Chemical Properties of Heavy Metal-Contaminated Soils from a Korean Military Shooting Range: Evaluation of Pb Sources Using Pb Isotope Ratios
Abstract
:1. Introduction
2. Materials and Methods
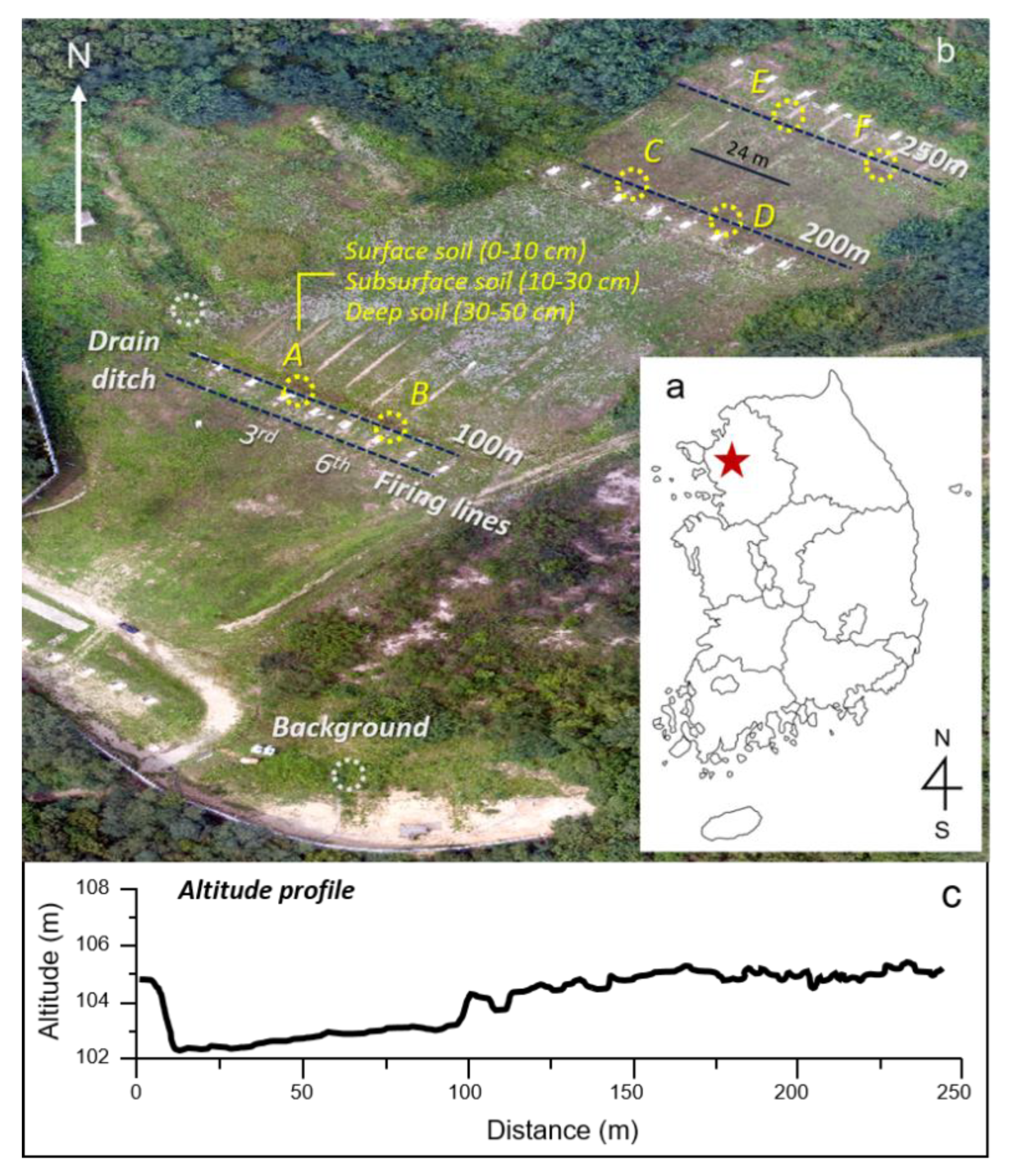
2.1. Study Area and Sampling Site
2.2. Analytical Methods
3. Results and Discussion
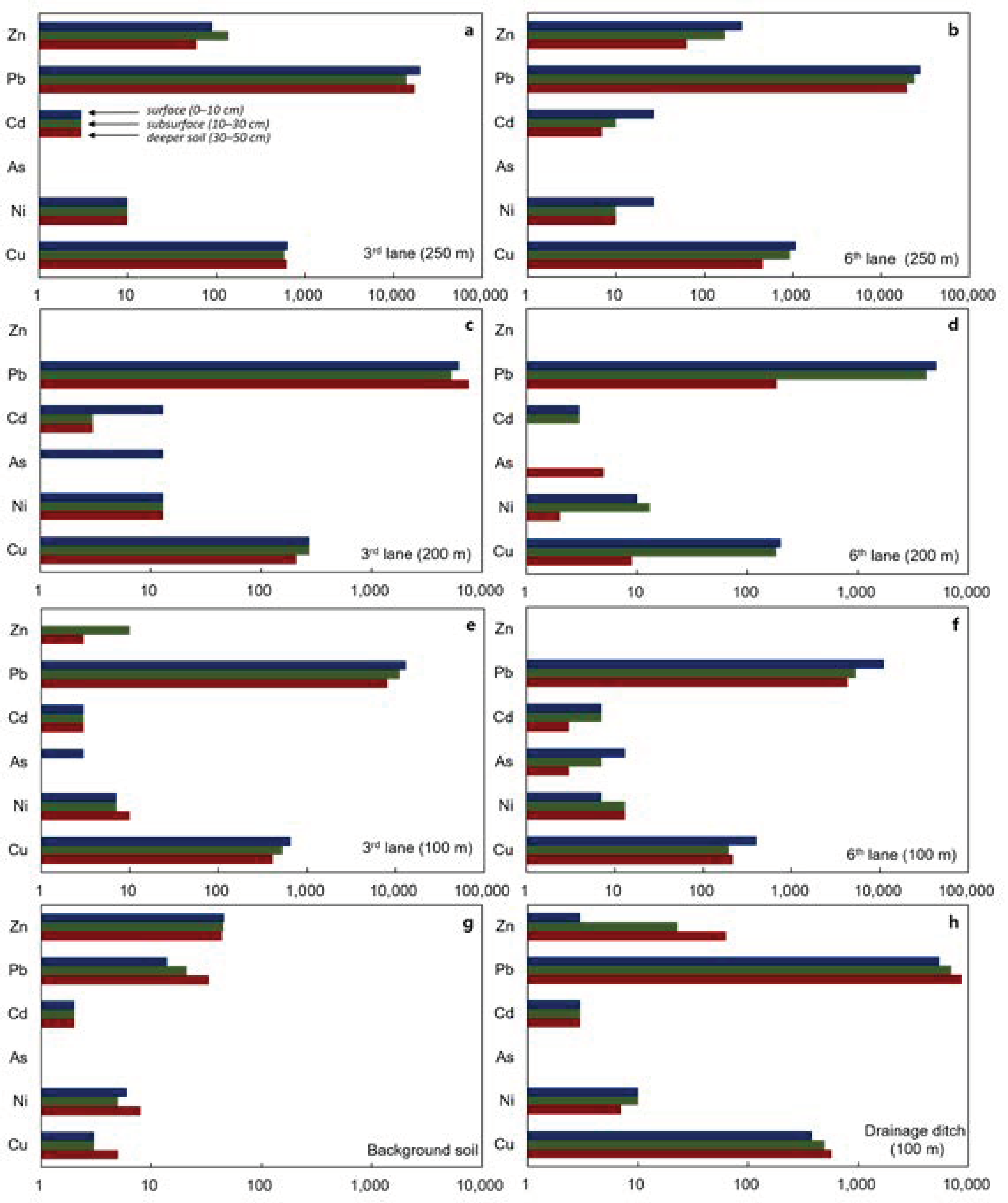
3.1. Geochemical Properties of Soil
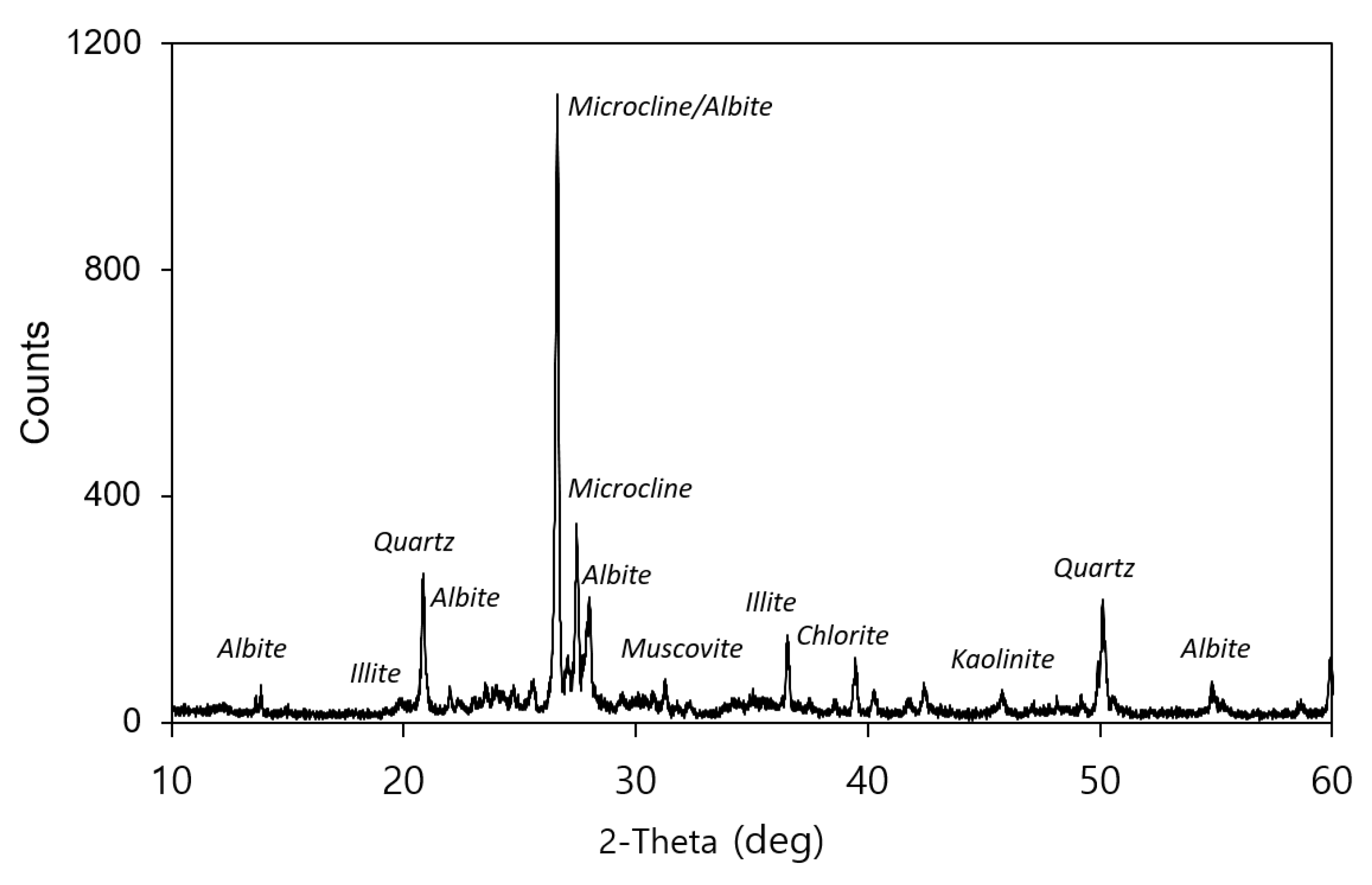
3.2. Mineralogical Properties of Soil
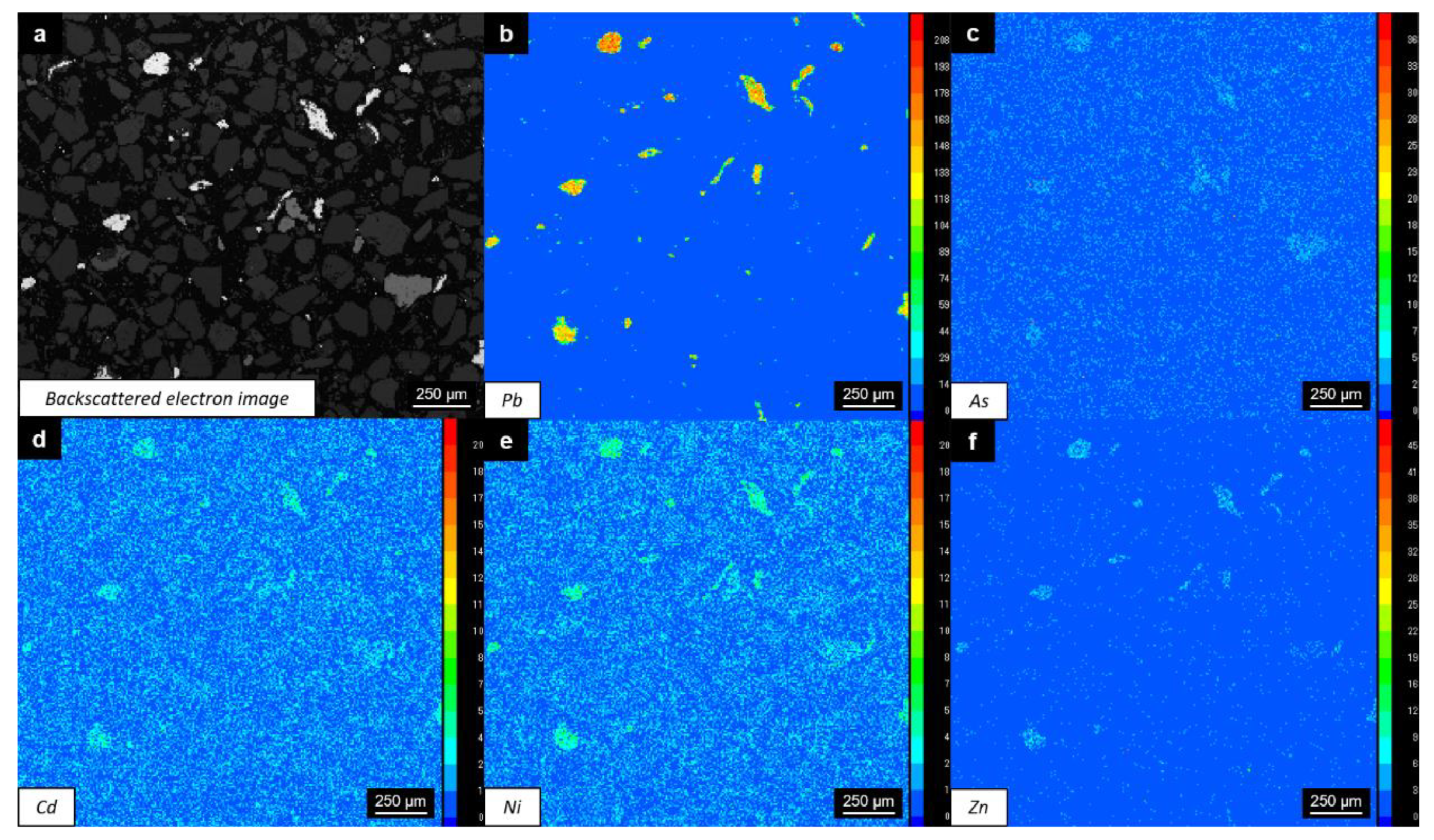
3.3. Potential Pb Contamination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basunia, S.; Landsberger, S. Contents and leachability of heavy metals (Pb, Cu, Sb, Zn, As) in soil at the Pantex firing range, Amarillo, Texas. J. Air Waste Manag. Assoc. 2001, 51, 1428–1435. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, P.; Bolan, N.; Bowman, M.; Naidu, R. Distribution and availability of metal contaminants in shooting range soils around Australia. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 65–67. [Google Scholar]
- Islam, M.N.; Nguyen, X.P.; Jung, H.-Y.; Park, J.-H. Chemical speciation and quantitative evaluation of heavy metal pollution hazards in two army shooting range backstop soils. Bull. Environ. Contam. Toxicol. 2016, 96, 179–185. [Google Scholar] [CrossRef]
- Lee, K.-L.; Hyun, J.-H. Modality of Heavy Metal Contamination of Soil in Military Rifle Shooting Range. J. Soil Groundw. 2016, 21, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ma, L.Q.; Chen, M.; Hardison, D.W., Jr.; Harris, W.G. Weathering of lead bullets and their environmental effects at outdoor shooting ranges. J. Environ. Qual. 2003, 32, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Chrastný, V.; Komárek, M.; Hájek, T. Lead contamination of an agricultural soil in the vicinity of a shooting range. Environ. Monit. Assess. 