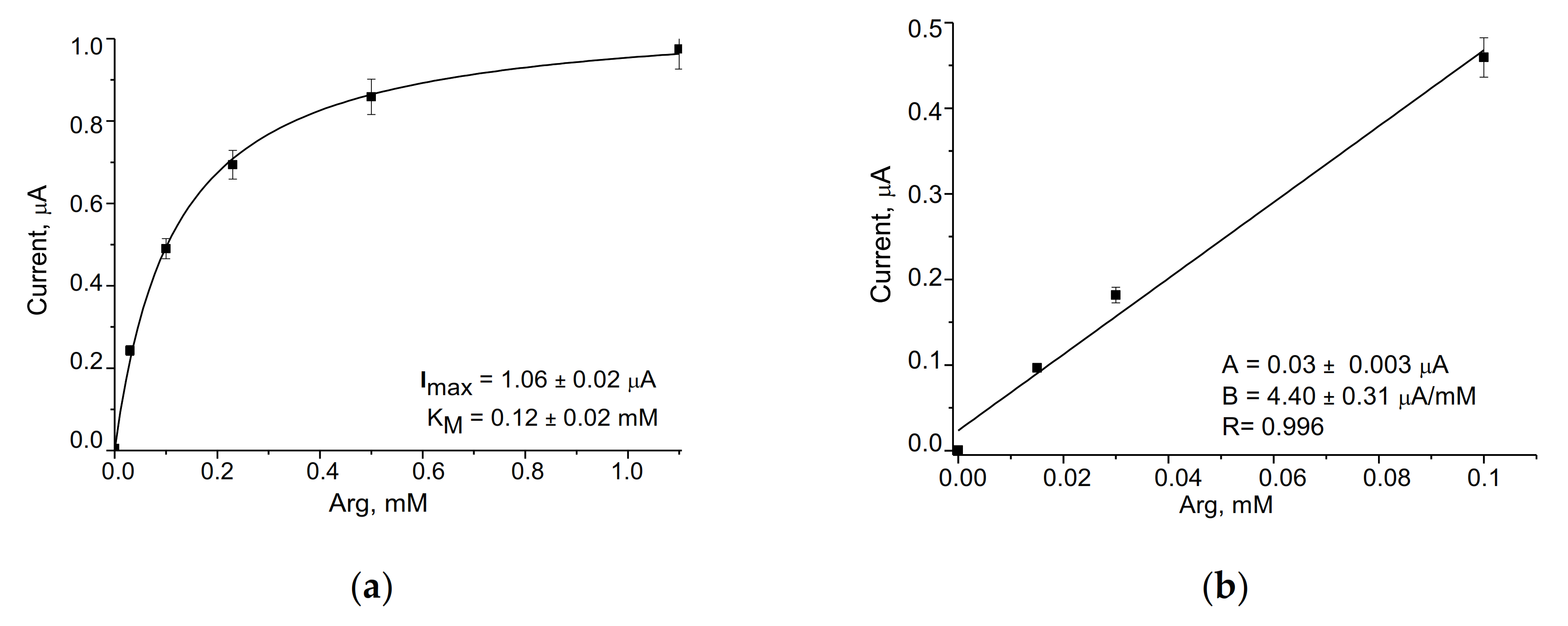
3.2. Evaluation and Optimization of the Arg-Sensitive Bioelectrodes
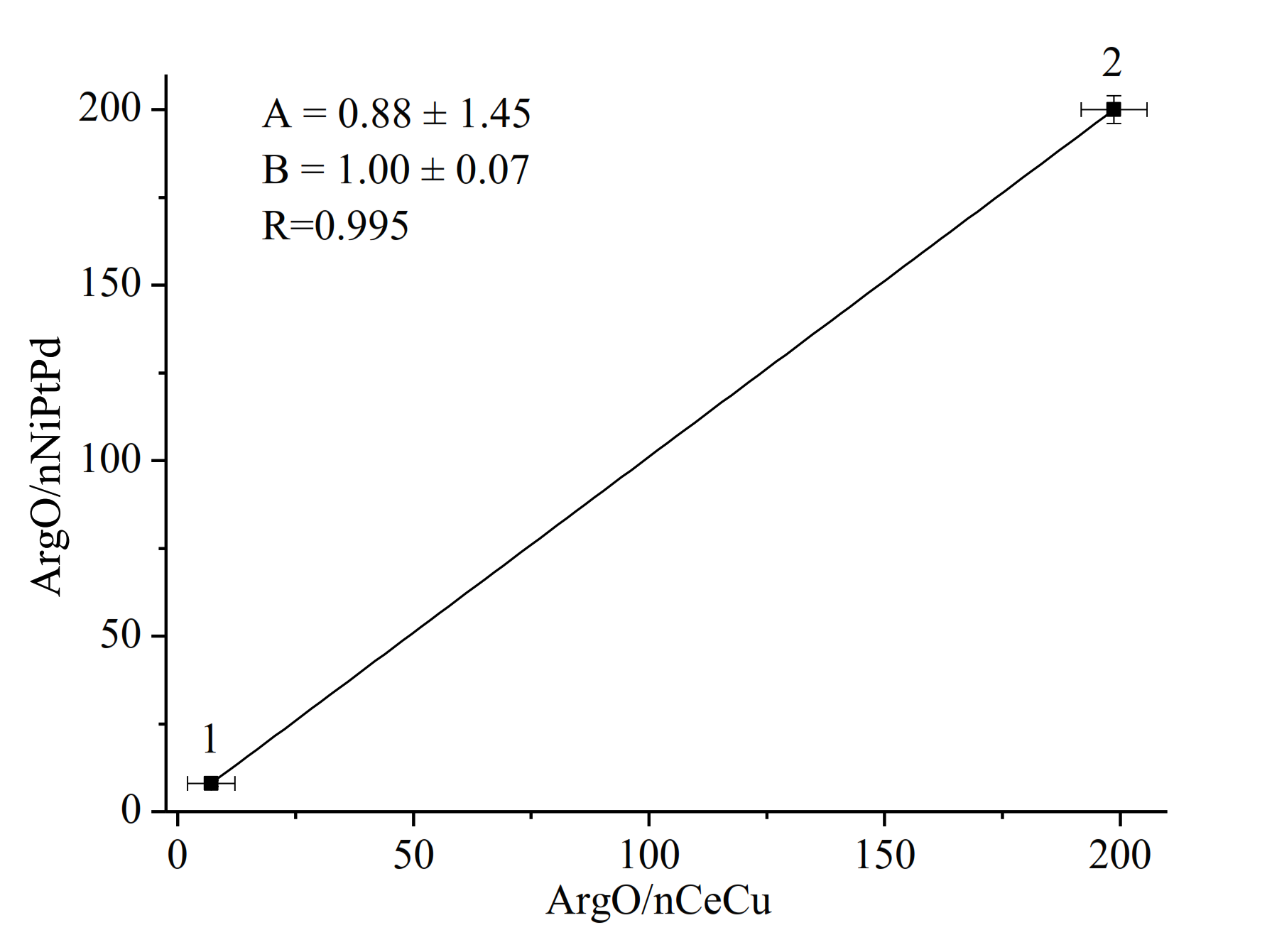
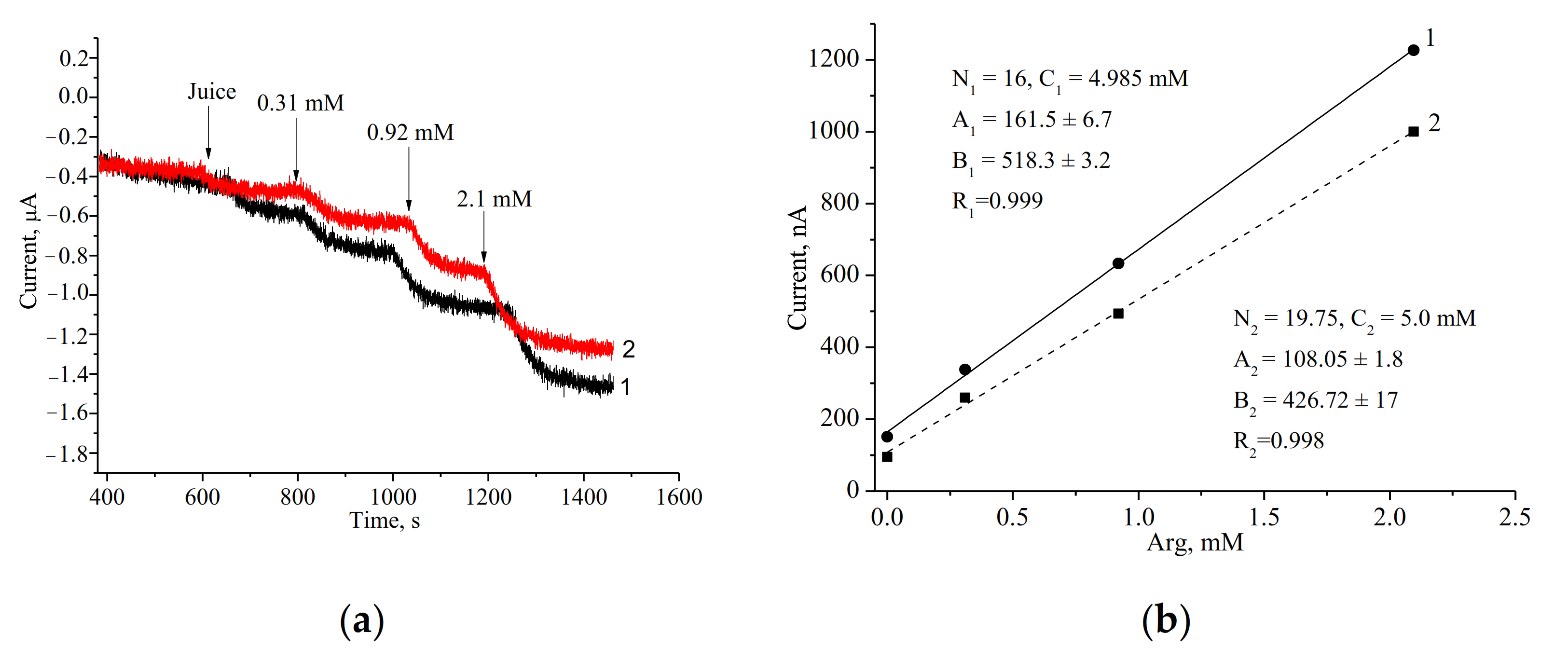
3.3. Assay of Arg in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Wu, G.B.; F, W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ladeiras, D.; Yu, Y.; Ming, X.F.; Yang, Z. Detrimental Effects of Chronic L-Arginine Rich Food on Aging Kidney. Front. Pharmacol. 2021, 11, 582155. [Google Scholar] [CrossRef] [PubMed]
- McNeal, C.J.; Meininger, C.J.; Reddy, D.; Wilborn, C.D.; Wu, G. Safety and effectiveness of arginine in adults. J. Nutr. 2016, 146, 2587S–2593S. [Google Scholar] [CrossRef] [PubMed]
- Bednarz-Misa, I.; Fleszar, M.G.; Zawadzki, M.; Kapturkiewicz, B.; Kubiak, A.; Neubauer, K.; Witkiewicz, W.; Krzystek-Korpacka, M. L-Arginine/NO pathway metabolites in colorectal cancer: Relevance as disease biomarkers and predictors of adverse clinical outcomes following surgery. J. Clin. Med. 2020, 9, 1782. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Azizi, F. Habitual intake of dietary L-arginine in relation to risk of type 2 diabetes: A prospective study. BMC Endocr. Disord. 2021, 21, 113. [Google Scholar] [CrossRef]
- Grimes, J.M.; Khan, S.; Badeaux, M.; Rao, R.M.; Rowlinson, S.W.; Carvajal, R.D. Arginine depletion as a therapeutic approach for patients with COVID-19. Int. J. Infect. Dis. 2021, 102, 566–570. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Zakalskiy, A.; Zakalska, O.; Fayura, L.; Vovk, O.; Stasyk, O.; Sibirny, A.; Gonchar, M. Recombinant Forms of Arginase and Arginine Deiminase as Catalytic Components of Argitest Enzymatic Kit. Sci. Innov. 2017, 13, 56–63. [Google Scholar] [CrossRef]
