Nutritional Improvement of Bean Sprouts by Using Chitooligosaccharide as an Elicitor in Germination of Soybean (Glycine max L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Chitooligosaccharide, and Chemicals
2.2. Soybean Germination
2.3. Chitooligosaccharide Treatment
2.4. Determination of Vitamin C
2.5. Phytochemical Extraction
2.5.1. Determination of Total Phenolics Content
2.5.2. Determination of Total Flavonoid Content
2.5.3. HPLC Analysis of Phenolic Components and Isoflavones
2.5.4. Analysis of DPPH Radical Scavenging Activity
2.5.5. Analysis of Hydroxyl Radical Scavenging Activity
2.6. Enzyme Assays
2.7. Statistical Analysis
3. Results and Discussion
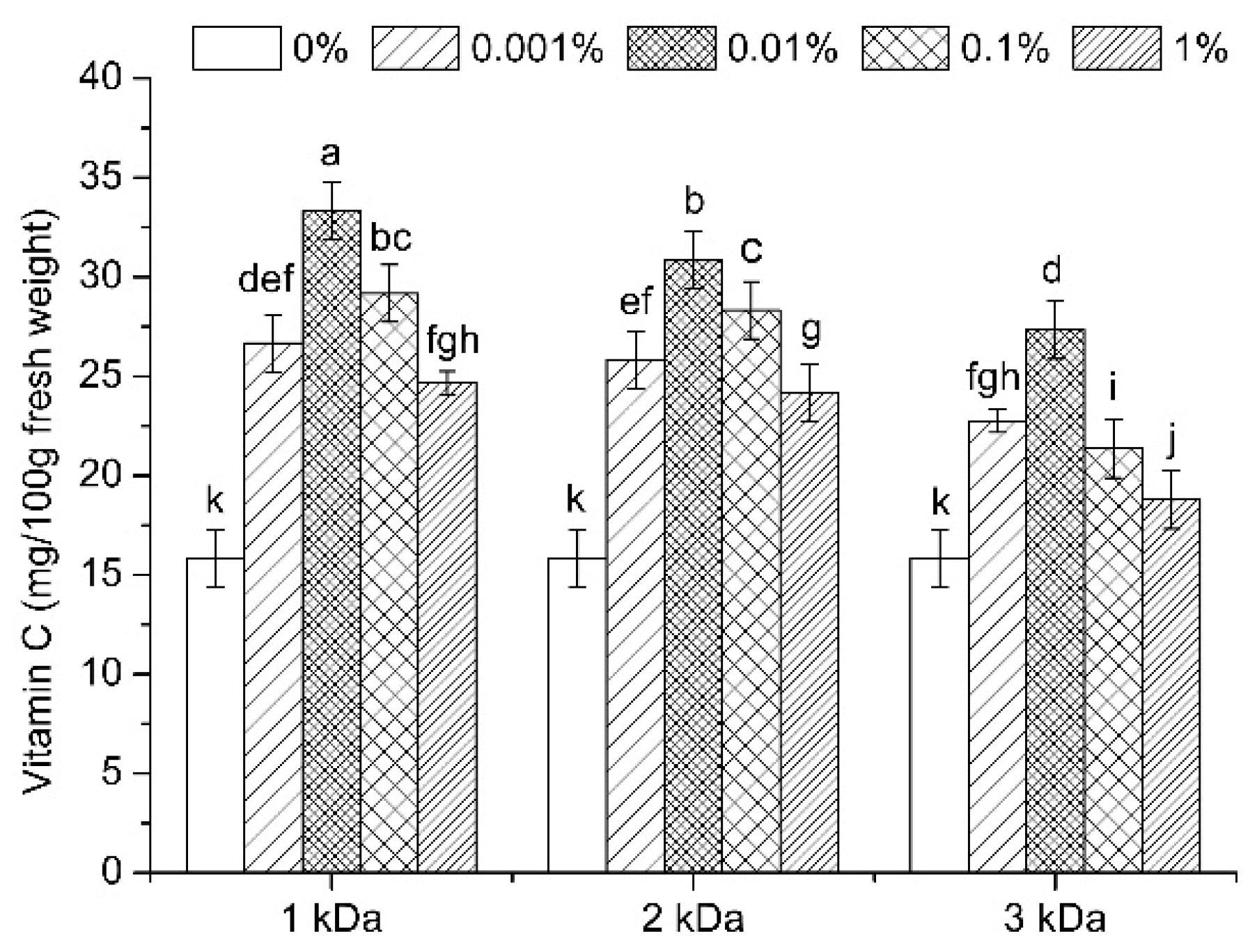
3.1. Vitamin C Content
3.2. Total Phenolics Content
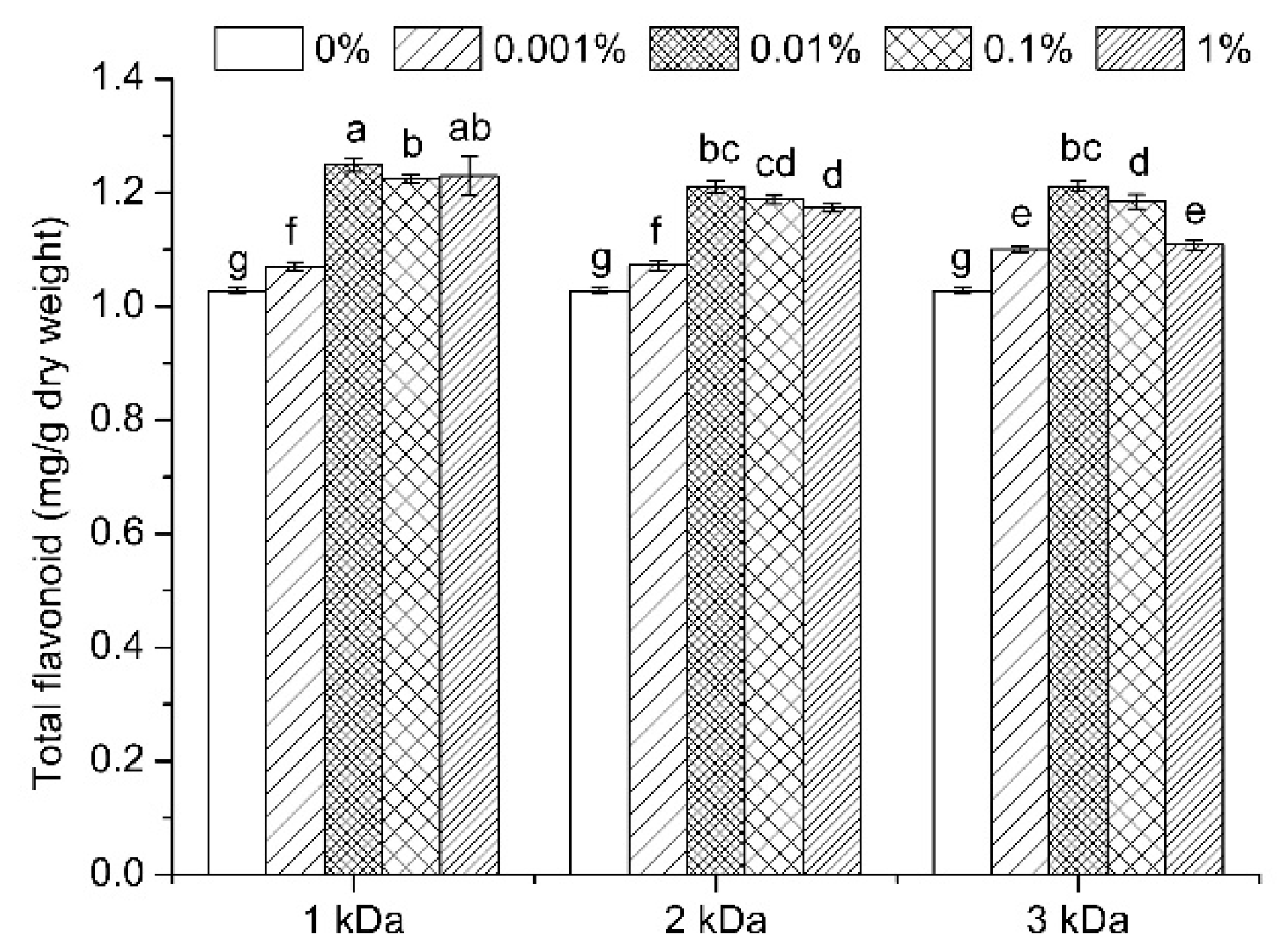
3.3. Total Flavonoid Content
3.4. Quantification of Phenolic Components
3.5. Quantification of Isoflavone Components
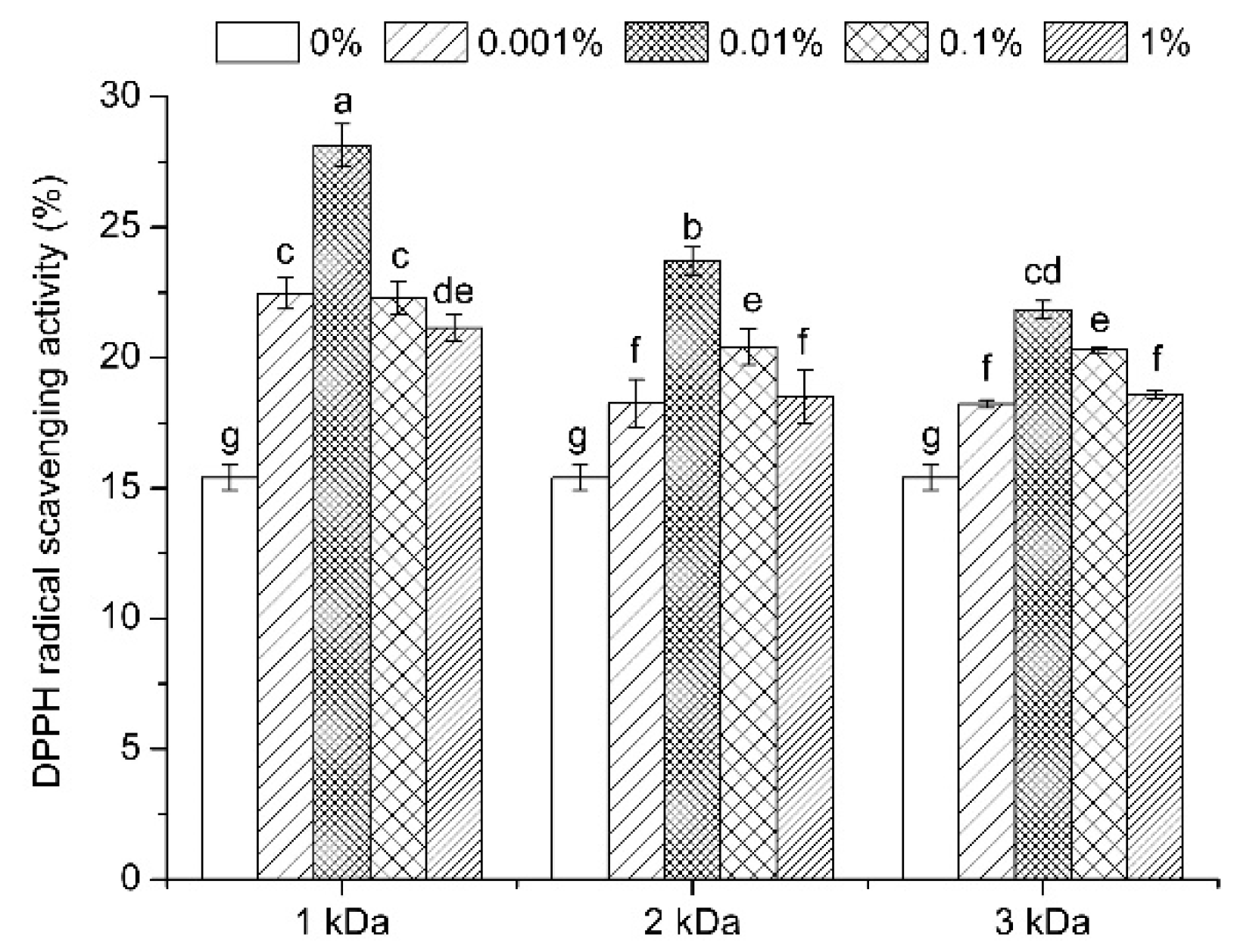
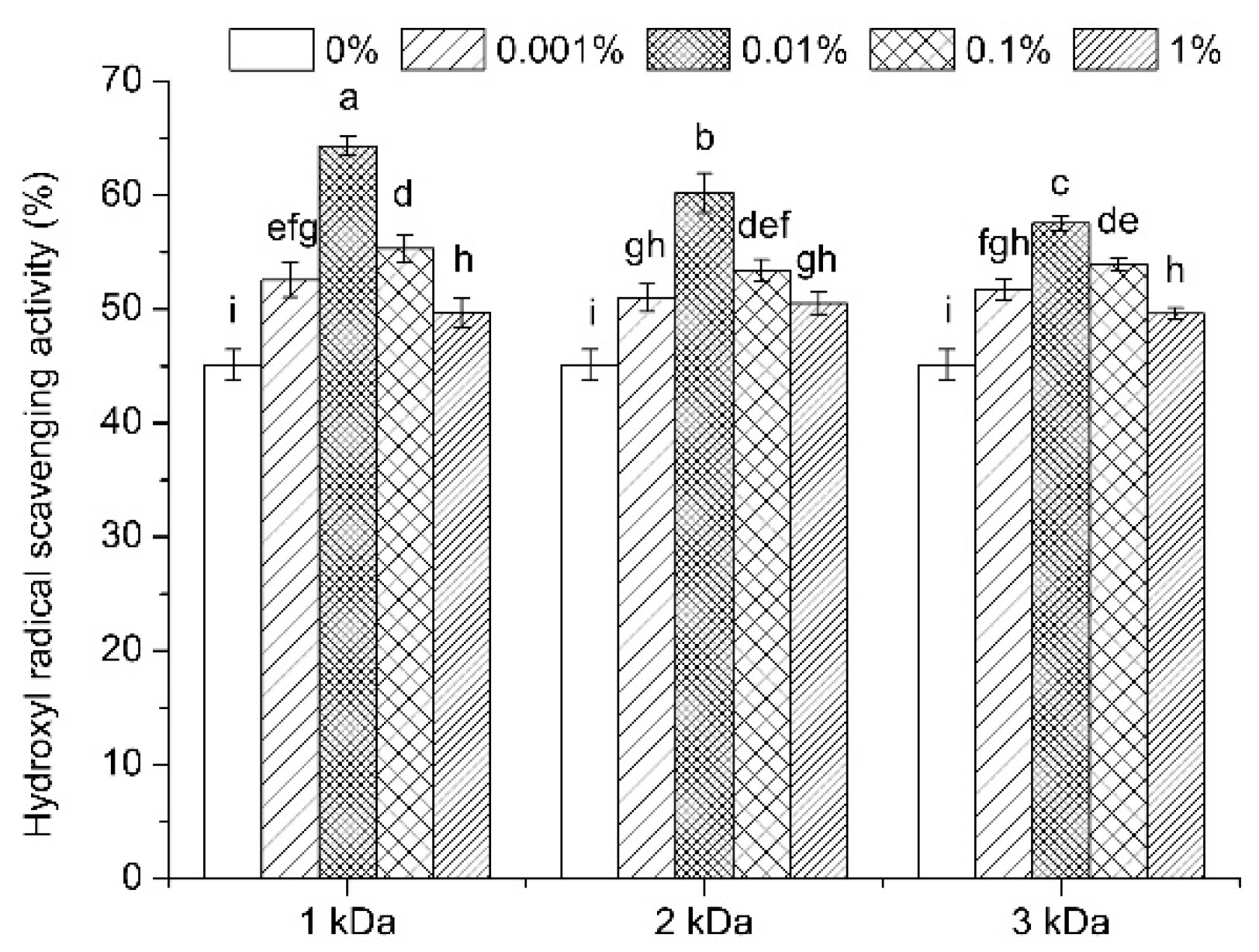
3.6. Antioxidant Activity Evaluation
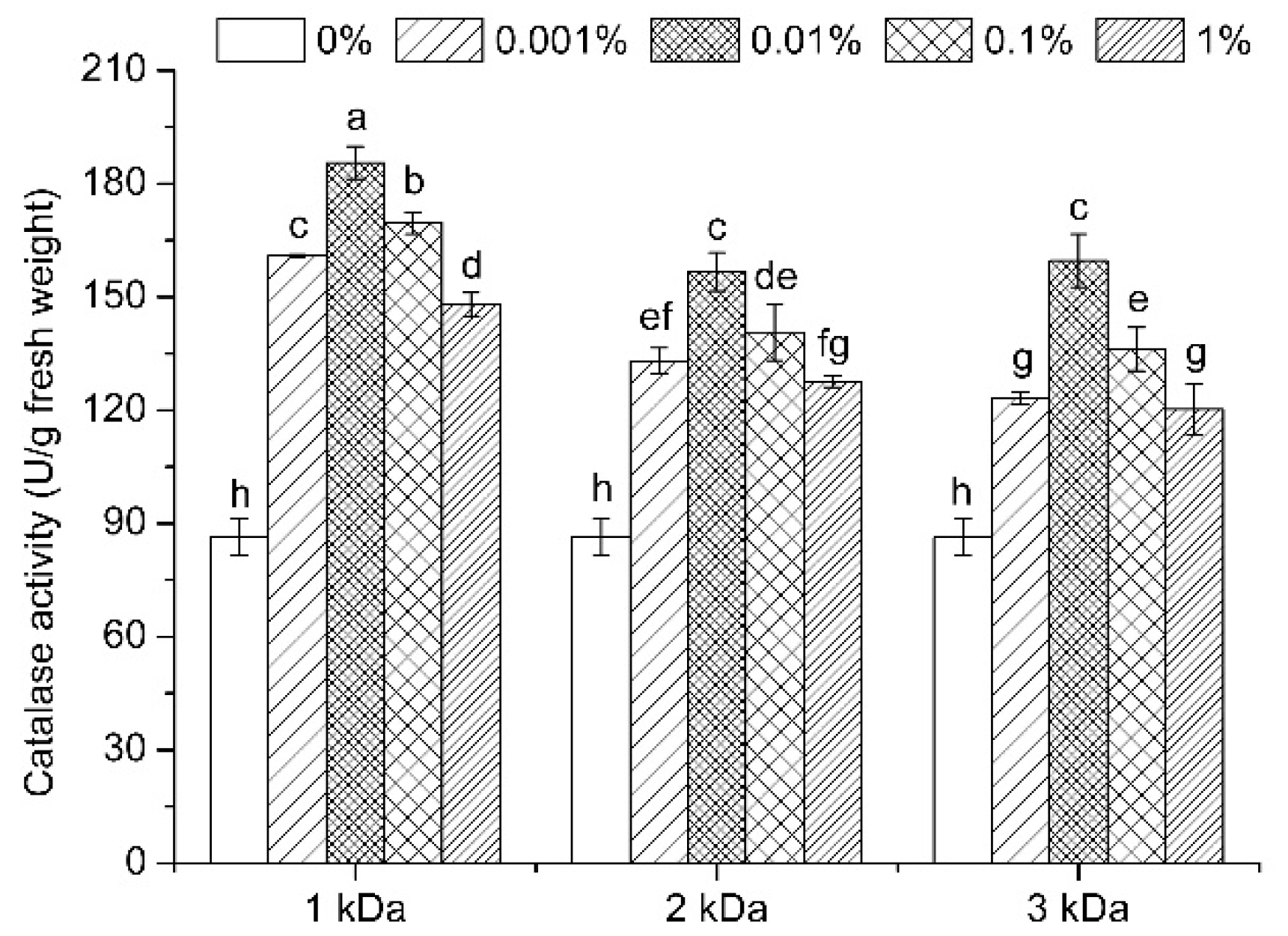
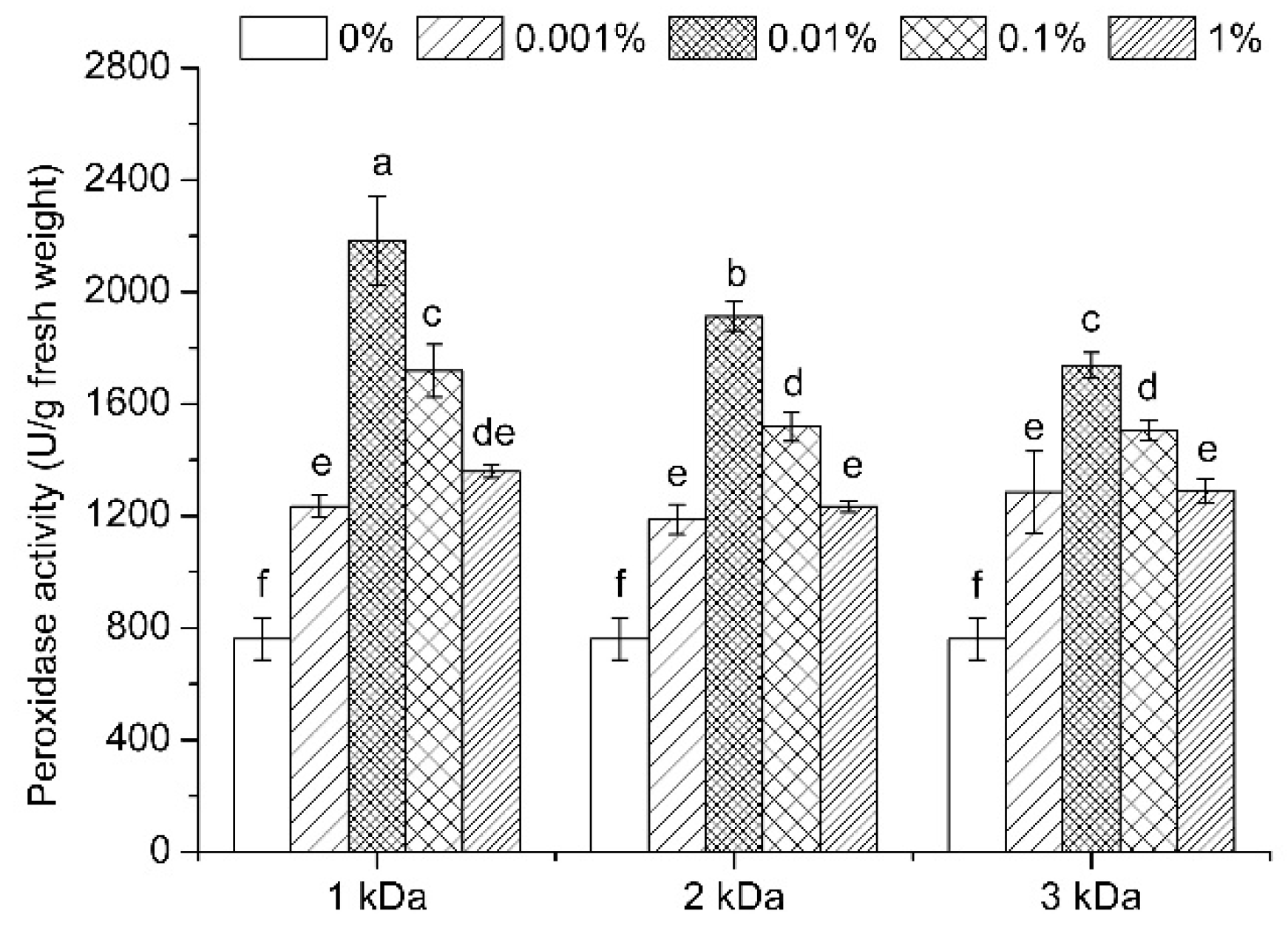
3.7. Enzymatic Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, M.J.; Dong, J.F.; Zhu, M.Y. Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. J. Sci. Food Agric. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Pilšáková, L.; Riečanský, I.; Jagla, F. The physiological actions of isoflavone phytoestrogens. Physiol. Res. 2010, 59, 651–664. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Valverde, C.; Frias, J.; Sierra, I.; Blazquez, I.; Lambein, F.; Kuo, Y.H. New functional legume foods by germination: Effect on the nutritive value of beans, lentils and peas. Eur. Food Res. Technol. 2002, 215, 472–477. [Google Scholar] [CrossRef]