2010, 162, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hardison, D.W., Jr.; Ma, L.Q.; Luongo, T.; Harris, W.G. Lead contamination in shooting range soils from abrasion of lead bullets and subsequent weathering. Sci. Total Environ. 2004, 328, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, M.A.; Filippelli, G.; Mielke, H.; Gulson, B.; Ball, A.S. Lead exposure at firing ranges—A review. Environ. Health 2017, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, Y.; Zhao, S.; Liu, X. The weathering and transformation process of lead in China’s shooting ranges. Environ. Sci. Process. Impacts 2015, 17, 1620–1633. [Google Scholar] [CrossRef]
- Walraven, N.; Bakker, M.; van Os, B.; Klaver, G.T.; Middelburg, J.J.; Davies, G.R. Factors controlling the oral bioaccessibility of anthropogenic Pb in polluted soils. Sci. Total Environ. 2015, 506, 149–163. [Google Scholar] [CrossRef]
- Tyszka, R.; Pietranik, A.; Kierczak, J.; Ettler, V.; Mihaljevič, M.; Medyńska-Juraszek, A. Lead isotopes and heavy minerals analyzed as tools to understand the distribution of lead and other potentially toxic elements in soils contaminated by Cu smelting (Legnica, Poland). Environ. Sci. Pollut. Res. 2016, 23, 24350–24363. [Google Scholar] [CrossRef] [Green Version]
- Scheuhammer, A.M.; Norris, S.L. A Review of the Environmental Impacts of Lead Shotshell Ammunition and Lead Fishing Weights in Canada; Occas. Pap. Can. Wildl. Serv.: Ottawa, Canada, 1995. [Google Scholar]
- Fayiga, A.; Saha, U. Soil pollution at outdoor shooting ranges: Health effects, bioavailability and best management practices. Environ. Pollut. 2016, 216, 135–145. [Google Scholar] [CrossRef]
