Application of a Novel Attachable Magnetic Nerve Stimulating Probe in Intraoperative Lumbar Pedicle Screw Placement: A Porcine Model Study
Abstract
:1. Introduction
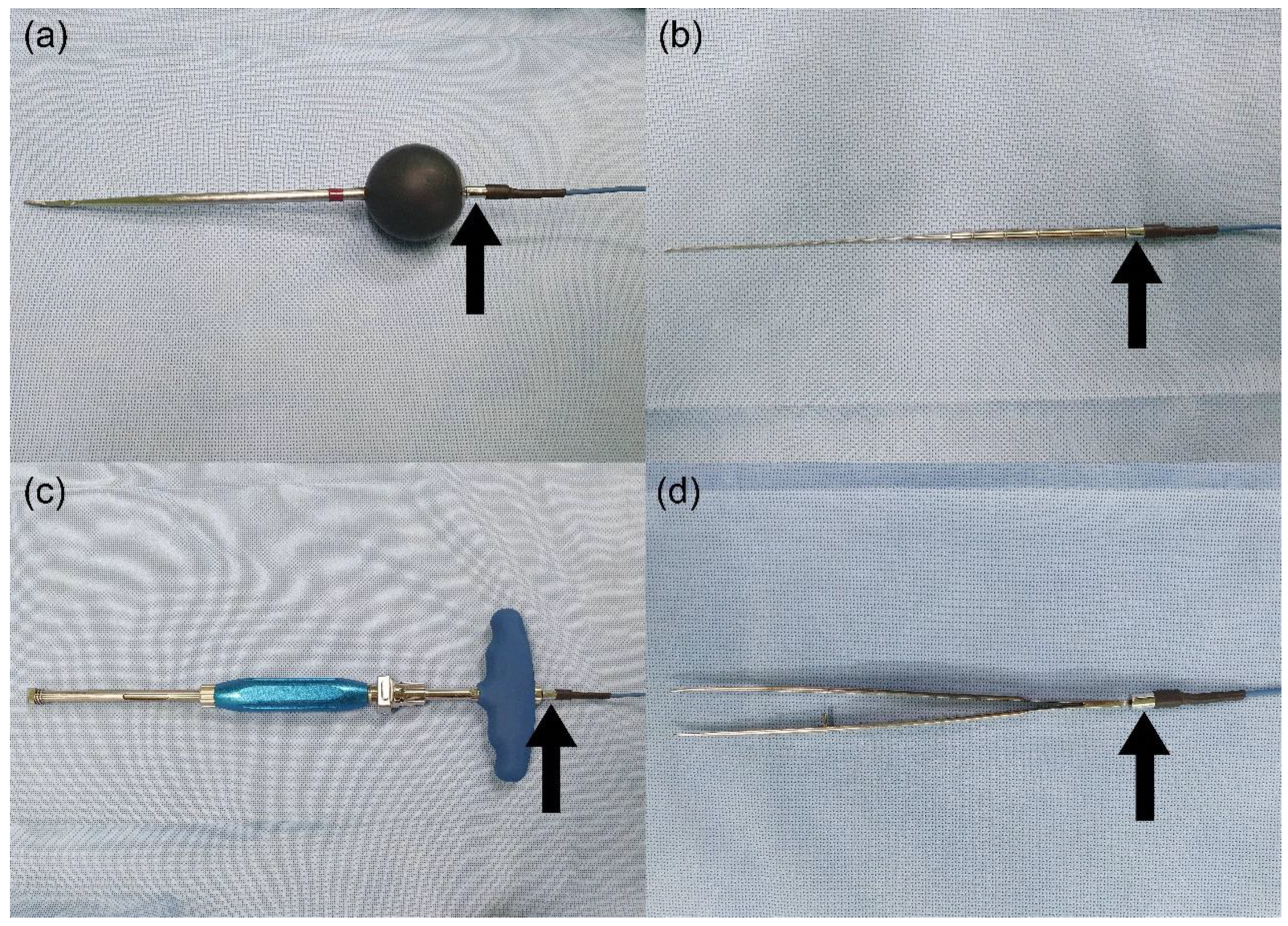
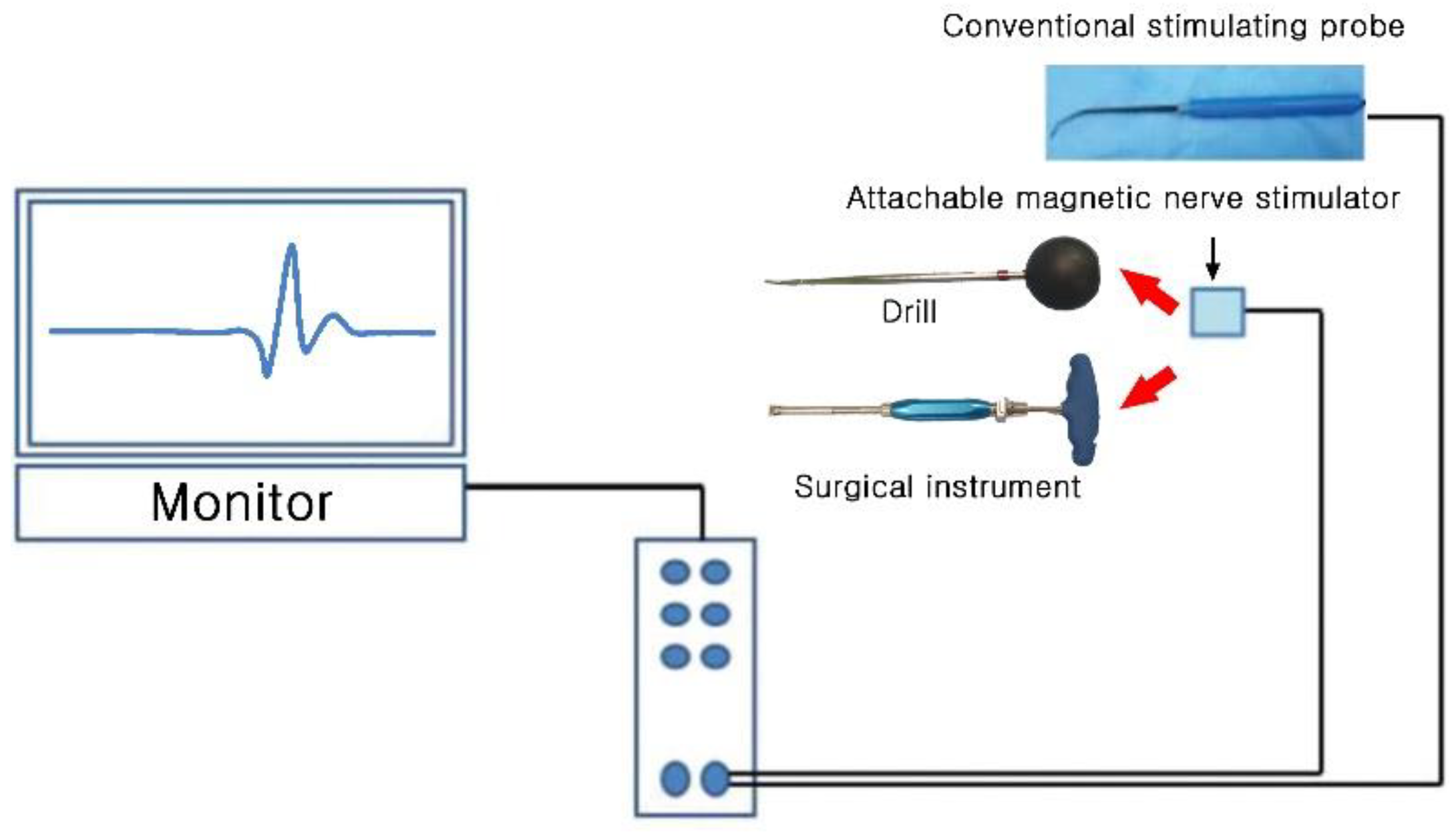
2. Materials and Methods
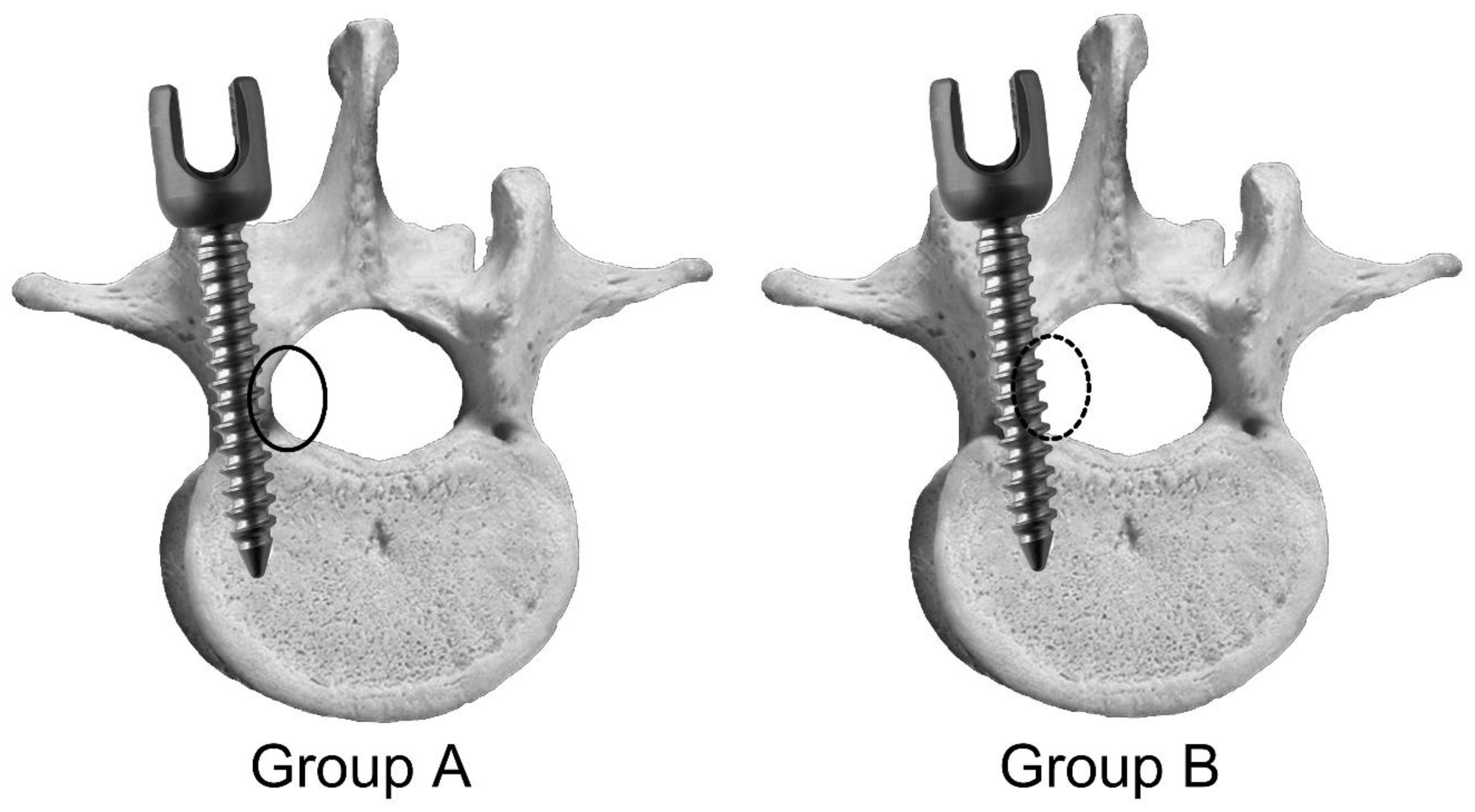
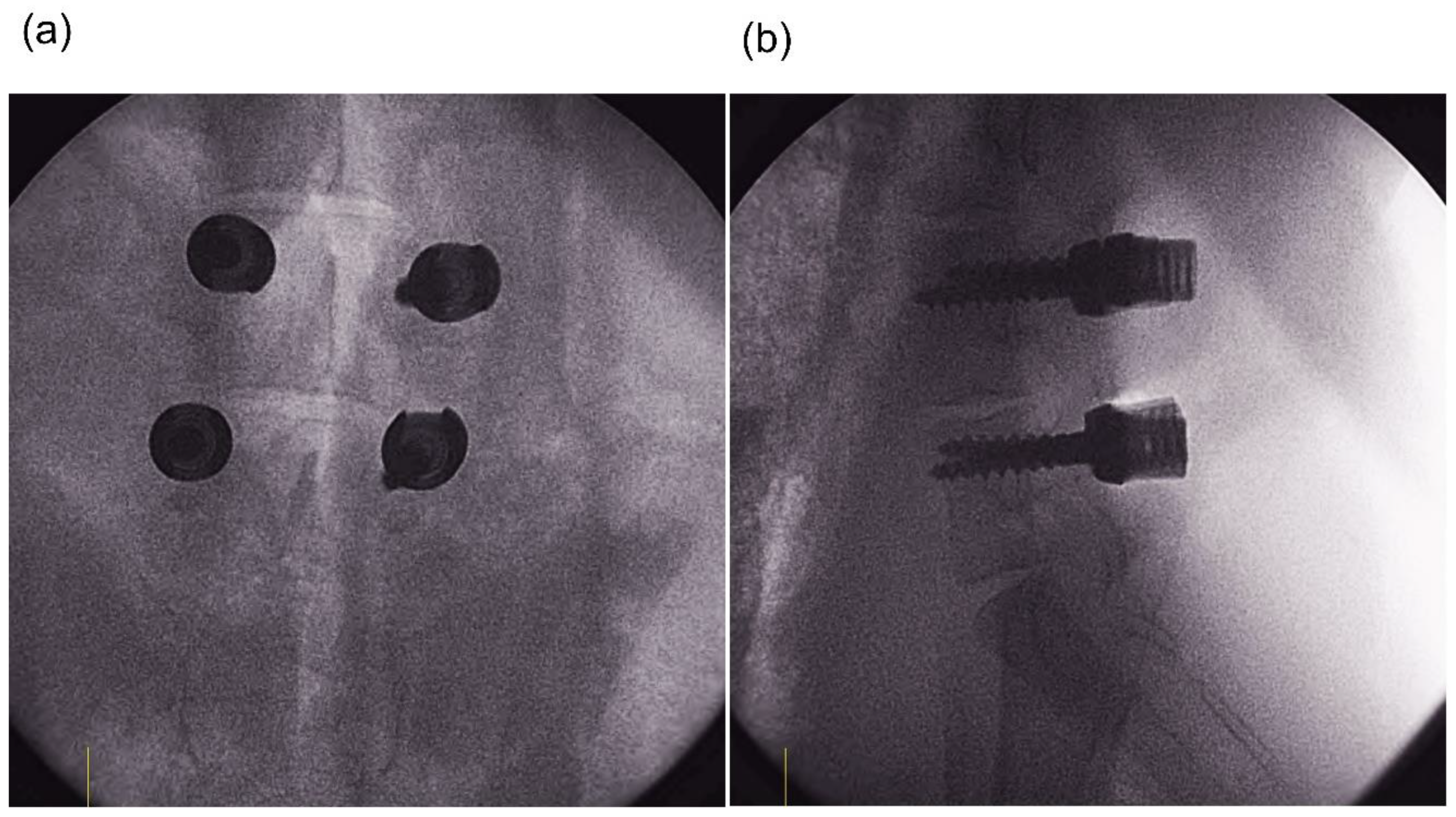
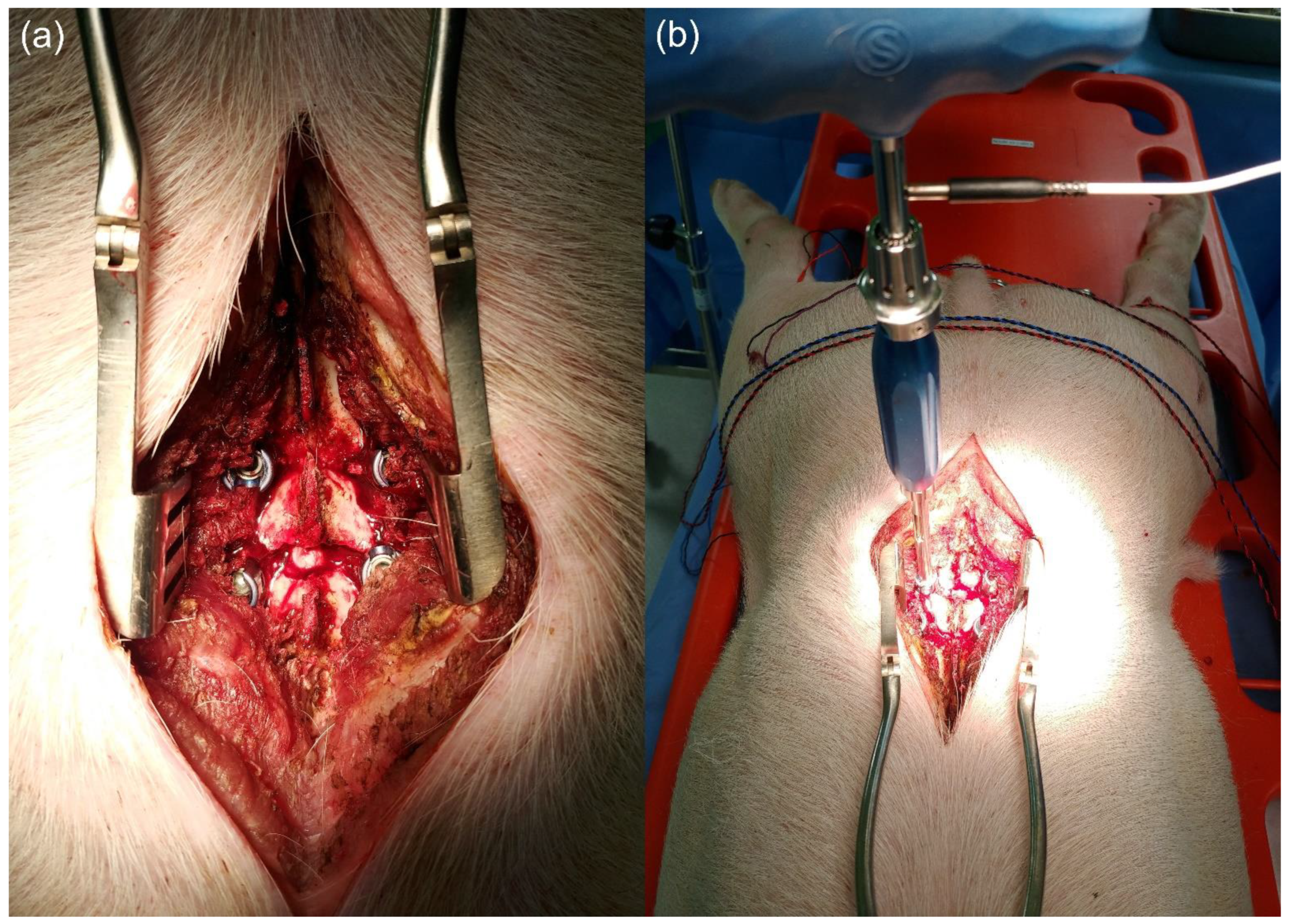
2.1. Animal Experiment
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boachie-Adjei, O.; Girardi, F.P.; Bansal, M.; Rawlins, B.A. Safety