Abstract
Significant quantities of onion are cultivated annually, such that industrial processing leads to an appreciable amount of by-products, estimated at around 500,000 tons. Onion skins are considered an important source of naturally occurring antioxidant compounds, particularly flavonoid compounds. Our study follows the development of a sustainable solution in order to manage the by-products of yellow onion skins by designing ingredients with multifunctional activities. A green solvent aqueous extraction of flavonoids was applied to obtain a safe, flavonoid-enriched extract, yielding a total flavonoid content of 50.21 ± 0.09 mg quercetin equivalent (QE)/g dry weight (DW), and an antioxidant activity of 250.81 ± 6.76 mM Trolox/g DW. Complex biopolymeric matrices consisting of whey protein isolates, whey protein hydrolysates, maltodextrin, and pectin were further dissolved in the flavonoid-enriched aqueous extract, followed by freeze-drying. Two powders were obtained, both showing satisfactory phytochemical content and good stability during storage. The application of confocal microscopy revealed that the microscopic structure of the powders have a distribution of the bioactive compounds within the biopolymeric matrices. The in vitro digestion suggested remarkable stability in the gastric tract and a flavonoid-controlled release in the intestinal phase. A significant compatibility range of up to 1 mg/mL for both powders was found, whereas concentrations between 10 and 250 µg/mL stimulated cell proliferation after 24 h of cultivation. The powders showed satisfactory thermal and pH stability, which favors their addition to different food matrices.
1. Introduction
Yellow onion skins (Allium cepa L.) are considered valuable raw material for the extraction of bioactives because they are rich, economical, sustainable, ubiquitous, and available worldwide; additionally, they are not considered a component of the global food supply. A significant amount of onion is cultivated annually, making it one of the most grown vegetables around the world, with an estimated total production of around 66–85.7 million tons per year [1]. Industrial processing leads to a significant amount of onion by-products, estimated to be around 500,000 tons [2], which is considered an important source of polyphenols. Furthermore, onions also represent a considerable amount of dietary phytochemicals such as organosulfur compounds, phenolic acids, flavonoids, thiosulfinates, and anthocyanins [3].
Flavonoids are well-known antioxidant compounds owing to their high redox potential, which allows them to act as reducing agents and singlet oxygen quenchers [4]. It has been reported that flavonoid levels in the edible portions of onions vary from 0.03 to 1 g/kg, whereas onion skins contain significantly higher levels of flavonoids at 2–10 g/kg [5].
Therefore, onion skins represent a valuable resource for flavonoid extraction. In most cases, solid–liquid conventional and assisted extraction methods are the most exploited technologies for the extraction of flavonoids from vegetables, but these techniques employ high volumes of solvent and energy [6]. However, high extraction yield, with its energetic feasibility and usage of environmentally friendly solvents, should be considered as an extraction technology. Hence, new solvents are being studied in order to carry out more selective extractions of flavonoid compounds from plants [7,8], while considering the reduction of extraction time, solvent, and energy.
When extracted from their natural matrices, polyphenols are highly unstable such that they undergo various chemical reactions because of the presence of unsaturated bonds in their molecular structure and are affected by oxidants, heat, light, and enzymatic reactions during storage [9]. Therefore, protective technologies should be applied to improve polyphenol stability and safeguard them against chemical damage before their industrial application. This can be achieved by applying suitable techniques such as microencapsulation [10].
It has been suggested that the use of microencapsulated flavonoids instead of the free compounds can overcome the drawbacks of their instability, improve their bioavailability as well as their shelf life [11], thus contributing to the widening of their industrial applications in food and in related fields such as the biopharmaceutical and cosmeceuticals industries [12].
Therefore, the objective of the present study was to test the use of a green, low-cost solvent to extract flavonoids from yellow onion skins. In order to provide a more stable, multifunctional, and safer powder—with multiple uses such as food ingredients, in biopharmaceuticals or in cosmeceuticals—a unique combination of biopolymeric matrices such as whey protein isolates (WPI), whey protein hydrolysates (WPH), maltodextrin (MD), and pectin (P) were dissolved in the flavonoid-enriched aqueous extract, followed by freeze-drying. The powders were evaluated for their functional properties, in terms of the flavonoids’ entrapping efficiency, total flavonoid and polyphenol contents, antioxidant activity, their microstructural pattern by confocal microscopy, in vitro digestion as well as the thermal and pH stability of the phytochemicals. The cytocompatibility of the powders was tested on mouse fibroblasts to evaluate the release of possible toxic compounds and their proliferative effect. The results obtained in this study could bring certain benefits in terms of exploiting the bioactive potential of yellow onion skin flavonoids or for the development of ingredients with health benefits, thus upgrading their functional characteristics.
2. Materials and Methods
2.1. Chemicals
Whey protein isolates (protein content of 95%) and whey protein hydrolysates were purchased from Fonterra (Auckland, New Zealand). Quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), apple pectin and maltodextrin (DE dextrose equivalent 16.5–19.5), 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), ethanol, sodium hydroxide, Folin-Ciocalteu reagent, and gallic acid were obtained from Sigma Aldrich, Steinheim, Germany.
2.2. Flavonoid Extraction from Yellow Onion Skins
Yellow onions (Allium cepa) were purchased from the local market (Galați, Romania) in May 2020. As a first step, onion skins were washed and manually detached, further washed with distilled water, and then blotted on paper towels. Skins were then dried for 2 h at 40 °C and further stored at 4 °C until extraction. Before extraction, the onion skins were ground to obtain a homogeneous batch with a particle size smaller than 0.5 × 0.5 mm. Furthermore, in order to obtain the flavonoid extract, about 40 g of milled onion skin was weighed and mixed with 1000 mL of hot water (70 °C). The extraction was performed at 500 rpm and 70 ± 2 °C for 2 h, followed by centrifugation at 6000 rpm for 15 min and 4 °C. The supernatant was collected, and the extraction was repeated 5 times. The supernatant was further used for the characterization and experiments in powder development.
2.3. Powder Formula
The materials were dissolved in an aqueous extract, with a volume of 180 mL, at a ratio of WPI:WHP:MD:P (2:0.5:1:0.5) (Variant 1), and WPI:MD:P (2:1:0.5) (Variant 2). The two variants were allowed to hydrate by maintaining them overnight on a magnetic stirrer. The samples were further frozen at −70 °C, and the ice crystals were then removed by freeze-drying (CHRIST Alpha 1–4 LD plus, Germany) at −42 °C under a pressure of 0.10 mBar for 48 h. Finally, the powders were collected and packed into metalized bags and stored in the freezer at 4 °C for further analysis. Each experiment was duplicated.