- Jørgensen, S.S.; Willems, M. The fate of lead in soils: The transformation of lead pellets in shooting-range soils. Ambio 1987, 16, 11–15. [Google Scholar]
- Lin, Z. Secondary mineral phases of metallic lead in soils of shooting ranges from Örebro County, Sweden. Environ. Geol. 1996, 27, 370–375. [Google Scholar] [CrossRef]
- Craig, J.; Rimstidt, J.; Bonnaffon, C.; Collins, T.K.; Scanlon, P.F. Surface water transport of lead at a shooting range. Bull. Environ. Contam. Toxicol. 1999, 63, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.; McCartney, C. The effects of lead shot deposition on soils and crops at a clay pigeon shooting site in northern England. Soil Use Manage. 1994, 10, 124–129. [Google Scholar] [CrossRef]
- Van Bon, J.; Boersema, J. Sources, Effects and Management of Metallic Lead Pollution. The Contribution of Hunting, Shooting and Angling. In Contaminated Soil’88; Springer: Dordrecht, The Netherlands, 1988; pp. 269–271. [Google Scholar]
- Lin, Z.; Comet, B.; Qvarfort, U.; Herbert, R. The chemical and mineralogical behaviour of Pb in shooting range soils from central Sweden. Environ. Pollut. 1995, 89, 303–309. [Google Scholar] [CrossRef]
- MOE. The Development of Hybrid Electrokinetic Remediation Technique Using Solar Energy on Shooting Range Soils Contaminated by Heavy Metals, Gwachun, Kyunggi Republic of Korea; MOE: Gwachun, Korea, 2005; pp. 40–63. [Google Scholar]
- Moon, D.H.; Cheong, K.H.; Khim, J.; Wazne, M.; Hyun, S.; Park, J.-H.; Chang, Y.-Y.; Ok, Y.S. Stabilization of Pb2+ and Cu2+ contaminated firing range soil using calcined oyster shells and waste cow bones. Chemosphere 2013, 91, 1349–1354. [Google Scholar] [CrossRef]
- Kim, H.-H.; Jeong, S. Heavy Metal Pollution and Management Direction of Small Arms Firing Ranges. J. KIMS Technol. 2019, 22, 724–734. [Google Scholar]