- He, Y.; Zhou, L.; Deng, L.; Feng, Z.; Cao, Z.; Yin, Y. An electrochemical impedimetric sensing platform based on a peptide aptamer identified by high-throughput molecular docking for sensitive L-arginine detection. Bioelectrochemistry 2021, 137, 107634. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, R.; Singh, M.; Verma, N. Electrochemical determination of L-arginine in leukemic blood samples based on a polyaniline-multiwalled carbon nanotube-magnetite nanocomposite film modified glassy carbon electrode. Instrum. Sci. Technol. 2020, 48, 400–416. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Fayura, L.; Boretskyy, Y.R.; Gonchar, M.V.; Sibirny, A.A. Novel arginine deiminase-based method to assay L-arginine in beverages. Food Chem. 2016, 201, 320–326. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Yepremyan, H.; Stepien, A.; Gonchar, M. Fluorometric enzymatic assay of L-arginine. Spectrochim. Acta A 2017, 70, 184–190. [Google Scholar] [CrossRef]
- Berninger, T.; Bliem, C.; Piccinini, E.; Azzaroni, O.; Knoll, W. Cascading reaction of arginase and urease on a graphene-based FET for ultrasensitive, real-time detection of arginine. Biosens. Bioelectron. 2018, 115, 104–110. [Google Scholar] [CrossRef]
- Verma, N.; Singh, A.K.; Singh, M. L-Arginine biosensors: A comprehensive review. Biochem. Biophys. Rep. 2017, 12, 228–239. [Google Scholar] [CrossRef]
- Saiapina, O.Y.; Dzyadevych, S.V.; Jaffrezic-Renault, N.; Soldatkin, O.P. Development and optimization of a novel conductometric bi-enzyme biosensor for L-Arginine determination. Talanta 2012, 92, 58–64. [Google Scholar] [CrossRef]
