Theoretical Methane Emission Estimation from Volatile Fatty Acids in Bovine Rumen Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Materials
2.2. Conventional Method
2.3. Catalytic Esterification Reaction Method
3. Results and Discussion
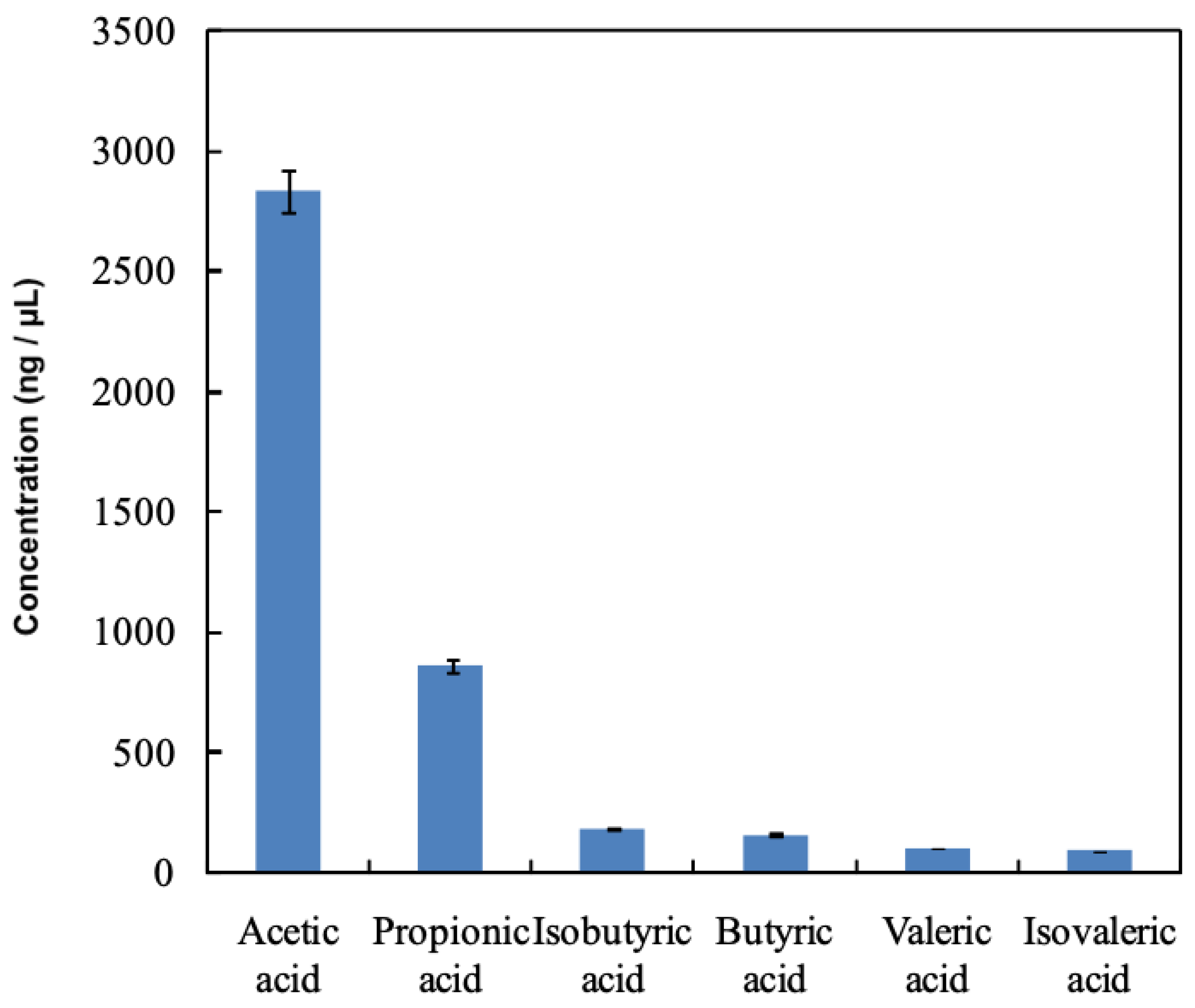
3.1. VFA Concentrations in Rumen Fluid Using the Conventional Method
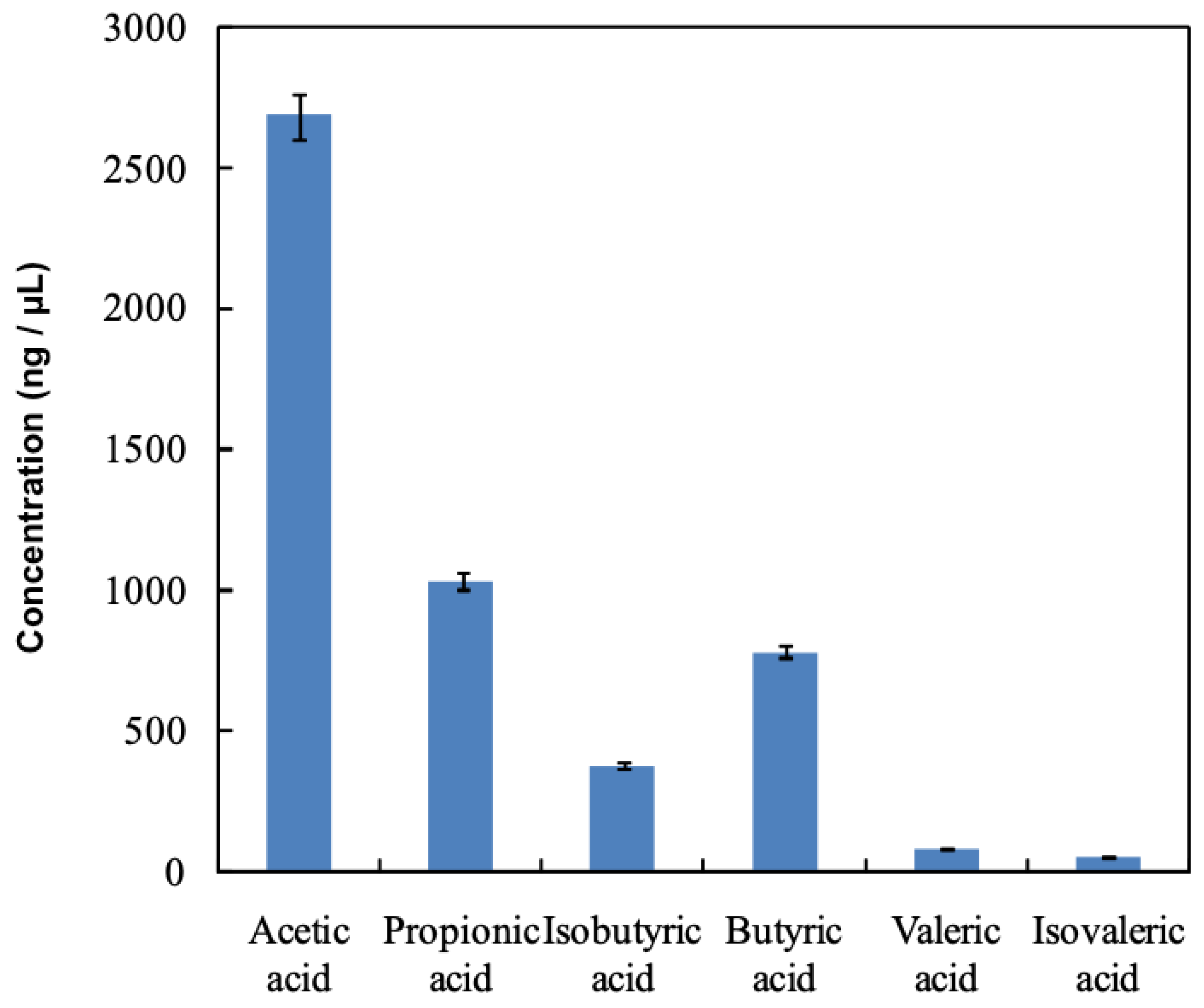
3.2. VFA Concentrations in Rumen