- Ma, L.Q.; Hardison, D.W.; Harris, W.G.; Cao, X.; Zhou, Q. Effects of soil property and soil amendment on weathering of abraded metallic Pb in shooting ranges. Water Air Soil Pollut. 2007, 178, 297–307. [Google Scholar] [CrossRef]
- Murray, K.; Bazzi, A.; Carter, C.; Ehlert, A.; Ham’s, A.; Kopec, M.; Richardson, J.; Sokol, H. Distribution and mobility of lead in soils at an outdoor shooting range. Soil Sediment Contam. 1997, 6, 79–93. [Google Scholar] [CrossRef]
- Sjåstad, K.-E.; Simonsen, S.L.; Andersen, T.H. Lead isotope ratios for bullets, a descriptive approach for investigative purposes and a new method for sampling of bullet lead. Forensic Sci. Int. 2014, 244, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, A.; Ehrlich, S.; Shoshani, E.; Halicz, L. Application of lead isotope analysis in shooting incident investigations. Forensic Sci. Int. 2006, 158, 52–64. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, and Water Environment Federation: Washington, DC, USA, 1998; pp. 3–37. [Google Scholar]
- Mann, A.; Deutscher, R. Solution geochemistry of lead and zinc in water containing carbonate, sulphate and chloride ions. Chem. Geol. 1980, 29, 293–311. [Google Scholar] [CrossRef]
- Bindler, R.; Renberg, I.; Klaminder, J.; Emteryd, O. Tree rings as Pb pollution archives? A comparison of 206Pb/207Pb isotope ratios in pine and other environmental media. Sci. Total Environ. 2004, 319, 173–183. [Google Scholar] [CrossRef]
- Rooney, C.; McLaren, R.; Cresswell, R. Distribution and phytoavailability of lead in a soil contaminated with lead shot. Water Air Soil Pollut. 1999, 116, 535–548. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, K.-S. Heavy metal distribution in soils from the Maehyang-ri inland shooting range area. J. Korean Soc. Water Environ. 2008, 24, 407–414. [Google Scholar]