- Stasyuk, N.E.; Gaida, G.Z.; Gonchar, M.V. L-arginine assay with the use of arginase I. Appl. Biochem. Microbiol. 2013, 49, 529–534. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Dzyadevych, S.V.; Soldatkin, A.P. Electrochemical biosensors based on multienzyme systems: Main groups, advantages and limitations—A review. Anal. Chim. Acta 2020, 1111, 114–131. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Biosensors—Recent Advances and Future Challenges. Sensors 2020, 20, 6645. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Senda, M. Amperometric ammonium ion sensor and its application to biosensors. Sens. Actuators B Chem. 1993, 13, 57–60. [Google Scholar] [CrossRef]
- Stasyk, O.V.; Boretsky, Y.R.; Gonchar, M.V.; Sibirny, A.A. Recombinant arginine-degrading enzymes in metabolic anticancer therapy and bioanalytics. Cell Biol. Int. 2015, 39, 246–252. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Gayda, G.; Gonchar, M.; Koval’chuk, Y. A new bi-enzyme potentiometric sensor for arginine analysis based on recombinant human arginase I and commercial urease. J. Mater. Sci. Eng. 2011, 1, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Stasyuk, N.; Smutok, O.; Gayda, G.; Vus, B.; Koval’chuk, Y.; Gonchar, M. Bi-enzyme L-arginine-selective amperometric biosensor based on ammonium-sensing polyaniline-modified electrode. Biosens. Bioelectron. 2012, 37, 46–52. [Google Scholar] [CrossRef]