Fluid Using the Catalytic Esterification Reaction Method
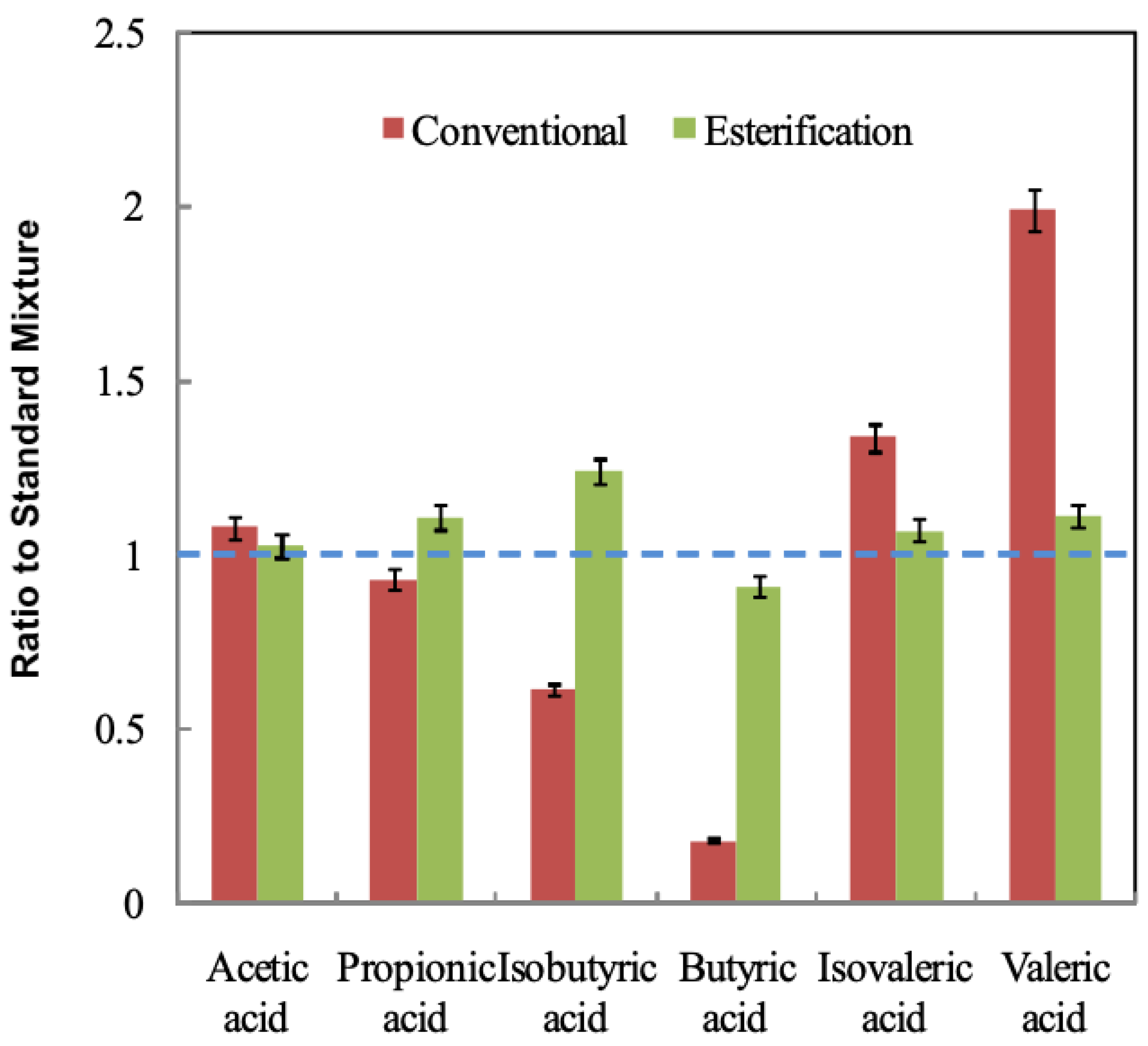
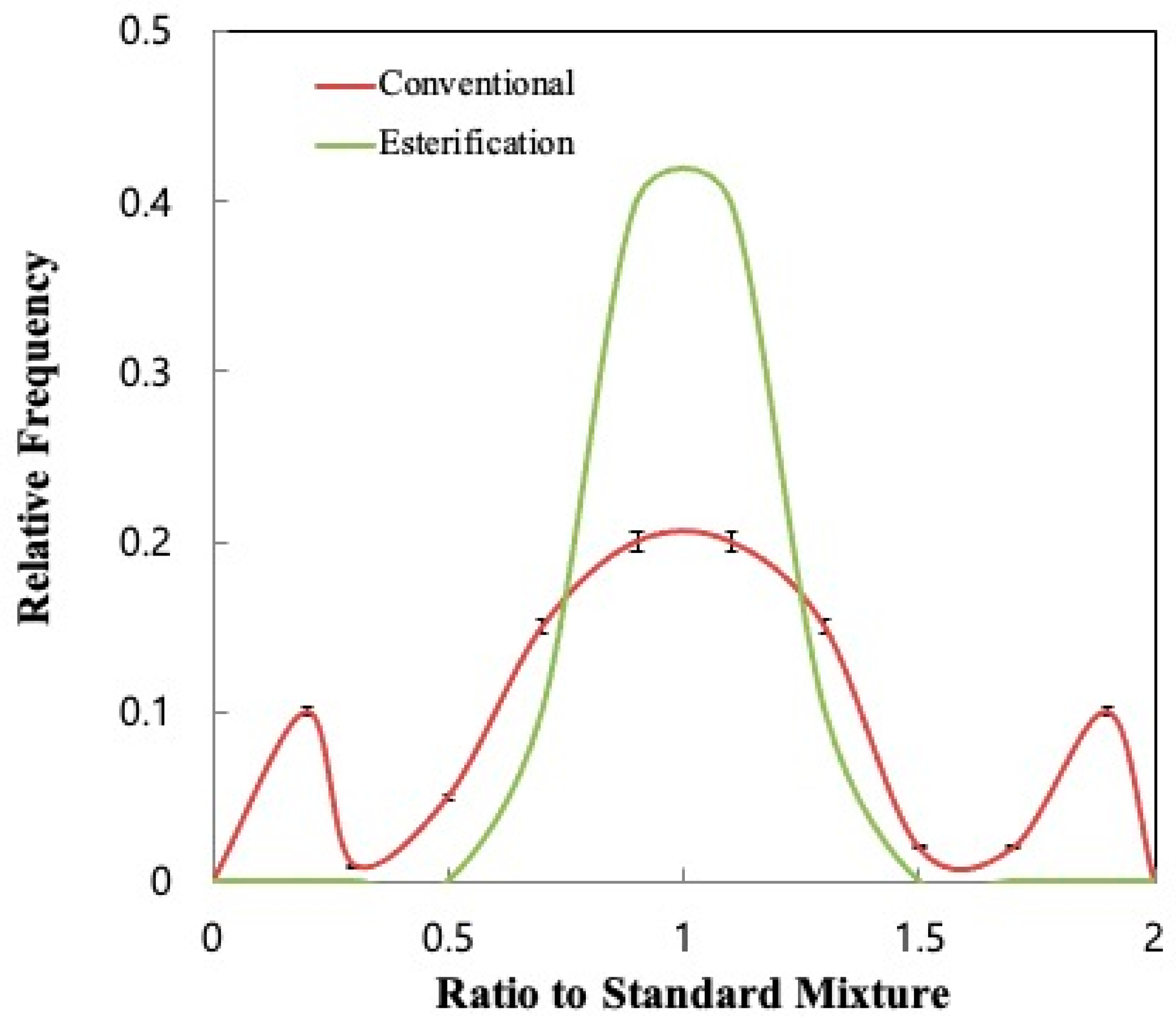
3.3. Confirmation Using Standard Mixtures Prepared with Purified VFAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. FAO Statistics, Food and Agriculture Organization of the United Nations. 2014. Available online: http://fao.org/faostat/en/#data/QCL (accessed on 1 March 2021).