- Shi, H.L.; Nam, P.K.; Ma, Y.F. Comprehensive Profiling of Isoflavones, Phytosterols, Tocopherols, Minerals, Crude Protein, Lipid, and Sugar during Soybean (Glycine max) Germination. J. Agric. Food Chem. 2010, 58, 4970–4976. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Ancos, B.D.; Cano, M.P. Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum. L) and alfalfa (Medicago sativa) treated by a new drying method. Eur. Food Res. Technol. 2003, 216, 138–144. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants 2020, 9, 558. [Google Scholar] [CrossRef]
- Bau, H.M.; Villaume, C.; Nicolas, J.P.; Méjean, L. Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soya bean (Glycine max) seeds. J. Sci. Food Agric. 1997, 73, 1–9. [Google Scholar] [CrossRef]
- Zhong, L.Y.; Niu, B.; Tang, L.; Chen, F.; Zhao, G.; Zhao, J.L. Effects of polysaccharide elicitors from endophytic Fusarium oxysporum Fat9 on the growth, flavonoid accumulation and antioxidant property of Fagopyrum tataricum sprout cultures. Molecules 2016, 21, 1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, S.R.; Shuai, X.T.; Unger, F.; Simon, M.; Bi, D.Z.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef]
- Shibuya, N.; Minami, E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001, 59, 223–233. [Google Scholar] [CrossRef] [Green Version]
- No, H.K.; Lee, K.S.; Kim, I.D.; Park, M.J.; Kim, S.D.; Meters, S.P. Chitosan treatment affects yield, ascorbic acid content, and hardness of soybean sprouts. J. Food Sci. 2003, 68, 680–685. [Google Scholar] [CrossRef]