2.4. Extract and Powder Characterization
The methods described in a previous study by Milea et al. [13] were further performed to determine the entrapping efficiency of flavonoids, and to quantify the phytochemical contents and antioxidant activity of the powders. Briefly, the extract and the powders were characterized in terms of total flavonoids, total polyphenols, and antioxidant activity by using the aluminum chloride method, the Folin-Ciocâlteu method, and the DPPH method, respectively. The colorimetric method, based on the aluminum chloride capacity of forming stable acid complexes with the flavones or flavonols, was used to measure the total flavonoids. In order to determine antioxidant activity, the protocol for measuring antiradical activity on DPPH was used. Discolorations were measured after incubation for 90 min at 25 °C in the dark. The entrapping efficiency was determined by assessing the surface flavonoid content (SFC) and total flavonoid content (TFC) of the powders, as described by Milea et al. [13]. The entrapping efficiency (EE, %) was calculated using the following equation:
2.5. In Vitro Digestion
To test the protective effect of biopolymeric matrices upon flavonoids, a static digestion model was applied using the method described prior by Oancea et al. [14], a method that reproduces the digestion process in gastrointestinal conditions. Briefly, for the in vitro digestion experiments, the powders were first mixed with a Tris-HCl buffer. The simulated gastric juice, consisting of pepsin and HCl, was added to the mixture and the pH was adjusted to 2.0. After two hours of incubation, the previous mixture was mixed with the simulated intestinal juice consisting of pancreatin and sodium bicarbonate, and the pH was adjusted to 7.8. During the entire experiment, the samples were incubated for 4 h at 150 rpm and 37 °C, while the total flavonoid content of the samples was measured every 30 min.
2.6. Thermal and pH Stability of the Extracted Phytochemicals
In order to test the thermal and pH stability of the microencapsulated bioactives, considering their introduction into different types of food, which involves ensuring their preservability by heat treatment at high temperatures and/or the use of different pH values, the behavior of flavonoids were studied at increasing temperatures and pH levels. The powders were dissolved in various ratios ranging from 0.1–0.6% in distillated water, and 10 mL of the resulting solutions were treated at different temperatures ranging from 25 to 100 °C for 15 min. Additionally, an amount of 100 mg was dissolved in 10 mL buffer, at a pH varying from 2.0 to 8.0, in order to test the stability of the phytochemicals in terms of total flavonoids, total polyphenols, and antioxidant activity.
2.7. Cell Culture and Treatment
Minimum Essential Medium (MEM) supplemented with 10% (v/v) fetal calf serum (FCS), 2 mM L-glutamine, and 1% (v/v) antibiotic mixture (penicillin–streptomycin–neomycin) was used for the culture and treatment of the mouse fibroblasts from the NCTC clone L929 cell line (ECACC, Sigma-Aldrich, Darmstadt, Germany). The culture medium was humidified with 5% CO2 at 37 °C, until subconfluence. The stock solutions were prepared by adding the powders to the culture medium as stock solutions, at a concentration of 1 mg/mL, followed by incubation at 37 °C for 24 h, then filtered through 0.22 µm membrane (MilliporeSigma, Merck, Darmstadt, Germany). The preparation of the cell suspension was performed using trypsin over the cells in order to detach them. The cells were seeded in 96-well microplates at a density of 4 × 104 cells/mL and incubated for 24 h at 37 °C in a humidified atmosphere with 5% CO2. Afterwards, the culture medium was replaced with fresh medium containing various concentrations of powders ranging from 10 to 1000 µg/mL. The samples were again incubated for 24 and 48 h, respectively. A negative control culture without any sample and a positive control using H2O2 were considered.
2.8. Cell Viability
The cell viability was evaluated using a spectrophotometric method described by Gaspar et al. [15], with neutral red (NR) as an Indicator. After the incubation time, the culture medium was removed from the wells and the cells were incubated in a 50 μg/mL NR solution, at 37 °C for 3 h. The cells were washed, and the colorant was released by gentle shaking for 15 min into 1% (v/v) acetic acid solution with 50% (v/v) ethanol. The amount of the uptaken colorant was directly proportional to the number of viable cells. A Sunrise microplate reader (Tecan, Austria) was used for measuring the optical density of the cells. A control culture with 100% viability was considered and the obtained results were reported as the percentage relative to it. The release of possible toxic compounds from the powders and the cell viability were evaluated in the non-tumorigenic L929 fibroblast cell culture by NR assay.
2.9. Cell Morphology
After 48 h of incubation, the morphology of the cells in the presence of the powders was analyzed using light microscopy. Firstly, the cells were washed and fixed with methanol and the identification was made using a Giemsa solution. The micrographs were acquired at an Axio Observer D1 optical microscope equipped with a digital camera (Carl Zeiss, Oberkochen, Germany).
2.10. Confocal Laser Microscope Spectroscopy
The confocal laser scanning microscopy (CLSM) analysis was used to determine the structural features and characteristics of the two powders. The CLSM analysis was employed by capturing the images with a type of Zeiss confocal laser scanning system (LSM710), equipped with various types of lasers such as the DPSS laser (diode pumped solid state—561 nm), diode laser (405 nm), Ar-laser (458,488,514 nm), and HeNe-laser (633 nm). The structural and morphological pattern of the powders was detected with a 20× apochromatic objective, at 0.6 zoom; the samples were subsequently dyed using Red Congo as a fluorescent marker. The obtained 3D images were rendered and analyzed using ZEN 2012 SP1 software (Black Edition, Jena, Germany).
2.11. Statistical Analysis
Unless otherwise stated, the data reported in this study represent the average of triplicate analyses and were reported as mean ± standard deviation (SD). After running the normality and homoscedasticity test, the data were analyzed using a one-way analysis of variance (ANOVA). The Tukey method with a 95% confidence interval was employed for the post hoc analysis; p < 0.05 was statistically significant. The statistical analysis was carried out using the Minitab 18 software. The statistical analysis of the data was performed using the one-tailed paired Student’s t-test for the cytocompatibility tests. Significant differences were considered at p < 0.05.
