Is the Synthetic Fungicide Fosetyl-Al Safe for the Ecotoxicological Models Danio rerio and Enchytraeus crypticus?
Abstract
:1. Introduction
2. Material and Methods
2.1. Test Chemical: Fosetyl-Al (FOS)
2.2. Test Species: Danio rerio and Enchytraeus crypticus
2.3. Bioassays
2.3.1. Danio rerio
Fish Embryo Acute Toxicity (FET) Test
Locomotor Behavior Assay
Biochemical Markers’ Assessment
2.3.2. Enchytraeus crypticus
Enchytraeid Reproduction Test (ERT)
2.4. Data Analysis
3. Results and Discussion
3.1. Danio rerio
3.1.1. Fish Embryo Acute Toxicity (FET) Test
3.1.2. Locomotor Behavior Assay
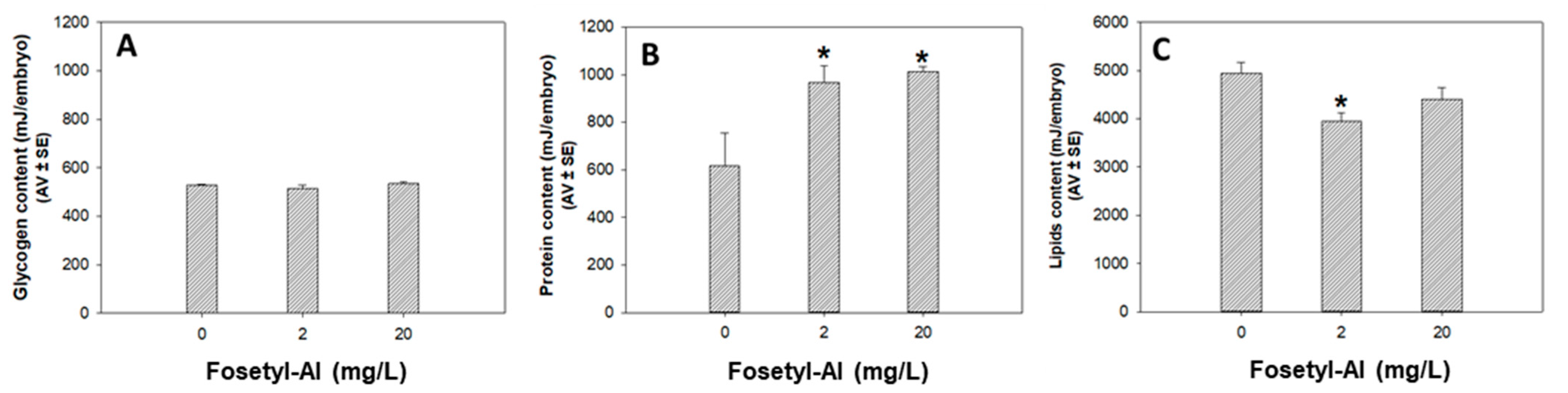
3.1.3. Biochemical Markers
3.2. Enchytraeus crypticus
Enchytraeid Reproduction Test (ERT)
3.3. Integration of Knowledge for Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiaia-Hernandez, A.C.; Keller, A.; Wächter, D.; Steinlin, C.; Camenzuli, L.; Hollender, J.; Krauss, M. Long-Term Persistence of Pesticides and TPs in Archived Agricultural Soil Samples and Comparison with Pesticide Application. Environ. Sci. Technol. 2017, 51, 10642–10651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, B.; Pereira Dos Santos, S.; Gustavsen, J.A.; Imfeld, G.; Lamy, F.; Mitchell, E.A.D.; Mota, M.; Noll, D.; Planchamp, C.; Heger, T.J. Impact of a synthetic fungicide (fosetyl-Al and propamocarb-hydrochloride) and a biopesticide (Clonostachys rosea) on soil bacterial, fungal, and protist communities. Sci. Total Environ. 2020, 738, 139635. [Google Scholar] [CrossRef]
- Channabasava, A.; Lakshman, H.C.; Jorquera, M.A. Effect of fungicides on association of arbuscular mycorrhiza fungus Rhizophagus fasciculatus and growth of Proso millet (Panicum miliaceum L.). J. Soil Sci. Plant Nutr. 2015, 15, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Prashar, P.; Shah, S. Impact of Fertilizers and Pesticides on Soil Microflora in Agriculture. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Germany, 2016; Volume 19, pp. 331–361. ISBN 978-3-319-26777-7. [Google Scholar]
- Vryzas, Z. Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Curr. Opin. Environ. Sci. Health 2018, 4, 5–9. [Google Scholar] [CrossRef]
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA; Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; et al. Peer review of the pesticide risk assessment of the active substance phenmedipham. EFSA J. 2018, 16, e05150. [Google Scholar]
- Almeida, P.; Coutinho, J.; Rocha, J.; Gomes-Laranjo, J. Study of the effect of Aliette on the activity of spinach (Spinacea oleracea L.) chloroplasts. Braz. J. Plant Physiol. 2007, 19, 77–81. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, S.M.M.; Haque, A.; Shahjahan, M. Toxicity of the organophosphate insecticide sumithion to embryo and larvae of zebrafish. Toxicol. Rep. 2020, 7, 317–323. [Google Scholar] [CrossRef]
- De la Paz, J.F.; Beiza, N.; Paredes-Zúñiga, S.; Hoare, M.S.; Allende, M.L. Triazole Fungicides Inhibit Zebrafish Hatching by Blocking the Secretory Function of Hatching Gland Cells. Int. J. Mol. Sci. 2017, 18, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterauer, R.; Köhler, H.-R. Temperature-dependent effects of the pesticides thiacloprid and diazinon on the embryonic development of zebrafish (Danio rerio). Aquat. Toxicol. 