- No, H.K.; Kim, S.D.; Prinyawiwatkul, W.; Meyers, S.P. Growth of soybean sprouts affected by chitosans prepared under various deproteinization and demineralization times. J. Sci. Food Agric. 2006, 86, 1365–1370. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, Y.H.; Kim, S.B. Changes in the respiration, growth, and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. HortScience 2005, 40, 1333–1335. [Google Scholar] [CrossRef] [Green Version]
- Rui, Y.; Yu, J.; Xiu, L.; Huang, J. Effect of chitosan pre-soaking on the growth and quality of yellow soybean sprouts. J. Sci. Food Agric. 2019, 99, 1596–1603. [Google Scholar]
- Lan, W.Q.; Wang, W.; Yu, Z.M.; Qin, Y.X.; Luan, J.; Li, X.Z. Enhanced germination of barley (Hordeum vulgare L.) using chitooligosaccharide as an elicitor in seed priming to improve malt quality. Biotechnol. Lett. 2016, 38, 1935–1940. [Google Scholar] [CrossRef]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Wang, J.H.; Ye, Y.T.; Li, Q.; Abbasi, A.M.; Guo, X.B. Assessment of phytochemicals, enzymatic and antioxidant activities in germinated mung bean (Vigna radiata L. Wilezek). Int. J. Food Sci. Technol. 2017, 52, 1276–1282. [Google Scholar] [CrossRef]
- Yang, F.; Luan, B.; Sun, Z.; Yang, C.; Yu, Z.M.; Li, X.Z. Application of chitooligosaccharides as antioxidants in beer to improve the flavour stability by protecting against beer staling during storage. Biotechnol. Lett. 2016, 39, 305–310. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.Z.; Jiang, L.L.; Hou, Y.M.; Yang, F.; Chen, W.F.; Li, X.Z. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol. Biotechnol. Equip. 2015, 29, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Hamel, L.P.; Beaudoin, N. Chitooligosaccharide sensing and downstream signaling: Contrasted outcomes in pathogenic and beneficial plant-microbe interaction. Planta 2010, 232, 787–806. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Chirkov, S.N.; Il’ina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. Microbiol. 2006, 42, 200–203. [Google Scholar] [CrossRef]