- Stasyuk, N.Y.; Gayda, G.Z.; Gonchar, M.V. L-arginine-selective microbial amperometric sensor based on recombinant yeast cells over-producing human liver arginase I. Sens. Actuators B Chem. 2014, 204, 515–521. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Soldatkin, O.O.; Velychko, T.P.; Prilipko, V.O.; Kuibida, M.A.; Dzyadevych, S.V. Conductometric biosensor for arginine determination in pharmaceutics. Bioelectrochemistry 2018, 124, 40–46. [Google Scholar] [CrossRef]
- Zakalskiy, A.E.; Zakalska, O.M.; Rzhepetskyy, Y.A.; Potocka, N.; Stasyk, O.V.; Horak, D.; Gonchar, M.V. Overexpression of (His)6-tagged human arginase I in Saccharomyces cerevisiae and enzyme purification using metal affinity chromatography. Protein Expr. Purif. 2012, 81, 63–68. [Google Scholar] [CrossRef]
- Karkovska, M.I.; Stasyuk, N.Y.; Gayda, G.Z.; Smutok, O.V.; Gonchar, M.V. Nanomaterials in construction of biosensors of biomedical purposes. In Multifunctional Nanomaterials for Biology and Medicine: Molecular Design, Synthesis, and Application; Stoika, R., Ed.; Naukova Dumka: Kyiv, Ukraine, 2017; pp. 165–177. ISBN 978-966-00-1564-7. (In Ukrainian) [Google Scholar]
- Zhybak, M.T.; Fayura, L.Y.; Boretsky, Y.R.; Gonchar, M.V.; Sibirny, A.A.; Dempsey, E.; Turner, A.; Korpan, Y.I. Amperometric L-Arginine biosensor based on a novel recombinant Arginine deiminase. Microchim. Acta 2017, 184, 2679–2686. [Google Scholar] [CrossRef] [Green Version]
- Zarei, M.; Rahbar, M.R.; Morowvat, M.H.; Nezafat, N.; Negahdaripour, M.; Berenjian, A.; Ghasemi, Y. Arginine Deiminase: Current Understanding and Applications. Recent Pat. Biotechnol. 2019, 13, 124–136. [Google Scholar] [CrossRef]
- Fayura, L.R.; Boretsky, Y.R.; Pynyaha, Y.V.; Wheatley, D.N.; Sibirny, A.A. Improved method for expression and isolation of the Mycoplasma hominis arginine deiminase from the recombinant strain of Escherichia coli. J. Biotechnol. 2013, 167, 420–426. [Google Scholar] [CrossRef]