3. Results
3.1. Yellow Onion Skin Extract and Powder Characterization
By using hot water as a solvent for the extraction of polyphenolic compounds from yellow onion skins, the extract showed a total flavonoid content of 50.21 ± 0.09 mg QE/g DW, a total polyphenol content of 21.68 ± 0.69 mg GAE/g DW, and an antioxidant activity of 250.81 ± 6.76 mM Trolox/g DW. Piechowiak et al. [16] demonstrated that the total yield of the antioxidant compounds from onion skin extraction, using methanol as solvent, varied depending on temperature and process time as well as on the ratio of onion skin mass to the methanol volume. The obtained onion skin extract was characterized by a high antioxidant activity (ranging from 441.4 to 593.9 mg/g), due to a high concentration of flavonoid compounds such as quercetin (315.6 mg/g), quercetin-3-glucoside (40.3 mg/g), isorhamnetin (14.8 mg/g), and kaempferol (10.9 mg/g).
Although freeze-drying involves higher cost, lower rate, and industrial flexibility, the technique brings certain advantages when considering the preservation of high quality products with minimal thermal and oxidative degradation and higher entrapment efficiency (Ballesteros et al., 2017; Pasrija et al., 2015). In our study, the resulting powders obtained by freeze-drying displayed a different phytochemical profile, as shown in Table 1.

Table 1.
Global phytochemical profile of the powders.
From the data presented in Table 1, it can be observed that using WPI, MD, and P of the yellow-skin flavonoid-enriched extract by freeze-drying allowed for a higher entrapping efficiency of flavonoids at approximately 84% when compared with the biopolymeric coating, including WPI, MD, WPH, and P. The variant 1 biopolymeric combination allowed for a better retention of polyphenols, whereas variant 2 yielded a higher concentration of flavonoids. Based on the results showed in Table 1, it can be concluded that when considering the effect of freeze-drying on the phytochemical profile of the powders, several factors should be examined such as the core-to-coating ratio, chemical properties, and interaction effects. Although higher total flavonoid content was found in variant 1, the availability of surface flavonoid content was higher when compared with variant 2, thus favoring degradation. In contrast, the surface flavonoid content in variant 2 contributed to the total increment of entrapping efficiency, in spite of the lower phenolic content. The freeze-dried ingredients are expected to have a favored phytochemical profile and antioxidant activity as well as a protective effect, especially during the simulated digestion, thus facilitating their addition to different food matrices.
The matrices were selected in terms of developing a more stable, multifunctional, and safer powder. A brief analysis of previous work highlighted the preponderant use of gum arabic, MD, or whey protein, in single form or in combination, for the protection and stabilization of polyphenols. The selection of WPI was based on several selected characteristics such as good physicochemical properties, thus contributing to the intermolecular cross-links with other biopolymers as well as to its high nutritional value, biodegradability, biocompatibility, and pepsin-resistant ability. Moreover, due to its high surface activity, this protein acts as film-forming and emulsifying agent. MD is often used as coating material due to its good water solubility, low viscosity, and low sugar content. MD with a DE value of 16.5–19.5 is easily digestible, either moderately sweet or almost flavorless. Solubility, hydroscopicity, osmolality, and its effectiveness in reducing the freezing point increases with increasing DE. Additionally, WPH were used as a source of bioactive peptides for functional foods, with antibacterial and antivirus activity and anti-inflammatory properties.
The results showed that variant 2 displayed lower flavonoid and polyphenol content and a higher antioxidant activity. This could be a result of the influence of the bioactive peptides, which might display a potentially higher antioxidant activity, and also to the possible entrapping of some other polyphenol classes that might likewise be responsible for the higher antioxidant activity. The powders were tested for phytochemical stability after 30 days of storage at 4 °C. The results are shown in Table 2.

Table 2.
Phytochemical stability in the powders during storage.
From Table 2, it can be observed that variant 2 showed a higher phytochemical stability, leading to an increase in flavonoids by approximately 10%, whereas in variant 1, a decrease in flavonoids by 17% was found. Polyphenols were found to be stable in variant 2, while they decreased by approximately 8% in variant 1. The antioxidant activity decreased in both variants by 20%.
Hamid et al. [17] studied the effect of maltodextrin concentrations on the microencapsulation of wild pomegranate flavedo (peel) phenolics through freeze-drying based on various functional properties and structural morphology. These authors reported that microencapsulation allowed for the retention of higher phenolics (78.47 ± 0.39 GAE/g) but lower flavonoids (4.18 ± 0.03 QE/g) as compared to our study. In another study, Horincar et al. [18] used WPI and different combinations of polymers, including chitosan, MD, and P to microencapsulate yellow onion skin flavonoids by freeze-drying. These authors also suggested a significantly different phytochemical profile for the powders as a function of the coating materials with a lower flavonoid content at 5.84 ± 0.23 mg QE/g DW in the WPI-chitosan combination, and a higher one at 104.97 ± 5.02 mg QE/g DW in the WPI-MD-P combinations. When compared with the data reported in this study, Horincar et al. [18] showed a significantly higher antioxidant activity for the powders, at 175.93 ± 1.50 mM Trolox/g DW and 269.20 ± 3.59 mM Trolox/g DW, respectively, particularly due to the different flavonoid content of the initial extract.
In a previous study, Milea et al. [12] encapsulated flavonoids from yellow onion skins, using MD, P, and WHP in different ratios as coating materials. These authors reported that the obtained powder showed comparable levels of flavonoids, varying from 98.12 ± 0.55 to 103.75 ± 0.57 mg QE/g DW, whereas significantly higher polyphenol content, varying from 53.53 ± 1.71 to 69.26 ± 1.03 mg GAE/g DW, and antioxidant activity from 280.61 ± 3.08 to 337.57 ± 0.89 mM Trolox/g DW, were reported for different variants.