- Mendoza-Sanchez, M.; Guevara-Gonzalez, R.G.; Castano-Tostado, E.; Mercado-Silva, E.M.; Acosta-Gallegos, J.A.; Rocha-Guzman, N.E. Effect of chemical stress on germination of cv Dalia bean (Phaseolus vularis L.) as an alternative to increase antioxidant and nutraceutical compounds in sprouts. Food Chem. 2016, 212, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Cao, Y.; Huang, W.N.; Guo, Y.D.; Kang, Y.F. Effect of ethylene on total phenolics, antioxidant activity, and the activity of metabolic enzymes in mung bean sprouts. Eur. Food Res. Technol. 2013, 237, 755–764. [Google Scholar] [CrossRef]
- Kumari, S.; Krishnan, V.; Sachdev, A. Impact of soaking and germination durations on antioxidants and anti-nutrients of black and yellow soybean (Glycine max. L.) varieties. J. Plant Biochem. Biotechnol. 2015, 24, 355–358. [Google Scholar] [CrossRef]
- Guo, X.B.; Li, T.; Tang, K.X.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Jiratanan, T.; Liu, R.H. Antioxidant activity of processed table beets (Beta vulgaris var, conditiva) and green beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2004, 52, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, S.H.; Chung, J.I.; Chi, H.Y.; Kim, J.A.; Chung, I.M. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol. 2006, 222, 201. [Google Scholar] [CrossRef]
- Lee, A.H.; Su, D.D.; Pasalich, M.; Tang, L.; Binns, C.W.; Qiu, L.Q. Soy and isoflavone intake associated with reduced risk of ovarian cancer in southern Chinese women. Nutr. Res. 2014, 34, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Al-Tawaha, A.M.; Seguin, P.; Smith, D.L.; Beaulieu, C. Biotic elicitors as a means of increasing isoflavone concentration of soybean seeds. Ann. Appl. Biol. 2005, 146, 303–310. [Google Scholar] [CrossRef]