2008, 86, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Bezchlebová, J.; Černohlávková, J.; Lána, J.; Sochová, I.; Kobetičová, K.; Hofman, J. Effects of toxaphene on soil organisms. Ecotoxicol. Environ. Saf. 2007, 68, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.L.; Ammendola, A.; Casini, S.; Amorim, M.J.B. Toxicity of fungicides to terrestrial non-target fauna—Formulated products versus active ingredients (azoxystrobin, cyproconazole, prothioconazole, tebuconazole)—A case study with Enchytraeus crypticus (Oligochaeta). Sci. Total Environ. 2021, 754, 142098. [Google Scholar] [CrossRef]
- Camargo Carniel, L.S.; Niemeyer, J.C.; Iuñes de Oliveira Filho, L.C.; Alexandre, D.; Gebler, L.; Klauberg-Filho, O. The fungicide mancozeb affects soil invertebrates in two subtropical Brazilian soils. Chemosphere 2019, 232, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Velki, M.; Lackmann, C.; Barranco, A.; Ereño Artabe, A.; Rainieri, S.; Hollert, H.; Seiler, T.-B. Pesticides diazinon and diuron increase glutathione levels and affect multixenobiotic resistance activity and biomarker responses in zebrafish (Danio rerio) embryos and larvae. Environ. Sci. Eur. 2019, 31, 4. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Kirk, R.G.W. Recovering the Principles of Humane Experimental Technique: The 3Rs and the Human Essence of Animal Research. Sci. Technol. Human Values 2018, 43, 622–648. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, G.; Dai, D.; Xu, Z.; Cai, L.; Wang, Q.; Yu, Y. Individual and mixture effects of five agricultural pesticides on zebrafish (Danio rerio) larvae. Environ. Sci. Pollut. Res. 2017, 24, 4528–4536. [Google Scholar] [CrossRef]
- Caioni, G.; Merola, C.; Perugini, M.; d’Angelo, M.; Cimini, A.M.; Amorena, M.; Benedetti, E. An Experimental Approach to Study the Effects of Realistic Environmental Mixture of Linuron and Propamocarb on Zebrafish Synaptogenesis. Int. J. Environ. Res. Public Health 2021, 18, 4664. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ferreira, M.P.; Roelofs, D.; van Gestel, C.A.M.; Verweij, R.A.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeus crypticus as model species in soil ecotoxicology. Chemosphere 2012, 87, 1222–1227. [Google Scholar] [CrossRef]
- OECD. Test No. 220: Enchytraeid Reproduction Test; OECD Guidelines for the Testing of Chemicals, Section 2; Organization for Economic Cooperation and Development (OECD): Paris, France, 2016; ISBN 9789264264472. [Google Scholar]
- Testa, M.; da Silva, A.S.; Segat, J.C.; Maluche-Baretta, C.R.D.; Baretta, D. Impacts on reproduction of Enchytraeus crypticus in fertilized soils with chicken litter treated with synthetic and natural insecticide. Environ. Toxicol. Pharmacol. 2020, 78, 103386. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Pan, R.; Xu, T.; Zhao, J.; Huang, W.; Liu, Y.; Yin, D. Effects of three different embryonic exposure modes of 2, 2′, 4, 4′-tetrabromodiphenyl ether on the path angle and social activity of zebrafish larvae. Chemosphere 2017, 169, 542–549. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases, The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M. Acetylcholinesterase activity in juveniles of Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 1996, 57, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- De Coen, W.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV.Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Golovko, O.; Žlábek, V.; Barata, C.; Soares, A.M.V.M.; Pestana, J.L.T. Life history and biochemical effects of chlorantraniliprole on Chironomus riparius. Sci. Total Environ. 2015, 508, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.J.; Maria, V.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Fate and Effect of Nano Tungsten Carbide Cobalt (WCCo) in the Soil Environment: Observing a Nanoparticle Specific Toxicity in Enchytraeus crypticus. Environ. Sci. Technol. 2018, 52, 11394–11401. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N. Ecotoxicology of Aquatic System: A Review on Fungicide Induced Toxicity in Fishes. Pro Aqua Farm Mar. Biol. 2018, 1, 180001. [Google Scholar]
- EFSA. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Disodium Phosphonate. EFSA J. 2013, 11, 3213. [Google Scholar] [CrossRef]
- Baudy, P.; Zubrod, J.P.; Konschak, M.; Weil, M.; Schulz, R.; Bundschuh, M. Does long-term fungicide exposure affect the reproductive performance of leaf-shredders? A partial life-cycle study using Hyalella azteca. Environ. Pollut. 2017, 222, 458–464. [Google Scholar] [CrossRef]
- Ahmad, F.; Ali, S.; Richardson, M.K. Effect of pesticides and metals on zebrafish embryo development and larval locomotor activity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Colosio, C.; Tiramani, M.; Maroni, M. Neurobehavioral Effects of Pesticides: State of the Art. Neurotoxicology 2003, 24, 577–591. [Google Scholar] [CrossRef]
- Bjørling-Poulsen, M.; Andersen, H.R.; Grandjean, P. Potential developmental neurotoxicity of pesticides used in Europe. Environ. Health A Glob. Access Sci. Source 2008, 7, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenn, M.E.; Coffey, M.D. Studies on the In Vitro and In Vivo Antifungal Activity of Fosetyl-Al and Phosphorous Acid. Phytopathology 1984, 74, 606–611. [Google Scholar] [CrossRef]
- Smillie, R. The Mode of Action of Phosphite: Evidence for Both Direct and Indirect Modes of Action on Three Phytophthora spp. in Plants. Phytopathology 1989, 79, 921. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.R.; Salimian, M.; Ferro, M.; Marques, P.A.; Goncalves, G.; Titus, E.; Domingues, I. Biochemical and behavioral responses of zebrafish embryos to magnetic graphene/nickel nanocomposites. Ecotoxicol. Environ. Saf. 2019, 186, 109760. [Google Scholar] [CrossRef]
- Abe, F.R.; Soares, A.M.V.M.; de Oliveira, D.P.; Gravato, C. Toxicity of dyes to zebrafish at the biochemical level: Cellular energy allocation and neurotoxicity. Environ. Pollut. 2018, 235, 255–262. [Google Scholar] [CrossRef]
- Freitas, J.S.; Girotto, L.; Goulart, B.V.; Alho, L.D.O.G.; Gebara, R.C.; Montagner, C.C.; Schiesari, L.; Espíndola, E.L.G. Effects of 2,4-D-based herbicide (DMA® 806) on sensitivity, respiration rates, energy reserves and behavior of tadpoles. Ecotoxicol. Environ. Saf. 2019, 182, 109446. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.E.M.; Müller, T.E.; Murussi, C.; do Amaral, A.M.B.; Gomes, J.L.C.; Marins, A.T.; Leitemperger, J.; Rodrigues, C.C.R.; Fiuza, T.L.; Costa, M.D.; et al. Oxidative effects of the acute exposure to a pesticide mixture of cypermethrin and chlorpyrifos on carp and zebrafish—A comparative study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 206–207, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Almroth, B.C.; Sturve, J.; Berglund, Å.; Förlin, L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat. Toxicol. 2005, 73, 171–180. [Google Scholar] [CrossRef]