3.2. The Microstructural Design of the Powders by Confocal Microscopy
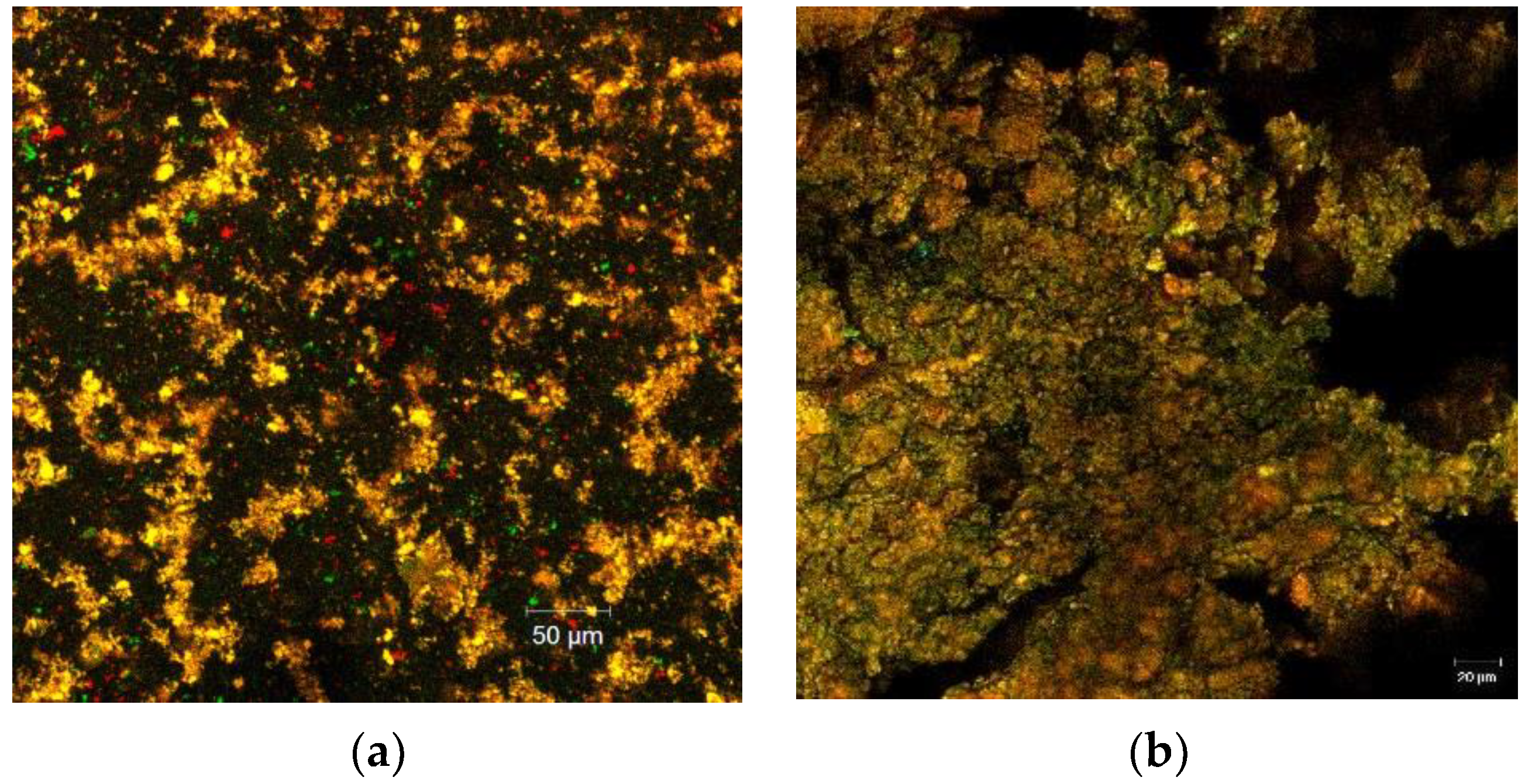
The CLSM analysis of the two variants of powders displayed important characteristics in regard to their morphology and structural pattern. The images were obtained by point-by-point scanning, at a high resolution within a wide field, with the help of digital focusing; the images were captured after the staining of the samples (Figure 1a,b).
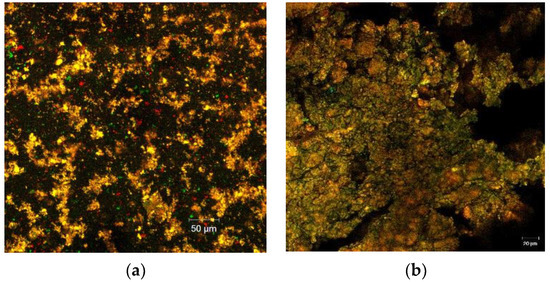
Figure 1.
Confocal laser scanning microscopy images of the two powder samples obtained with the ZEN 2012 SP1 software (Black Edition) by encapsulating the flavonoids extracted from the yellow onion skins within different materials: Variant 1—WPI:WHP:MD:P—2:0.5:1:0.5 (a), Variant 2—WPI:MD:P—2:1:0.5 (b).
Flavonoids represent a valuable large group of polyphenol compounds with a high antioxidant capacity; they are of plant origin with over 4500 different compounds, delivering a variable absorption-emission spectra, which depends on the biochemical profile of the extraction source. Through confocal microscopy analysis, the microstructural design of the obtained powders was assessed. By fluorescent labeling of the two fluorescently stained powders, a well-individualized matrix formed of very small microspherosomes filled with flavonoids and polyphenols could be observed, together with the biopolymeric matrix. Between the two obtained powders, the finest powder was variant 1 (Figure 1a), which displayed a rather uniform microscopic appearance in the form of small spherosomes, with diameters smaller than 2 µm, emitting predominantly around 550 nm. Thus, it can be stated that adding WPH in variant 1 modulated the microscopic appearance and behavior of the powder. For variant 2, the observed spherosomes displayed the tendency to assemble into bigger clusters. From the images, it can be observed that the addition of WPH in variant 1 imprinted slightly different characteristics in terms of the spherosomes’ tendency to assemble, which is well-correlated with the results of the entrapping efficiency. Therefore, it can be stated that this tendency of biopolymeric matrices to assemble is responsible for the higher efficiency of entrapping flavonoids. The agglutination process of the polyphenolic compounds and flavonoids in the form of coacervates of larger dimensions observed for the variant 2 powder could be correlated with the more complex biopolymeric coating material. Furthermore, the complexity of the emission spectra, ranging from the green (starting from 500 nm) to the red domain (until 650 nm), revealed the presence of polyphenolic compounds and flavonoids inside the coating material. This microscopic detail supports the results of the biochemical analysis, which showed a significantly high content of flavonoids and polyphenols associated with a remarkable antioxidant activity. Therefore, the confocal microscopy analysis highlighted the fact that the applied technique was efficient and generated multifunctional ingredients with a high bioactive content, which in turn produces high antioxidant activity.