- Stansley, W.; Widjeskog, L.; Roscoe, D.E. Lead contamination and mobility in surface water at trap and skeet ranges. Bull. Environ. Contam. Toxicol. 1992, 49, 640–647. [Google Scholar] [CrossRef]
- Komárek, M.; Chrastný, V.; Ettler, V.; Tlustoš, P. Evaluation of extraction/digestion techniques used to determine lead isotopic composition in forest soils. Anal. Bioanal. 2006, 385, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.; do Nascimento, C.; de Souza Júnior, V.; Southard, R.J.; de Olinda, R.A. Lead isotope distribution and enrichment factors in soil profiles around an abandoned Pb-smelter plant. Int. J. Environ. Sci. Technol. 2017, 14, 2331–2342. [Google Scholar] [CrossRef]
- Kelepertzis, E.; Komárek, M.; Argyraki, A.; Šillerová, H. Metal (loid) distribution and Pb isotopic signatures in the urban environment of Athens, Greece. Environ. Pollut. 2016, 213, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Ettler, V.; Chrastný, V.; Mihaljevic, M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008, 34, 562–577. [Google Scholar] [CrossRef]
- Patel, M.M.; Adrianne, H.; Jones, R.; Jarrett, J.; Berner, J.; Rubin, C.S. Use of lead isotope ratios to identify sources of lead exposure in Alaska Natives. Int. J. Circumpolar Health 2008, 67, 261–268. [Google Scholar] [CrossRef]
- Zhu, Y.; Kashiwagi, K.-i.; Sakaguchi, M.; Aoki, M.; Fujimori, E.; Haraguchi, H. Lead isotopic compositions of atmospheric suspended particulate matter in Nagoya City as measured by HR-ICP-MS. J. Nucl. Sci. Technol. 2006, 43, 474–478. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Šebek, O.; Molek, M.; Grygar, T.; Zeman, J. Geochemical and Pb isotopic evidence for sources and dispersal of metal contamination in stream sediments from the mining and smelting district of Příbram, Czech Republic. Environ. Pollut. 2006, 142, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hopper, J.; Ross, H.; Sturges, W. Regional source discrimination of atmospheric aerosols in Europe using the isotopic composition of lead. Tellus B 1991, 43, 45–60. [Google Scholar] [CrossRef]