- Chen, Y.M.; Chang, S.K.C. Macronutrients, phytochemicals, and antioxidant activity of soybean sprout germinated with or without light exposure. J. Food Sci. 2015, 80, 1391–1398. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Algar, E.; Ramos-Solano, B.; García-Villaraco, A.; Sierra, M.D.S.; Gómez, M.S.M.; Gutiérrez-Mañero, F.J. Bacterialbioeffectors modify bioactive profile and increase isoflavone content in soybean sprouts (Glycine max var Osumi). Plant Foods Hum. Nutr. 2013, 68, 299–305. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Yun, J.; Li, X.H.; Fan, X.T.; Li, W.L.; Jiang, Y.Q. Growth and quality of soybean sprouts (Glycine max L. Merrill) as affected by gamma irradiation. Radiat. Phys. Chem. 2013, 82, 106–111. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.C.; Liu, S.; Xing, R.E.; Qin, Y.K.; Yu, H.H.; Zhou, M.M.; Li, P.C. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydr. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1988, 28, 1057–1060. [Google Scholar] [CrossRef]
- Chung, S.K.; Osawa, T.; Kawakishi, S. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 1997, 61, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tian, S.P.; Meng, X.H.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Syeed, S.; Khan, N.A. Activities of carbonic anhydrase, catalase and ACC oxidase of mung bean (Vigna radiata) are differentially affected by salinity stress. J. Food Agric. Environ. 2004, 2, 241–249. [Google Scholar]