- Maasakkers, J.D.; Jacob, D.J.; Sulprizio, M.P.; Turner, A.J.; Weitz, M.; Wirth, T.; Hight, C.; DeFigueiredo, M.; Desai, M.; Schmeltz, R.; et al. Gridded National Inventory of U.S. Methane Emissions. Environ. Sci. Technol. 2016, 50, 13123–13133. [Google Scholar] [CrossRef] [PubMed]
- Thoma, G.; Popp, J.; Shonnard, D.; Nutter, D.; Matlock, M.; Ulrich, R.; Kellogg, W.; Kim, D.S.; Neiderman, Z.; Kemper, N.; et al. Regional analysis of greenhouse gas emissions from USA dairy farms: A cradle to farm-gate assessment of the American dairy industry circa 2008. Int. Dairy J. 2013, 31, S29–S40. [Google Scholar] [CrossRef] [Green Version]
- Sasu-Boakye, Y.; Cederberg, C.; Wirsenius, S. Localising livestock protein feed production and the impact on land use and greenhouse gas emissions. J. Anim. 2014, 8, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, M.A.; de Neergaard, A.; Jensen, L.S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.S.; Novak, J.M.; Johnson, M.G.; Szogi, A.A.; Libra, J.A.; Spokas, K.A.; Bae, S. Leachate water quality of soils amended with different swine manure-based amendments. Chemosphere 2016, 142, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Minato, K.; Kouda, Y.; Yamakawa, M.; Hara, S.; Tamura, T.; Osada, T. Determination of GHG and ammonia emissions from stored dairy cattle slurry by using a floating dynamic chamber. Anim. Sci. J. 2013, 84, 165–177. [Google Scholar] [CrossRef]
- Jarvis, S.C.; Pain, B.F. Greenhouse gas emissions from intensive livestock systems: Their estimation and technologies for reduction. Clim. Chang. 1994, 27, 27–38. [Google Scholar] [CrossRef]
- Chung, M.L.; Shilton, A.N.; Guieysse, B.; Pratt, C. Questioning the Accuracy of Greenhouse Gas Accounting from Agricultural Waste: A Case Study. J. Environ. Qual. 2013, 42, 654–659. [Google Scholar] [CrossRef]
- Tomkins, N.W.; McGinn, S.M.; Turner, D.A.; Charmley, E. Comparison of open-circuit respiration chambers with a micrometeorological method for determining methane emissions from beef cattle grazing a tropical pasture. Anim. Feed Sci. Technol. 2011, 166–167, 240–247. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Hernández, A.; Salem, A.Z.M.; Mellado, M.; Odongo, N.E. Effects of organic acid salts on ruminal biogas production and fermentation kinetics of total mixed rations with different maize silage to concentrate ratios. J. Clean. Prod. 2017, 147, 523–530. [Google Scholar] [CrossRef]
- Zhou, M.; Hernandez-Sanabria, E.; Luo Guan, L. Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl. Environ. Microbiol. 2010, 76, 3776–3786. [Google Scholar] [CrossRef] [Green Version]
- Hammond, K.J.; Jones, A.K.; Humphries, D.J.; Crompton, L.A.; Reynolds, C.K. Effects of diet forage source and neutral detergent fiber content on milk production of dairy cattle and methane emissions determined using GreenFeed and respiration chamber techniques. J. Dairy Sci. 2016, 99, 7904–7917. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Wattiaux, M.A.; Powell, J.M. Emissions of ammonia, nitrous oxide, methane, and carbon dioxide during storage of dairy cow manure as affected by dietary forage-to-concentrate ratio and crust formation. J. Dairy Sci. 2012, 95, 7409–7416. [Google Scholar] [CrossRef]
- Snelling, T.J.; Wallace, R.J. The rumen microbial metaproteome as revealed by SDS-PAGE. BMC Microbiol. 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kingston-Smith, A.H.; Davies, T.E.; Rees Stevens, P.; Mur, L.A.J. Comparative metabolite fingerprinting of the rumen system during colonisation of three forage grass (Lolium perenne L.) varieties. PLoS ONE 2013, 8, e82801. [Google Scholar] [CrossRef] [Green Version]