- Matsui, D.; Terai, A.; Asano, Y. L-A;rginine oxidase from Pseudomonas sp. TPU 7192: A;rginine determination. Enzym. Microb. Technol. 2016, 82, 151–157. [Google Scholar] [CrossRef]
- Nakano, S.; Niwa, M.; Asano, Y. Following the Evolutionary Track of a Highly Specific L-A;rginine Oxidase by Reconstruction and Biochemical Analysis of Ancestral and Native Enzymes. Appl. Environ. Microbiol. 2019, 30, 567–570. [Google Scholar] [CrossRef]
- Sarkar, P.; Tothill, I.E.; Setford, S.J.; Turner, A.P. Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids. Analyst 1999, 124, 865–870. [Google Scholar] [CrossRef]
- Kacaniklic, V.; Johansson, K.; Marko-Varga, G.; Gorton, L.; Jönsson–Pettersson, G.; Csöregi, E. Amperometric biosensors for detection of L- and D-amino acids based on co-immobilized peroxidase and L- and D-amino acid oxidases in carbon paste electrodes. Electroanalysis 1994, 6, 381–390. [Google Scholar] [CrossRef]
- Qin, M.; Li, F.; Huang, Y.; Ran, W.; Han, D.; Song, Y. Twenty Natural Amino Acids Identification by a Photochromic Sensor Chip. Anal. Chem. 2014, 87, 837–842. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensor 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; El-Moghazy, A.Y.; Hussin, M.; Abu-Saied, M.A.; El-Shanshory, A.A.; Solman, H.M.A. The nanomaterials and recent progress in biosensing systems: A review. Trends Environ. Anal. Chem. 2020, 26, e00087. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors 2020, 20, 4509. [Google Scholar] [CrossRef]