- Day, R.B.; Okada, M.; Ito, Y.; Tsukada, K.; Zaghouani, H.; Shibuya, N.; Stacey, G. Binding site for chitin oligosaccharides in the soybean plasma membrane. Plant Physiol. 2001, 126, 1162–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | COS Concentration (%) | Gallic Acid | Protocatechuic Acid | Syringic Acid | p-Coumaric Acid | Vanillic Acid | Ferulic Acid | Ellagic Acid | Cinnamic Acid | p-Hydroxybenzoic Acid |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | 18.698 ± 0.311 e | 0.539 ± 0.023 e | 0.073 ± 0.005 e | 0.771 ± 0.015 g | 1.021 ± 0.020 e | 4.845 ± 0.047 g | 0.459 ± 0.001 e | 0.053 ± 0.004 e | 0.251 ± 0.030 e |
| 1 kDa COS | 0.001 | 30.462 ± 0.212 b | 0.915 ± 0.017 d | 0.106 ± 0.002 cd | 1.261 ± 0.008 de | 1.268 ± 0.014 d | 7.296 ± 0.052 a | 0.652 ± 0.007 cd | 0.121 ± 0.003 d | 0.913 ± 0.044 d |
| 0.01 | 32.274 ± 0.547 a | 1.444 ± 0.032 a | 0.116 ± 0.001 a | 1.460 ± 0.019 a | 1.844 ± 0.105 a | 7.398 ± 0.078 a | 0.775 ± 0.012 a | 0.254 ± 0.003 a | 2.646 ± 0.014 a | |
| 0.1 | 25.660 ± 0.305 d | 0.809 ± 0.010 d | 0.100 ± 0.002 d | 1.194 ± 0.013 f | 1.307 ± 0.020 d | 6.477 ± 0.061 de | 0.710 ± 0.016 b | 0.126 ± 0.004 d | 1.061 ± 0.026 cd | |
| 1 | 26.855 ± 0.251 d | 0.988 ± 0.034 c | 0.105 ± 0.008 cd | 1.191 ± 0.022 f | 1.404 ± 0.024 d | 7.050 ± 0.024 a | 0.645 ± 0.0207 d | 0.185 ± 0.006 c | 1.170 ± 0.201 bc | |
| 2 kDa COS | 0.001 | 25.486 ± 0.481 d | 0.860 ± 0.027 d | 0.101 ± 0.002 d | 1.231 ± 0.009 e | 1.445 ± 0.016 d | 6.291 ± 0.087 f | 0.730 ± 0.056 ab | 0.195 ± 0.002 bc | 1.344 ± 0.037 bc |
| 0.01 | 31.020 ± 0.465 b | 1.116 ± 0.034 b | 0.110 ± 0.002 ab | 1.372 ± 0.031 a | 1.754 ± 0.037 a | 7.155 ± 0.117 a | 0.669 ± 0.009 bc | 0.239 ± 0.000 a | 1.660 ± 0.846 b | |
| 0.1 | 25.291 ± 0.540 d | 1.144 ± 0.018 b | 0.109 ± 0.001 bc | 1.286 ± 0.015 cd | 1.551 ± 0.031 c | 6.559 ± 0.029 e | 0.628 ± 0.008 d | 0.172 ± 0.006 c | 0.978 ± 0.518 cd | |
| 1 | 29.939 ± 0.260 c | 0.971 ± 0.012 c | 0.103 ± 0.004 d | 1.315 ± 0.008 bc | 1.549 ± 0.021 c | 6.682 ± 0.045 cd | 0.637 ± 0.008 cd | 0.142 ± 0.003 d | 1.083 ± 0.021 cd | |