- Zhang, P.; Ryan, J.A. Transformation of Pb (II) from cerrusite to chloropyromorphite in the presence of hydroxyapatite under varying conditions of pH. Environ. Sci. Technol. 1999, 33, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Violence Policy Center. The Health Risks of Shooting Ranges and Lead to Children, Families, and the Environment. In Poisonous Pastime; Environmental Working Group: Washington, DC, USA, 2001. [Google Scholar]
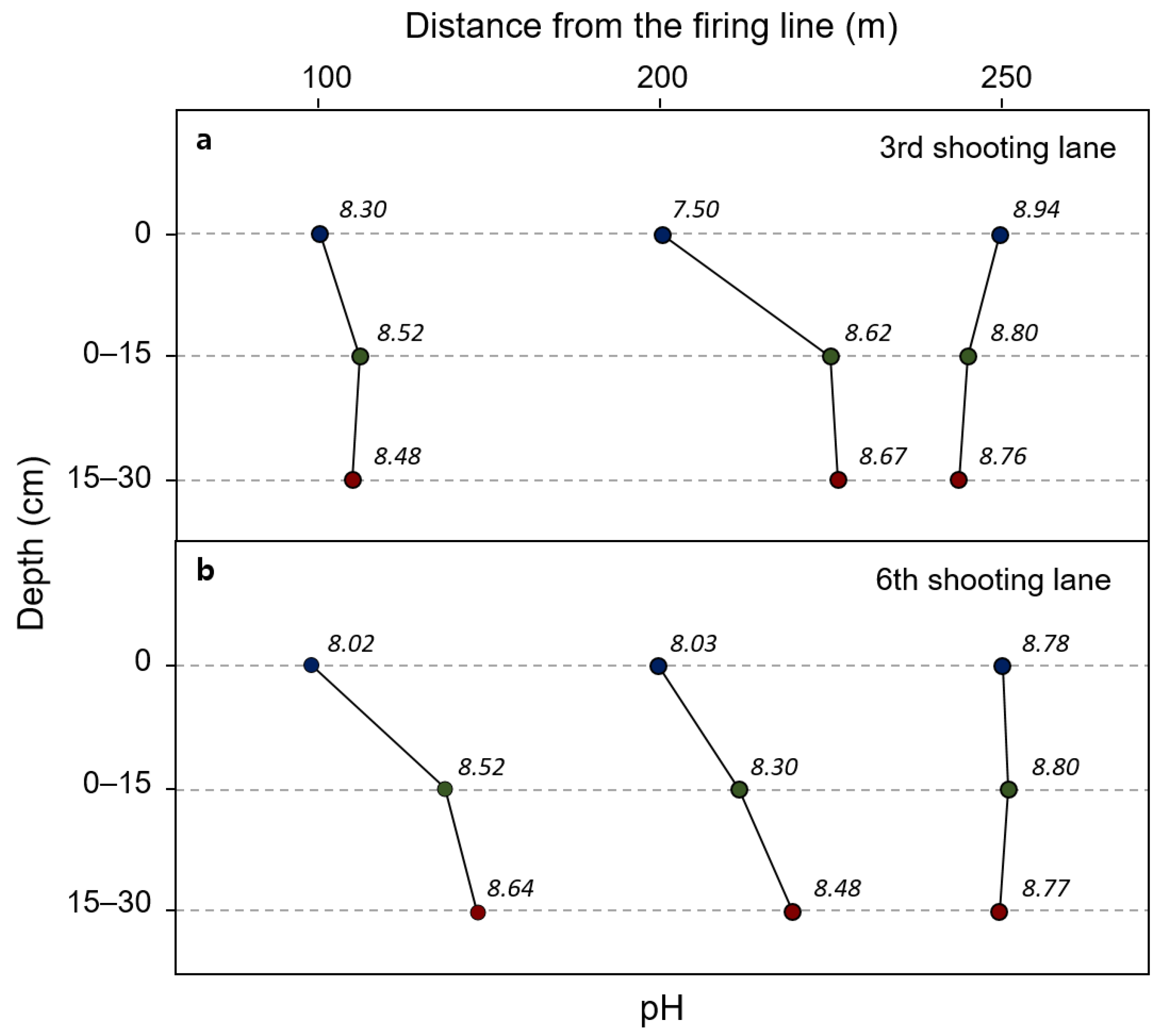

| Soil | Distance from Firing Line (m) | Depth (cm) | pH | Cu | Ni | As | Cd | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|
| 6th shooting range | 250 | 0 | 8.78 | 1,073 | 27 | 0 | 27 | 28,040 | 267 |
| 5–15 | 8.80 | 927 | 10 | 0 | 10 | 24,043 | 170 | ||
| 15–30 | 8.77 | 463 | 10 | 0 | 7 | 19,763 | 63 | ||
| 200 | 0 | 8.03 | 200 | 10 | 0 | 3 | 5127 | 0 | |
| 5–15 | 8.30 | 183 | 13 | 0 | 3 | 4147 | 0 | ||
| 15–30 | 8.48 | 9 | 2 | 5 | 0 | 185 | 0 | ||
| 100 | 0 | 8.02 | 400 | 7 | 13 | 7 | 11,137 | 0 | |
| 5–15 | 8.52 | 193 | 13 | 7 | 7 | 5297 | 0 | ||
| 15–30 | 8.64 | 217 | 13 | 3 | 3 | 4293 | 0 | ||
| 3rd shooting range | 250 | 0 | 8.94 | 643 | 10 | 0 | 3 | 20,030 | 90 |
| 5–15 | 8.80 | 580 | 10 | 0 | 3 | 13,987 | 137 | ||
| 15–30 | 8.76 | 623 | 10 | 0 | 3 | 17,303 | 60 | ||
| 200 | 0 | 7.50 | 273 | 13 | 13 | 13 | 6203 | 0 | |
| 5–15 | 8.62 | 273 | 13 | 0 | 3 | 5313 | 0 | ||
| 15–30 | 8.67 | 210 | 13 | 0 | 3 | 7557 | 0 | ||
| 100 | 0 | 8.30 | 650 | 7 | 3 | 3 | 13,043 | 0 | |
| 5–15 | 8.52 | 533 | 7 | 0 | 3 | 11,093 | 10 | ||