3.3. In Vitro Digestion of Flavonoids
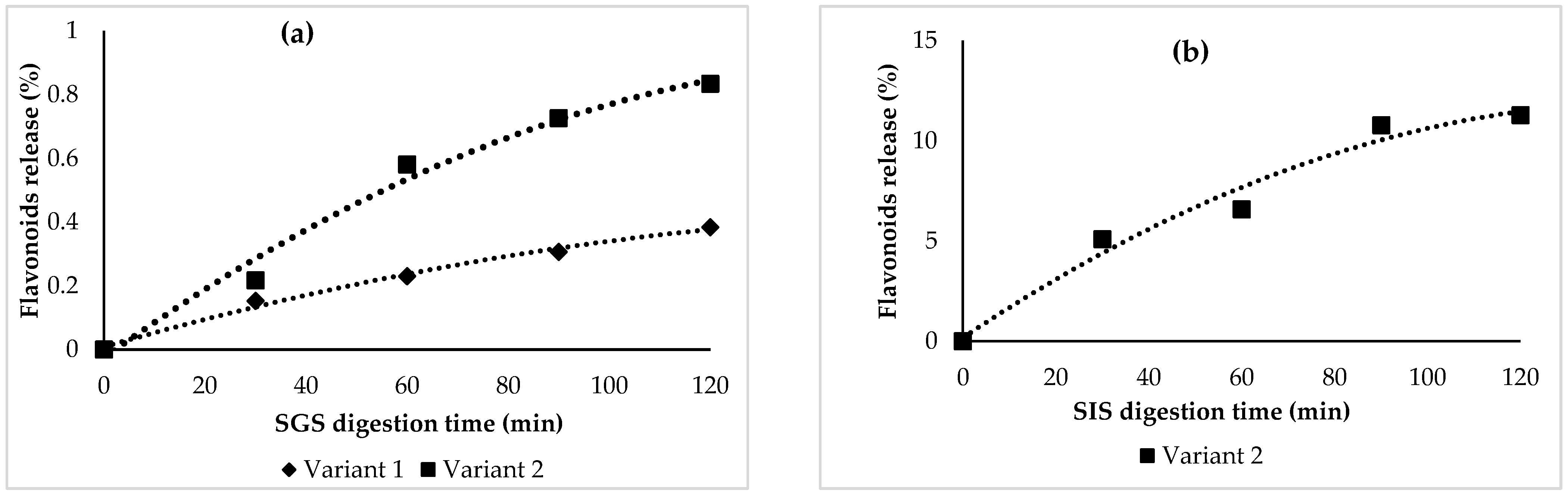
The in vitro behavior of the flavonoids under simulated gastric (SGS) and intestinal (SIF) conditions was observed. Significant flavonoid stability was found in SGS (Figure 2a) for both variants, with a slight increase in flavonoids, up to approximately 1% in variant 2. Therefore, it can be stated that the flavonoids were successfully protected by the selected coating materials.
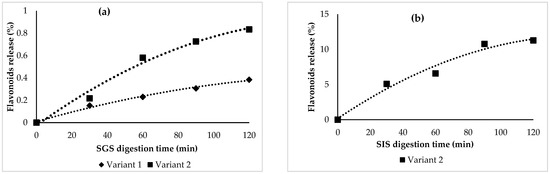
Figure 2.
Flavonoid release (%) after in vitro digestion in the simulated gastric (a) and intestinal juices (b).
No flavonoids were detected in variant 1 in SIS, suggesting a possible maximum absorption capacity. In variant 2, the results indicated that the concentration of the total flavonoids was released during the 120 min period under the simulated intestinal conditions, with a maximum value recorded at 11%.
3.4. In Vitro Cytotoxicity of the Microencapsulated Powders
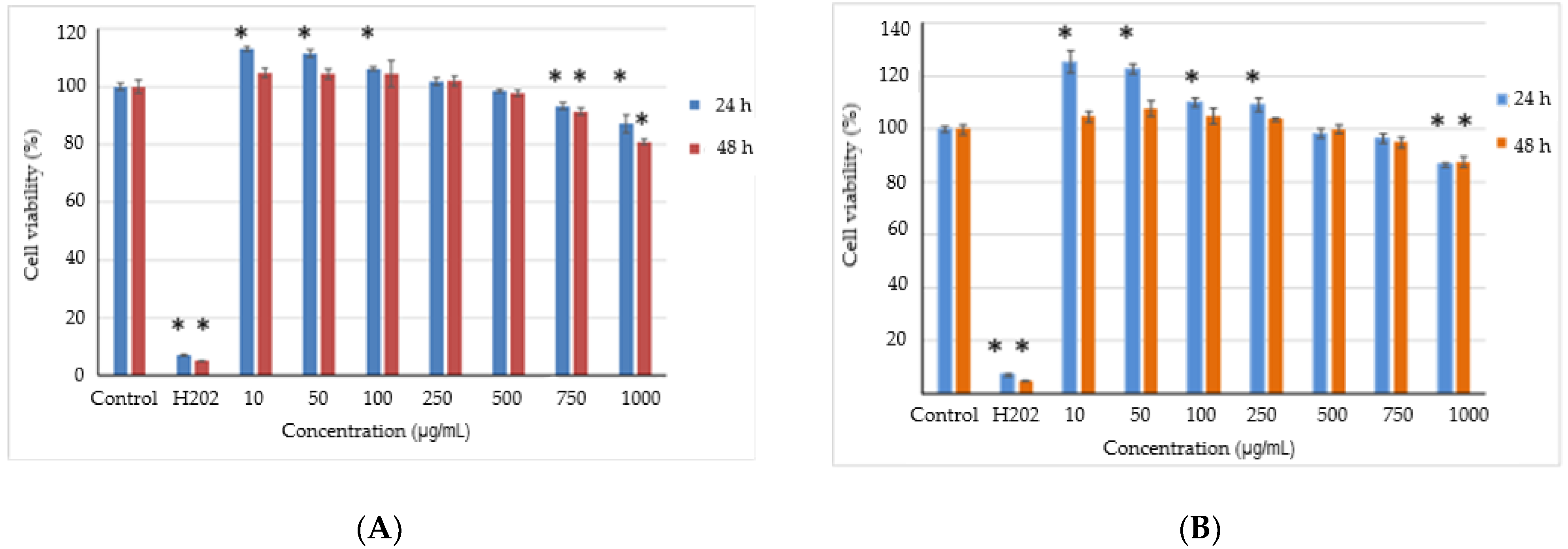
The possible presence and release of any toxic compounds from the powders and the cell viability were analyzed in the L929 fibroblast cell culture by NR assay. The results showed that the powders did not present a cytotoxic effect in the range of the tested concentrations after 24 h and 48 h of cultivation (>80% cell viability) (Figure 3).