| 3 kDa COS | 0.001 | 29.026 ± 0.078 c | 0.958 ± 0.036 d | 0.109 ± 0.001 bc | 1.331 ± 0.029 b | 1.626 ± 0.045 b | 6.839 ± 0.170 bc | 0.671 ± 0.005 bc | 0.197 ± 0.007 b | 1.074 ± 0.083 cd |
| 0.01 | 32.066 ± 0.585 a | 1.207 ± 0.006 b | 0.112 ± 0.002 ab | 1.381 ± 0.012 a | 1.619 ± 0.051 b | 6.973 ± 0.021 b | 0.736 ± 0.003 a | 0.230 ± 0.005 a | 1.546 ± 0.319 b | |
| 0.1 | 28.318 ± 0.672 c | 1.060 ± 0.028 c | 0.109 ± 0.003 bc | 1.259 ± 0.031 de | 1.600 ± 0.048 b | 6.307 ± 0.099 ef | 0.658 ± 0.014 bc | 0.151 ± 0.010 cd | 1.056 ± 0.120 cd | |
| 1 | 31.068 ± 0.323 b | 1.012 ± 0.014c | 0.105 ± 0.003 cd | 1.266 ± 0.014 de | 1.619 ± 0.024 b | 6.440 ± 0.036 e | 0.690 ± 0.010 b | 0.148 ± 0.002 cd | 0.914 ± 0.077 d |
| Treatment | COS Concentration (%) | Genistein | Daidzein | Glycitein | Genistin | Daidzin | Glycitin |
|---|---|---|---|---|---|---|---|
| Control | 0 | 0.497 ± 0.007 e | 0.461 ± 0.001 e | 0.012 ± 0.004 cd | 0.089 ± 0.001 h | 0.079 ± 0.001 h | 0.019 ± 0.001 h |
| 1 kDa COS | 0.001 | 0.521 ± 0.007 c | 0.463 ± 0.001 d | 0.088 ± 0.026 b | 0.122 ± 0.003 d | 0.088 ± 0.003 g | 0.025 ± 0.001 d |
| 0.01 | 0.575 ± 0.003 a | 0.490 ± 0.001 a | 0.251 ± 0.006 a | 0.144 ± 0.001 a | 0.150 ± 0.001 a | 0.032 ± 0.002 a | |
| 0.1 | 0.551 ± 0.013 b | 0.462 ± 0.000 de | 0.102 ± 0.007 b | 0.138 ± 0.004 b | 0.132 ± 0.003 c | 0.026 ± 0.001 d | |
| 1 | 0.507 ± 0.012 cd | 0.466 ± 0.001 c | 0.041 ± 0.014 cd | 0.102 ± 0.002 g | 0.137 ± 0.001 bc | 0.020 ± 0.001 gh | |
| 2 kDa COS | 0.001 | 0.511 ± 0.007 cd | 0.461 ± 0.001 e | 0.043 ± 0.007 c | 0.118 ± 0.003 e | 0.095 ± 0.004 fg | 0.022 ± 0.001 ef |
| 0.01 | 0.555 ± 0.003 b | 0.467 ± 0.001 c | 0.220 ± 0.008 ab | 0.136 ± 0.001 b | 0.135 ± 0.003 c | 0.029 ± 0.001 c | |