- Tricot, C.; Stalon, V.; Legrain, C. Isolation and characterization of Pseudomonas putida mutants affected in arginine, ornithine and citrulline catabolism: Function of the arginine oxidase and arginine succinyltransferase pathways. J. Gen. Microbiol. 1991, 137, 2911–2918. [Google Scholar] [CrossRef] [Green Version]
- Hossain, G.S.; Li, J.; Shin, H.D.; Du, G.; Liu, L.; Chen, J. L-Amino acid oxidases from microbial sources: Types, properties, functions, and applications. Appl. Microbiol. Biotechnol. 2014, 98, 1507–1515. [Google Scholar] [CrossRef]
- Castellano, F.; Molinier-Frenkel, V. An Overview of L-Amino Acid Oxidase Functions from Bacteria to Mammals: Focus on the Immunoregulatory Phenylalanine Oxidase IL4I1. Molecules 2017, 22, 2151. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.W. A brief review of the scientific history of several lesser-known snake venom proteins: L-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon 2013, 62, 75–82. [Google Scholar] [CrossRef]
- Pollegioni, L.; Motta, P.; Molla, G. L-amino acid oxidase as biocatalyst: A dream too far? Appl. Microbiol. Biotechnol. 2013, 9, 9323–9341. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and L-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Gayda, G. Oxidases of L-amino acids: Obtaining, properties and prospects of utilization. Inter. Conf. 2021, 63, 228–237. [Google Scholar] [CrossRef]
- Demkiv, O.; Stasyuk, N.; Serkiz, R.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Peroxidase-Like Metal-Based Nanozymes: Synthesis, Catalytic Properties, and Analytical Application. Appl. Sci. 2021, 11, 777. [Google Scholar] [CrossRef]