- De Menezes, C.C.; Leitemperger, J.; Santi, A.; Lópes, T.; Aline Veiverberg, C.; Peixoto, S.; Bohrer Adaime, M.; Zanella, R.; Vargas Barbosa, N.B.; Lucia Loro, V. The effects of diphenyl diselenide on oxidative stress biomarkers in Cyprinus carpio exposed to herbicide quinclorac (Facet®). Ecotoxicol. Environ. Saf. 2012, 81, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yonar, M.E. Protective effect of lycopene on oxidative stress and antioxidant status in Cyprinus carpio during cypermethrin exposure. Environ. Toxicol. 2013, 28, 609–616. [Google Scholar] [CrossRef]
- Steevens, J.A.; Benson, W.H. Toxicological interactions of chlorpyrifos and methyl mercury in the amphipod, Hyalella azteca. Toxicol. Sci. 1999, 52, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLoughlin, N.; Yin, D.; Maltby, L.; Wood, R.M.; Yu, H. Evaluation of sensitivity and specificity of two crustacean biochemical biomarkers. Environ. Toxicol. Chem. 2000, 19, 2085–2092. [Google Scholar] [CrossRef]
- Thompson, H.M. Esterases as Markers of Exposure to Organophosphates and Carbamates. Ecotoxicology 1999, 8, 369–384. [Google Scholar] [CrossRef]
- Bicho, R.C.; Santos, F.; Gonçalves, M.; Soares, A.; Amorim, M. Enchytraeid Reproduction TestPLUS: Hatching, growth and full life cycle test—an optional multi-endpoint test with Enchytraeus crypticus. Ecotoxicology 2015, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.; Maria, V.L.; Römbke, J.; Amorim, M.J.B. Multigenerational exposure of Folsomia candida to ivermectin—Using avoidance, survival, reproduction, size and cellular markers as endpoints. Geoderma 2019, 337, 273–279. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Alternative test methods for (nano)materials hazards assessment: Challenges and recommendations for regulatory preparedness. NanoToday 2021, 40, 101242. [Google Scholar] [CrossRef]
- Amorim, M.; Fernández-Cruz, M.L.; Hund-Rinke, K.; Scott-Fordsmand, J.J. Environmental hazard testing of nanobiomaterials. Environ. Sci. Eur. 2020, 32, 101. [Google Scholar] [CrossRef]
- Amorim, M.J.B.; Scott-Fordsmand, J.J. Plastic pollution—A case study with Enchytraeus crypticus—From micro-to nanoplastics. Environ. Pollut. 2021, 271, 116363. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. Is the Synthetic Fungicide Fosetyl-Al Safe for the Ecotoxicological Models Danio rerio and Enchytraeus crypticus? Appl. Sci. 2021, 11, 7209. https://doi.org/10.3390/app11167209
Barreto A, Santos J, Amorim MJB, Maria VL. Is the Synthetic Fungicide Fosetyl-Al Safe for the Ecotoxicological Models Danio rerio and Enchytraeus crypticus? Applied Sciences. 2021; 11(16):7209. https://doi.org/10.3390/app11167209
Chicago/Turabian StyleBarreto, Angela, Joana Santos, Mónica J. B. Amorim, and Vera L. Maria. 2021. "Is the Synthetic Fungicide Fosetyl-Al Safe for the Ecotoxicological Models Danio rerio and Enchytraeus crypticus?" Applied Sciences 11, no. 16: 7209. https://doi.org/10.3390/app11167209
APA StyleBarreto, A., Santos, J., Amorim, M. J. B., & Maria, V. L. (2021). Is the Synthetic Fungicide Fosetyl-Al Safe for the Ecotoxicological Models Danio rerio and Enchytraeus crypticus? Applied Sciences, 11(16), 7209. https://doi.org/10.3390/app11167209