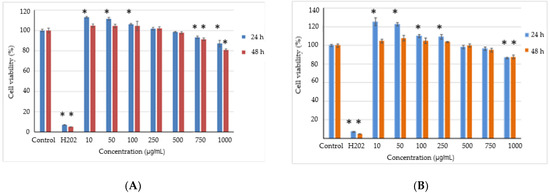
Figure 3.
Cell viability of L929 fibroblasts cultivated in the presence of the powders variant 1 (A) and variant 2 (B) for 24 h and 48 h, respectively, determined by NR assay. The results were expressed as a percentage relative to the control culture (untreated), considered 100% viable. The values represent mean ± SD (n = 3). * p < 0.05, compared to the control.
The values of the cell viability were ranked between 81 and 113% for variant 1 and between 87 and 125% for variant 2, and then decreased by increasing the concentration. These data indicate that the cytocompatibility of the variants are up to 1 mg/mL. Moreover, concentrations between 10 and 100 µg/mL for variant 1 and between 10 and 250 µg/mL for variant 2 stimulated cell proliferation after 24 h of cultivation, in contrast to the control culture, with the highest values for variant 2. A slight decrease in the cell viability was recorded for higher concentrations of 750–1000 µg/mL for variant 1 and 1000 µg/mL for variant 2, until 80% and 87%, respectively.
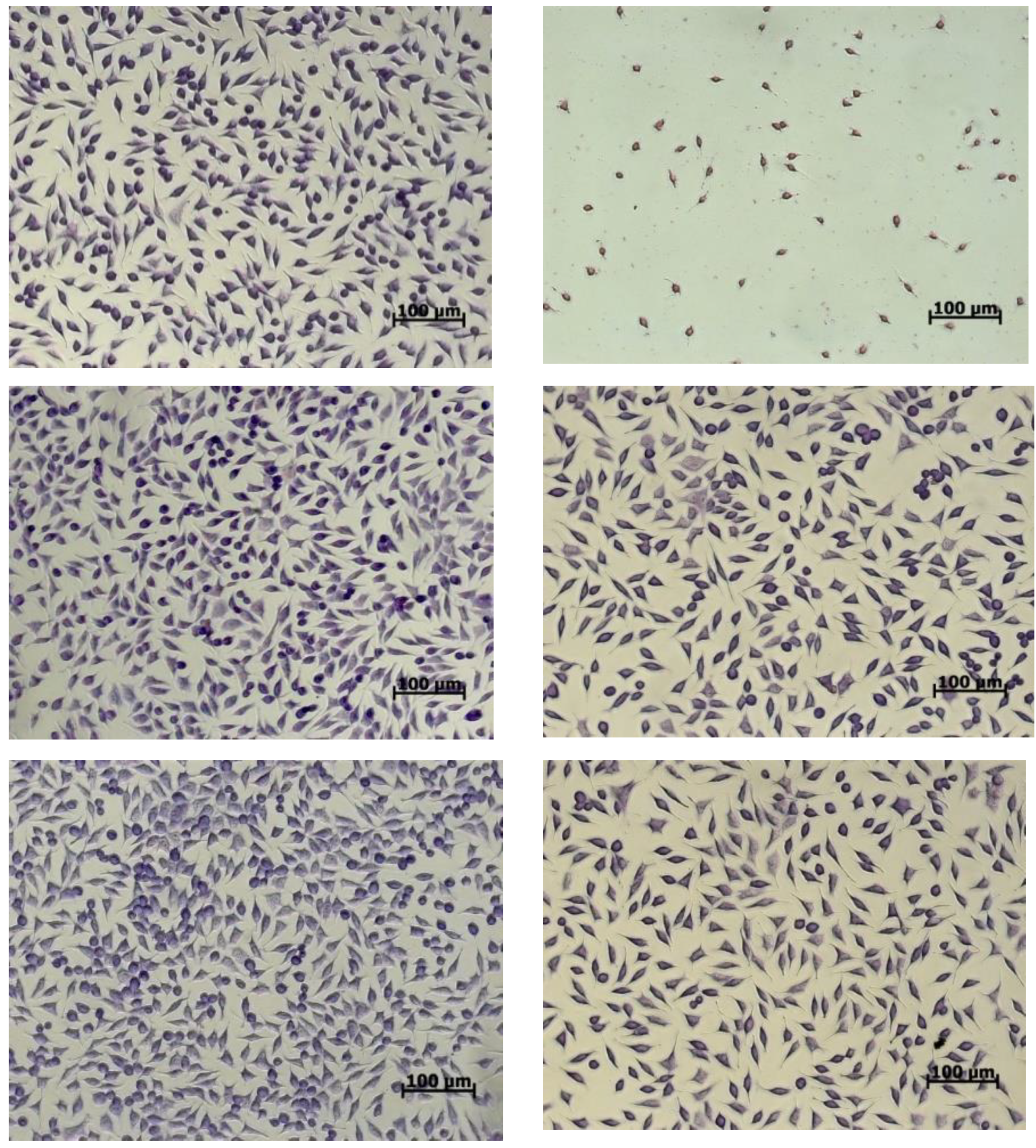
Cell morphology examinations were correlated with the NR quantitative data (Figure 4). The images showed that the cells treated with the variants maintained their normal fusiform phenotype, a characteristic of fibroblast cells, similar to the untreated culture.