- Gayda, G.Z.; Demkiv, O.M.; Gurianov, Y.; Serkiz, R.Y.; Klepach, H.M.; Gonchar, M.V.; Nisnevitch, M. “Green” Prussian Blue Analogues as Peroxidase Mimetics for Amperometric Sensing and Biosensing. Biosensors 2021, 11, 193. [Google Scholar] [CrossRef]
- Austin, K.T.; Butzke, C.E. Spectrophotometric Assay for Arginine in Grape Juice and Must. Am. J. Enol. Vitic. 2000, 51, 227–232. [Google Scholar]
- Mira De Orduña, R.; Liu, S.Q.; Patchett, M.L.; Pilone, G.J. Ethyl carbamate precursor citrulline formation from Arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 183, 31–35. [Google Scholar] [CrossRef]
- Petropoulos, S.; Metafa, M.; Kotseridis, Y.; Paraskevopoulos, I.; Kallithraka, S. Amino acid content of Agiorgitiko (Vitis vinifera L. cv.) grape cultivar grown in representative regions of Nemea. Eur. Food Res. Technol. 2018, 244, 2041–2050. [Google Scholar] [CrossRef]

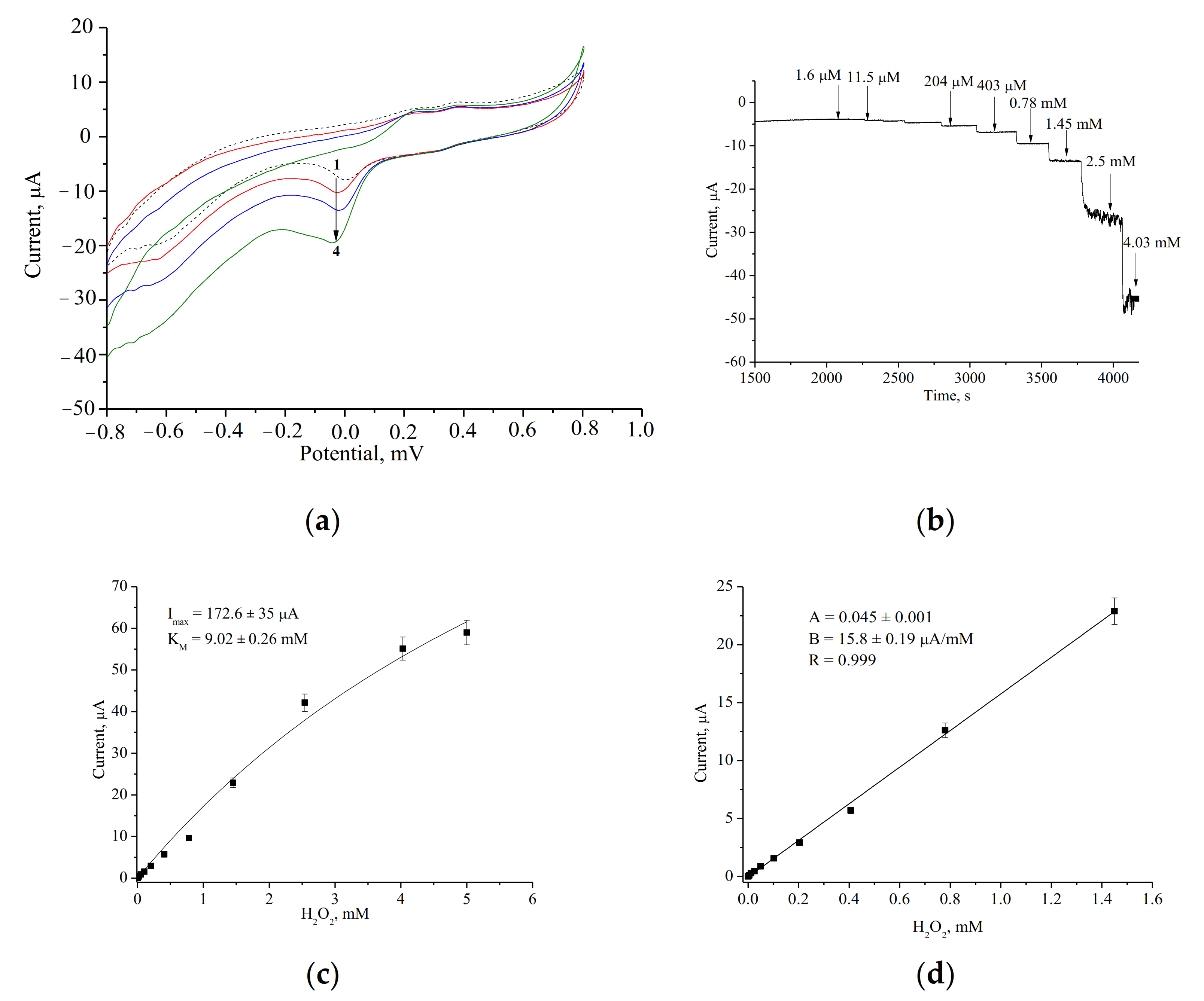
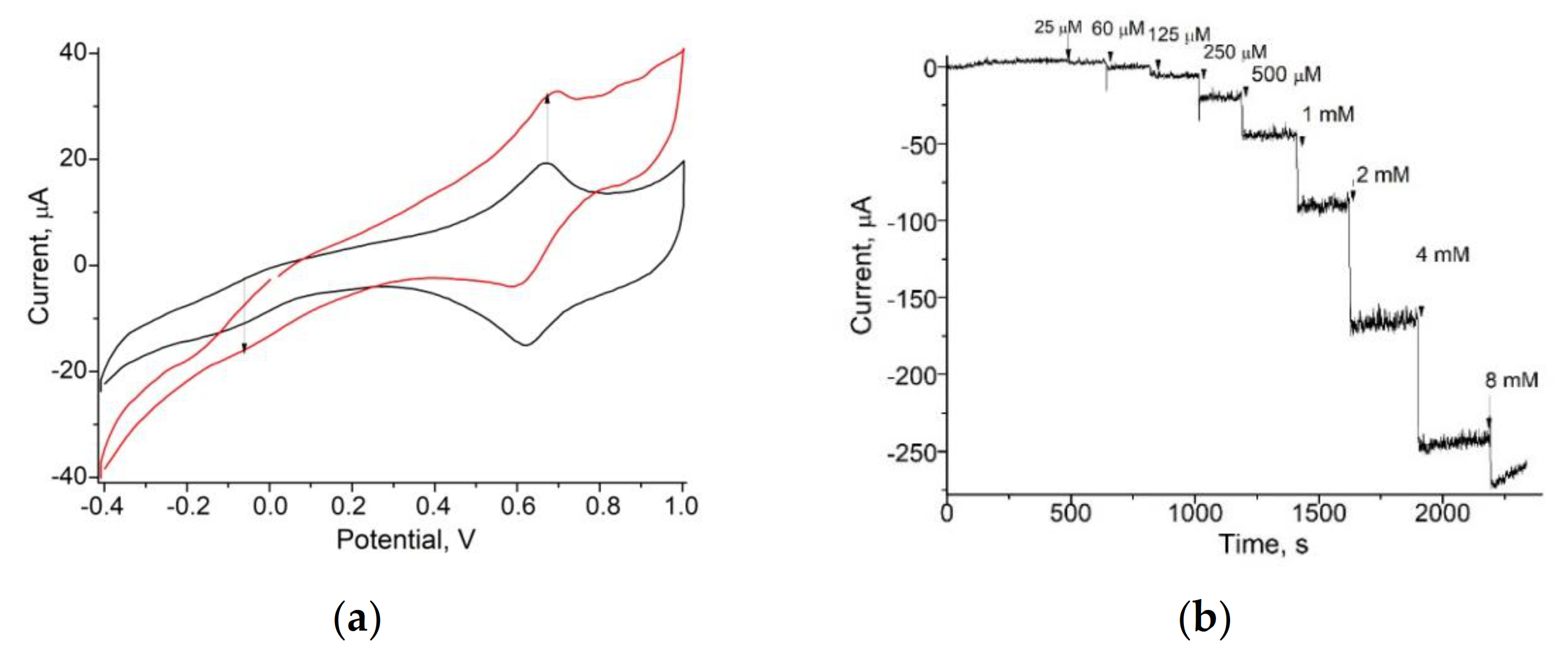
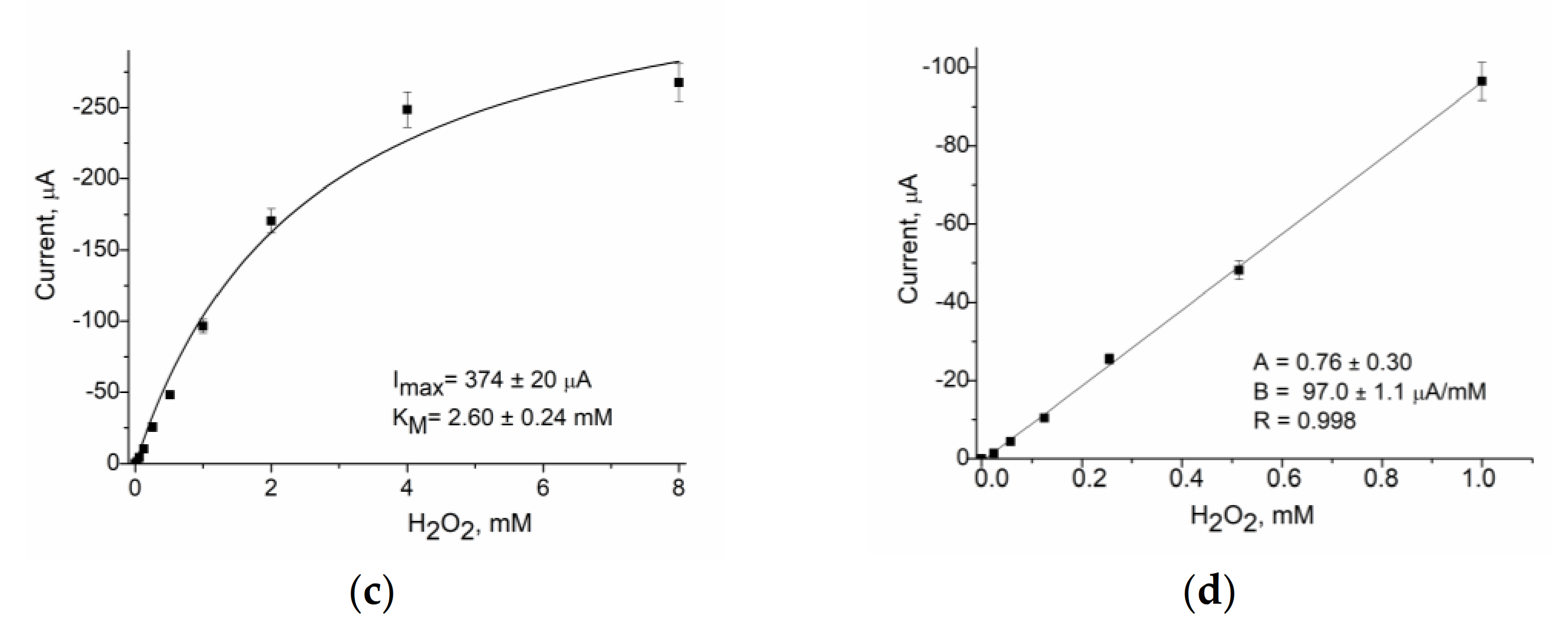
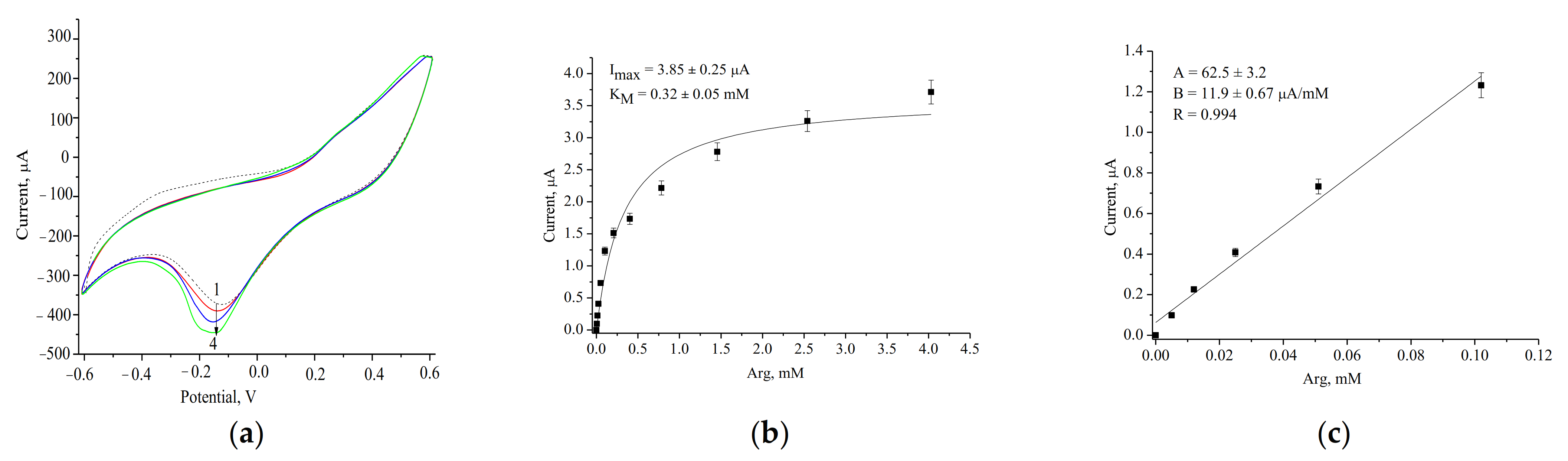

| PO-like NZ | Sensitivity, A·M−1·m−2 | Linear Range, Up to, mM | LOD, µM | Imax, µA | KMapp, mM | Reference |
|---|---|---|---|---|---|---|
| nCeCu | 2164 ± 70 | 1.5 | 0.5 | 172.6 ± 1.1 | 9.0 ± 0.05 | Current work |
| nNiPtPd | 13250 ± 150 | 1.0 | 5 | 374 ± 20 | 2.6 ± 0.24 | Current work |
| gCuHCF | 1620 | 0.8 | 10 | 138.0 ± 8.5 | 31.0 ± 4.4 | [47] |
| Bioelectrode | Sensitivity, A·M−1·m−2 | Linear Range, µM | Response Time, s (95%) | KMapp, mM | Reference |
|---|---|---|---|---|---|
| Arginase/ urease/PANi/Pt | 110 ± 1.3 | 70–600 | 10 | 1.27 ± 0.29 | [21] |
| 1 p-cells/urease/PANi/Pt | 14 ± 1.2 | up to 600 | 60 | 0.51 ± 0.05 | [22] |
| Arginase-nAu- p-cells/urease/PANi/Pt | 357 ± 24 | 10–700 | 30 | 0.45 ± 0.09 | [25] |