- Fernández, R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Critical analysis of methods for the measurement of volatile fatty acids. Crit. Rev. Environ. Sci. Technol. 2016, 46, 209–234. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Fontecha, J.; Rodríguez-Alcalá, L.M. A high-performance direct transmethylation method for total fatty acids assessment in biological and foodstuff samples. Talanta 2014, 128, 518–523. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals From Lipids. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, J.; Cho, S.H.; Kim, J.; Oh, J.I.; Tsang, D.C.W.; Jeong, K.H.; Kwon, E.E. Quantification of volatile fatty acids from cattle manure via non-catalytic esterification for odour indication. Sci. Total Environ 2018, 610–611, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage sludge to bio-fuel: A review on the sustainable approach of transforming sewage waste to alternative fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Luo, C.; Cai, S.; Jia, L.; Tang, X.; Zhang, R.; Jia, G.; Li, H.; Tang, J.; Liu, G.; Wu, C. Study on Accurate Determination of Volatile Fatty Acids in Rumen Fluid by Capillary Gas Chromatography. In Proceedings of the 5th International Conference on Information Engineering for Mechanics and Materials, Huhhot, China, 25–26 July 2015; Volume 21, pp. 386–391. [Google Scholar] [CrossRef] [Green Version]
- Filípek, J.; Dvořák, R. Determination of the volatile fatty acid content in the rumen liquid: Comparison of gas chromatography and capillary isotachophoresis. Acta Vet. Brno 2009, 78, 627–633. [Google Scholar] [CrossRef]
- Hofírek, B.; Haas, D. Comparative studies of ruminal fluid collected by oral tube or by puncture of the caudoventral ruminal. J. Acta Vet. Brno 2001, 70, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Sutton, J.D.; Dhanoa, M.S.; Morant, S.V.; France, J.; Napper, D.J.; Schuller, E. Rates of production of acetate, propionate, and butyrate in the rumen of lactating dairy cows given normal and low-roughage diets. J. Dairy Sci. 2003, 86, 3620–3633. [Google Scholar] [CrossRef] [Green Version]
- Loh, T.C.; Thanh, N.T.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Anim. Sci. J. 2010, 81, 205–214. [Google Scholar] [CrossRef]
- Nur, A.I.; Alimon, A.R.; Yaakub, H.; Abdullah, N.; Jahromi, M.F.; Ivan, M.; Samsudin, A.A. Profiling of rumen fermentation, microbial population and digestibility in goats fed with dietary oils containing different fatty acids. J. BMC Vet. Res. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Cottyn, B.G.; Boucque, C.V. Rapid Methods for the Gas-Chromatographic Determination of Volatile Fatty Acids in Rumen Fluid. J. Agric. Food Chem. 1968, 16, 105–107. [Google Scholar] [CrossRef]
- Ryan, J.P. Determination of volatile fatty acids and some related compounds in ovine rumen fluid, urine, and blood plasma, by gas-liquid chromatography. Anal. Biochem. 1980, 108, 374–384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-R.; Cho, Y.; Ju, H.K.; Kim, E. Theoretical Methane Emission Estimation from Volatile Fatty Acids in Bovine Rumen Fluid. Appl. Sci. 2021, 11, 7730. https://doi.org/10.3390/app11167730
Lee S-R, Cho Y, Ju HK, Kim E. Theoretical Methane Emission Estimation from Volatile Fatty Acids in Bovine Rumen Fluid. Applied Sciences. 2021; 11(16):7730. https://doi.org/10.3390/app11167730
Chicago/Turabian StyleLee, Sang-Ryong, Yunseo Cho, Hyuck K. Ju, and Eunjeong Kim. 2021. "Theoretical Methane Emission Estimation from Volatile Fatty Acids in Bovine Rumen Fluid" Applied Sciences 11, no. 16: 7730. https://doi.org/10.3390/app11167730