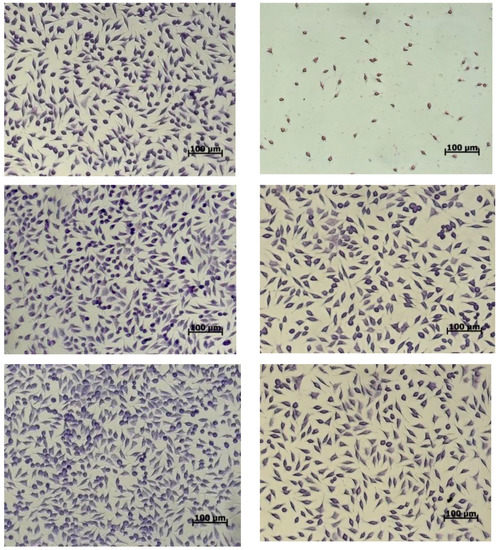
Figure 4.
Light micrographs of L929 cells treated with microencapsulated variants of yellow onion skin extracts for 48 h.
The cell distribution on the culture plate was uniform, whereas the density was comparable to that of the untreated culture. At concentrations between 10 and 100 µg/mL, a cell density higher than that of the control was observed. When higher concentrations of powders were added, the cell density decreased in the culture plates.
Similar studies have shown that flavonoid-rich extracts from onion skins [19] did not exhibit toxicity in a normal cell culture within the range of the tested concentrations of 50–200 µg/mL. Our results indicate that the freeze-drying of the extract into different biopolymeric matrices could lead to an increase of the cytocompatibility domain. Thus, the cell viability was higher than 80% for concentrations of up to 1 mg/mL, especially in variant 2.
3.5. Thermal and pH Stability
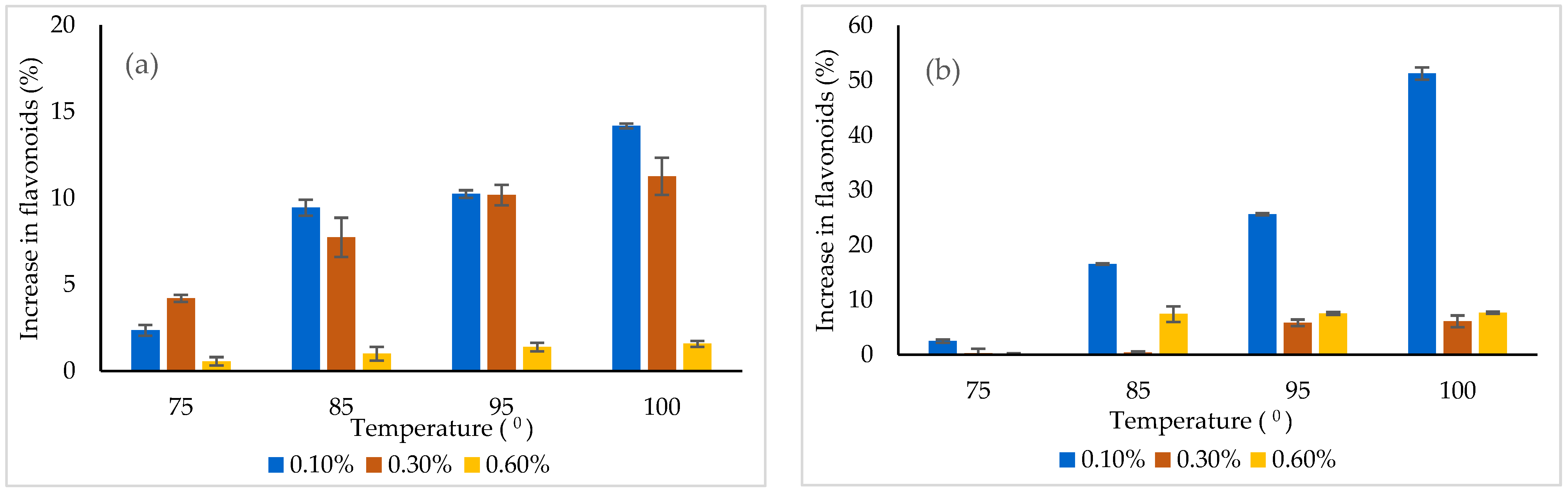
In order to use the powders as functional ingredients, it is imperative to investigate the thermal and pH behavior of the bioactive nutrients in a simulated system, due to the fact that many functional foods containing bioactive compounds susceptible to degradation are usually subjected to thermal treatment and pH variation. Therefore, the selected concentration of powders, varying from 0.1 to 0.6%, were dissolved in distillated water and subjected to various temperatures (from 25 °C to 100 °C) for 15 min. The flavonoid content was measured after the thermal treatment (Figure 5). Heating caused an increase in flavonoid content, probably due to the thermal degradation of the matrices and the release of the bioactives.
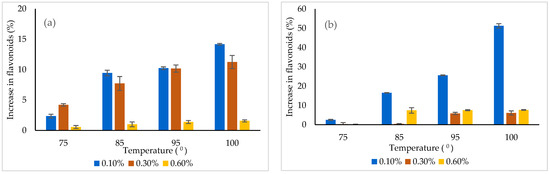
Figure 5.
The effect of thermal treatment on the flavonoids released from the powders (a) variant 1, (b) variant 2.
The flavonoids’ release from the powders was found to be temperature and concentration dependent. In variant 1, the highest release of approximately 14% was found at 0.1% and 100 °C, whereas in variant 2, a significantly higher release of 51% was highlighted under similar conditions. Therefore, it could be understood that variant 2 was less protective when a thermal treatment was applied.