| ADI/PANi/Cu | 684 ± 32 | 3–200 | 15 | 0.31 ± 0.05 | [26] |
| ArgO/nCeCu/GE | 1630 ± 92 | 5–100 | 60 | 0.32 ± 0.05 | Current work |
| ArgO/nNiPtPd/GE | 578 ± 5 | 10–250 | 50 | 0.35 ± 0.03 | Current work |
| ArgO/gCuHCF/GE | 602 ± 42 | 10–100 | 50 | 0.12 ± 0.02 | Current work |
| NZ As H2O2-Sensor | nCeCu | nNiPtPd | ||
|---|---|---|---|---|
| Sample | C, mM | 1 CV, % | C, mM | CV, % |
| Pharmaceutical “Tivortine” | 198.7 ± 6.22 | 3.1 | 199.5 ± 2.12 | 1.06 |
| Commercial apple-pear juice | 7.1 ± 0.08 | 1.1 | 7.4 ± 0.5 | 6.8 |
| Freshly prepared apple juice | 4.99 ± 0.001 | 0.02 | 2 ND | - |
| Commercial grape-apple juice | 8.71 ± 0.43 | 4.9 | ND | - |
| Commercial multifruit juice | 6.14 ± 0.25 | 4.1 | ND | - |
| Wine, red, dry | 3.26 ± 0.09 | 2.8 | ND | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasyuk, N.; Gayda, G.; Demkiv, O.; Darmohray, L.; Gonchar, M.; Nisnevitch, M. Amperometric Biosensors for L-Arginine Determination Based on L-Arginine Oxidase and Peroxidase-Like Nanozymes. Appl. Sci. 2021, 11, 7024. https://doi.org/10.3390/app11157024
Stasyuk N, Gayda G, Demkiv O, Darmohray L, Gonchar M, Nisnevitch M. Amperometric Biosensors for L-Arginine Determination Based on L-Arginine Oxidase and Peroxidase-Like Nanozymes. Applied Sciences. 2021; 11(15):7024. https://doi.org/10.3390/app11157024
Chicago/Turabian StyleStasyuk, Nataliya, Galina Gayda, Olha Demkiv, Lyubomyr Darmohray, Mykhailo Gonchar, and Marina Nisnevitch. 2021. "Amperometric Biosensors for L-Arginine Determination Based on L-Arginine Oxidase and Peroxidase-Like Nanozymes" Applied Sciences 11, no. 15: 7024. https://doi.org/10.3390/app11157024