| 15–30 | 8.48 | 413 | 10 | 0 | 3 | 8140 | 3 | ||
| Drain ditch | 100 | 0 | 7.95 | 377 | 10 | 0 | 3 | 5417 | 3 |
| 5–15 | 8.28 | 493 | 10 | 0 | 3 | 6977 | 23 | ||
| 15–30 | 8.62 | 573 | 7 | 0 | 3 | 8740 | 63 | ||
| Background | 0 | 0 | 6.09 | 3 | 6 | 0 | 2 | 14 | 46 |
| 5–15 | 6.04 | 3 | 5 | 0 | 2 | 21 | 45 | ||
| 15–30 | 6.03 | 5 | 8 | 1 | 2 | 33 | 44 |
| Soil | Distance from Firing Line (m) | Depth (cm) | 208Pb/204Pb | 207Pb/204Pb | 206Pb/204Pb | 206Pb/207Pb | 208Pb/207Pb |
|---|---|---|---|---|---|---|---|
| 6th shooting range | 250 | 0 | 38.77 | 15.69 | 18.53 | 1.18 | 2.47 |
| 5–15 | 38.21 | 15.45 | 18.26 | 1.18 | 2.47 | ||
| 15–30 | 38.96 | 15.65 | 18.50 | 1.18 | 2.49 | ||
| 200 | 0 | 38.09 | 15.53 | 18.46 | 1.19 | 2.45 | |
| 5–15 | 38.22 | 15.53 | 18.63 | 1.20 | 2.46 | ||
| 15–30 | 39.08 | 15.77 | 18.62 | 1.18 | 2.48 | ||
| 100 | 0 | 38.90 | 15.88 | 18.60 | 1.17 | 2.45 | |
| 5–15 | 35.98 | 15.11 | 15.14 | 1.00 | 2.38 | ||
| 15–30 | 39.62 | 16.56 | 16.67 | 1.01 | 2.39 | ||
| Drain ditch | 100 | 0 | 37.97 | 15.53 | 15.77 | 1.02 | 2.44 |
| 5–15 | 38.98 | 16.12 | 16.41 | 1.02 | 2.42 | ||
| 15–30 | 38.79 | 16.03 | 16.26 | 1.01 | 2.42 | ||
| Background | 0 | 0 | 40.70 | 16.10 | 18.82 | 1.17 | 2.53 |
| 5–15 | 38.73 | 15.55 | 18.21 | 1.17 | 2.49 | ||
| 15–30 | 40.03 | 15.95 | 18.55 | 1.16 | 2.51 |
| Soil | Distance from Firing Line (m) | Depth (cm) | Mineral Component |
|---|---|---|---|
| 6th shooting range | 250 | 0 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite, Calcite |
| 5–15 | Quartz, Microcline, Albite, Muscovite/Illite, Kaolinite, Chlorite, Calcite | ||
| 15–30 | Quartz, Microcline, Albite, Kaolinite, Muscovite/Illite, Chlorite, Calcite | ||
| 200 | 0 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite, Hematite | |