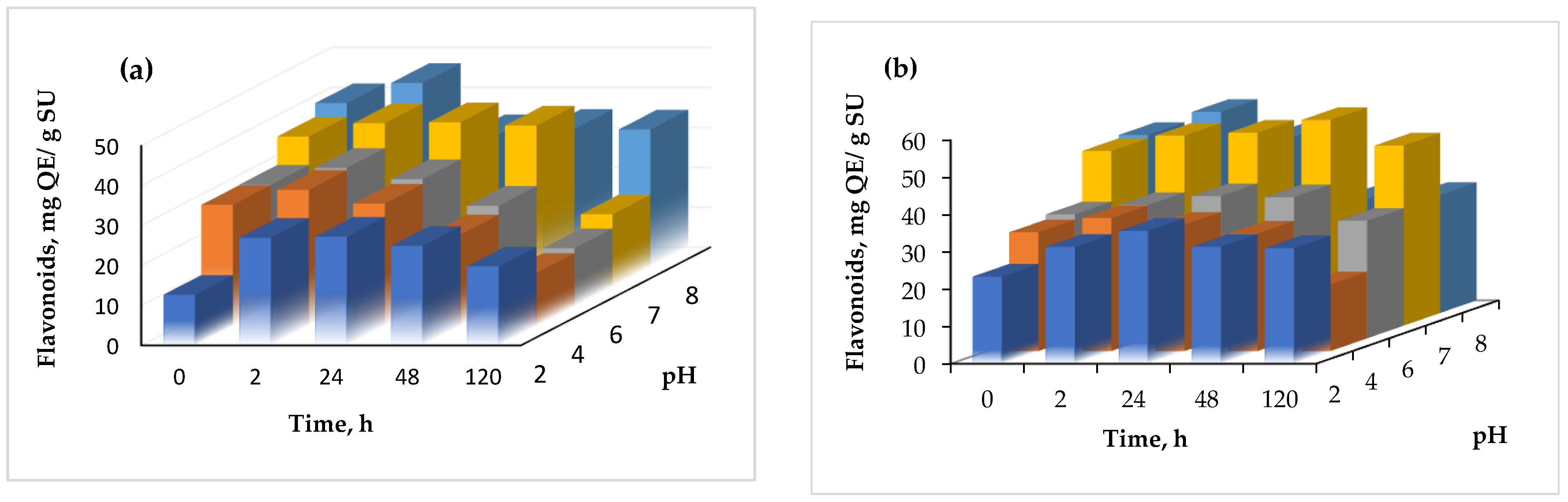
Regarding the stability at different pH values, the results are shown in Figure 6.
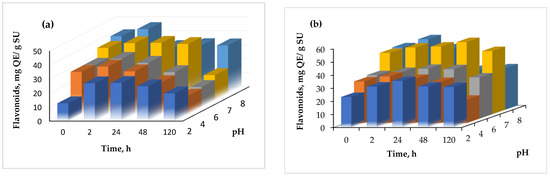
Figure 6.
The effect of pH on the flavonoid content of the powders (a) variant 1, (b) variant (2).
The pH stability was checked at a constant value for variable periods, ranging from 0 to 120 h. From Figure 6, it can be seen that in general, the flavonoid content increased with time, up to 24 h, and started to slowly decrease at a more prolonged time with every test, regardless of the variant. The highest increase of the flavonoid content was found at pH 2.0, after 2 h, when a two-fold increase of the flavonoid content was found in variant 1.
4. Conclusions
The extraction of the flavonoid compounds from yellow onion skins was successfully completed using hot-water extraction, thus allowing for the obtainment of a safe, flavonoid-enriched extract. The achieved results permitted us to conclude that a hot aqueous extraction of the flavonoids from onion skins can be used as an alternative solvent for the selective extraction of flavonoid compounds. The aqueous extract was successfully valorized into biopolymeric matrices based on proteins, peptides, and complex carbohydrates, with an entrapping efficiency higher than 76%. The freeze-dried powders showed a satisfactory phytochemical content and antioxidant activity, whereas the selected matrices provided a protective effect during the simulated digestion. The microstructural analysis showed that the microscopic structure of the powders was evenly distributed and presented a rather uniform structural pattern. A significant cytocompatibility range was found, up to 1 mg/mL, whereas at lower concentrations, the powders stimulated cell proliferation. The powders showed an acceptable thermal and pH stability, which favors their addition to different food matrices. The obtained results demonstrate that simple, green strategies for an efficient valorization of onion skins may be applied in the design of multifunctional ingredients for food, biopharmaceutical, or nutraceutical applications.
Author Contributions
Conceptualization, N.S.; methodology, N.S.; validation, Ș.A.M. and N.S.; formal analysis, Ș.A.M., E.E. and O.C.; investigation, Ș.A.M., E.E. and O.C.; resources, G.R., A.O. and G.E.B.; writing—original draft preparation, Ș.A.M. and N.S.; writing—review and editing, N.S.; visualization, N.S.; supervision, G.R., A.O. and G.E.B.; project administration, N.S.; funding acquisition, G.R., A.O. and G.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by a grant of the Romanian Ministry of Research and Innovation, CCCDI-UEFISCDI, project number PN-III-P1-1.2-PCCDI-2017-0569-PRO-SPER (10PCCI), within the PNCDI program.
Acknowledgments
The Integrated Center for Research, Expertise, and Technological Transfer in Food Industry and The Romanian Center for Modeling of Recirculating Aquaculture Systems (MORAS) are acknowledged for providing technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, F.; Niana, Y.; Perussello, C.A. Effect of storage, food processing and novel extraction technologies on onions flavonoid content: A review. Food Res. Int. 2020, 132, 108953. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Cho, E.; Moon, J.; Bae, H. Onion skin waste as a valorization resource for the by-product’s quercetin and biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, Y.; Yang, Z.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid. Based Complement. Alternat. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromat. A 2003, 1018, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N.; Sumnu, G.; Sahin, S. Microencapsulation of phenolic compounds extracted from onion (Allium cepa) skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharmaceut. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiki, S.; Kumar, N.; Charu, M.; Mahanta, L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze-drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; Cihatİçyer, N.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Milea, A.S.; Aprodu, I.; Vasile, A.M.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J. Food Eng. 2019, 251, 29–35. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.M.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Co-microencapsulation of flavonoids from yellow onion skins and lactic acid bacteria lead to a multifunctional ingredient for foods and pharmaceutics applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim., G.; Rapeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Craciunescu, O.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Central J. 2012, 6, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onionskin and their use in functional bread production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Thakur, H.N.S.; Thakur, A. Microencapsulation of wild pomegranate flavedo phenolics by lyophilization: Effect of maltodextrin concentration, structural morphology, functional properties, elemental composition, and ingredient for development of functional beverage. LWT 2020, 133, 110077. [Google Scholar] [CrossRef]
- Horincar, G.; Aprodu, I.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Interactions of flavonoids from yellow onion skins with whey proteins: Mechanisms of binding and microencapsulation with different combinations of polymers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 215, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.Q.; Yang, J.; Liu, J.; Liu, S.N.; Song, H.X.; Zhao, W.E.; Liu, Y.Q. Isolation of flavonoids from onion skin and their effects on K562 cell viability. Bangladesh J. Pharmacol. 2016, 11, S18–S25. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).