| 0.1 | 0.519 ± 0.008 c | 0.466 ± 0.001 c | 0.096 ± 0.041 b | 0.119 ± 0.002 de | 0.122 ± 0.002 e | 0.021 ± 0.001 fg | |
| 1 | 0.513 ± 0.007 cd | 0.462 ± 0.002 de | 0.019 ± 0.003 cd | 0.099 ± 0.002 g | 0.104 ± 0.003 f | 0.019 ± 0.001 h | |
| 3 kDa COS | 0.001 | 0.515 ± 0.003 cd | 0.466 ± 0.001 c | 0.023 ± 0.002 cd | 0.110 ± 0.000 f | 0.105 ± 0.002 f | 0.023 ± 0.001 e |
| 0.01 | 0.569 ± 0.010 a | 0.484 ± 0.001 b | 0.236 ± 0.021 a | 0.139 ± 0.001 b | 0.140 ± 0.001 b | 0.031 ± 0.001 b | |
| 0.1 | 0.503 ± 0.004 de | 0.466 ± 0.001 c | 0.073 ± 0.029 b | 0.131 ± 0.002 c | 0.128 ± 0.002 de | 0.025 ± 0.001 d | |
| 1 | 0.518 ± 0.006 c | 0.462 ± 0.001 de | 0.009 ± 0.005 d | 0.102 ± 0.001 g | 0.102 ± 0.002 f | 0.020 ± 0.001 gh |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Lei, X.; Liu, X.; Yang, F. Nutritional Improvement of Bean Sprouts by Using Chitooligosaccharide as an Elicitor in Germination of Soybean (Glycine max L.). Appl. Sci. 2021, 11, 7695. https://doi.org/10.3390/app11167695
Tang W, Lei X, Liu X, Yang F. Nutritional Improvement of Bean Sprouts by Using Chitooligosaccharide as an Elicitor in Germination of Soybean (Glycine max L.). Applied Sciences. 2021; 11(16):7695. https://doi.org/10.3390/app11167695
Chicago/Turabian StyleTang, Wenzhu, Xinting Lei, Xiaoqi Liu, and Fan Yang. 2021. "Nutritional Improvement of Bean Sprouts by Using Chitooligosaccharide as an Elicitor in Germination of Soybean (Glycine max L.)" Applied Sciences 11, no. 16: 7695. https://doi.org/10.3390/app11167695
APA StyleTang, W., Lei, X., Liu, X., & Yang, F. (2021). Nutritional Improvement of Bean Sprouts by Using Chitooligosaccharide as an Elicitor in Germination of Soybean (Glycine max L.). Applied Sciences, 11(16), 7695. https://doi.org/10.3390/app11167695