| 5–15 | Quartz, Microcline, Albite, Kaolinite, Muscovite/Illite, Chlorite, Calcite | ||
| 15–30 | Quartz, Muscovite/Illite, Albite, Microcline, Kaolinite, Chlorite, Calcite | ||
| 100 | 0 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite | |
| 5–15 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite | ||
| 15–30 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite, Calcite, Hematite | ||
| Drain ditch | 100 | 0 | Quartz, Microcline, Albite, Muscovite/Illite, Kaolinite, Chlorite, Calcite |
| 5–15 | Quartz, Muscovite/Illite, Albite Microcline, Kaolinite, Chlorite, Calcite | ||
| 15–30 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite | ||
| Background | 0 | 0 | Quartz, Albite, Microcline, Muscovite/Illite, Kaolinite, Chlorite |
| 5–15 | Quartz, Microcline, Albite, Muscovite/Illite, Kaolinite, Chlorite | ||
| 15–30 | Quartz, Microcline, Albite, Muscovite/Illite, Kaolinite, Chlorite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, I.; Kim, H.; Jeong, S.; Choi, H.; Park, J.; Lee, I. Chemical Properties of Heavy Metal-Contaminated Soils from a Korean Military Shooting Range: Evaluation of Pb Sources Using Pb Isotope Ratios. Appl. Sci. 2021, 11, 7099. https://doi.org/10.3390/app11157099
Moon I, Kim H, Jeong S, Choi H, Park J, Lee I. Chemical Properties of Heavy Metal-Contaminated Soils from a Korean Military Shooting Range: Evaluation of Pb Sources Using Pb Isotope Ratios. Applied Sciences. 2021; 11(15):7099. https://doi.org/10.3390/app11157099
Chicago/Turabian StyleMoon, Inkyeong, Honghyun Kim, Sangjo Jeong, Hyungjin Choi, Jungtae Park, and Insung Lee. 2021. "Chemical Properties of Heavy Metal-Contaminated Soils from a Korean Military Shooting Range: Evaluation of Pb Sources Using Pb Isotope Ratios" Applied Sciences 11, no. 15: 7099. https://doi.org/10.3390/app11157099