and efficacy of pedicle screw placement for adult spinal deformity with a pedicle-probing conventional anatomic technique. J. Spinal Disord. 2000, 13, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Liljenqvist, U.R.; Halm, H.F.; Link, T.M. Pedicle screw instrumentation of the thoracic spine in idiopathic scoliosis. Spine (Phila Pa 1976) 1997, 22, 2239–2245. [Google Scholar] [CrossRef]
- Kosmopoulos, V.; Theumann, N.; Binaghi, S.; Schizas, C. Observer reliability in evaluating pedicle screw placement using computed tomography. Int. Orthop. 2007, 31, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevzati, E.; Marbacher, S.; Soleman, J.; Perrig, W.N.; Diepers, M.; Khamis, A.; Fandino, J. Accuracy of pedicle screw placement in the thoracic and lumbosacral spine using a conventional intraoperative fluoroscopy-guided technique: A national neurosurgical education and training center analysis of 1236 consecutive screws. World Neurosurg. 2014, 82, 866–871.e2. [Google Scholar] [CrossRef]
- Shi, Y.B.; Binette, M.; Martin, W.H.; Pearson, J.M.; Hart, R.A. Electrical stimulation for intraoperative evaluation of thoracic pedicle screw placement. Spine (Philipa Pa 1976) 2003, 28, 595–601. [Google Scholar] [CrossRef]
- Esses, S.I.; Sachs, B.L.; Dreyzin, V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine (Philipa Pa 1976) 1993, 18, 2231–2238. [Google Scholar] [CrossRef]
- Dunn, C.; Faloon, M.; Milman, E.; Pourtaheri, S.; Sinah, K.; Hwang, K.; Emami, A. Accuracy and safety of percutaneous lumbosacral pedicle screw placement using dual-planar intraoperative fluoroscopy. Asian Spine J. 2018, 12, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.R.; Ledonio, C.G.; Castro, C.A.; Trong, W.H.; Sembrano, J.N. The accuracy of intraoperative O-arm images for the assessment of pedicle screw postion. Spine (Philipa Pa 1976) 2012, 37, E119–E125. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Lebwohl, N.; Madsen, P.; Klose, K.J. Intraoperative evoked EMG monitoring in an animal model. A new technique for evaluating pedicle screw placement. Spine (Philipa Pa 1976) 1992, 17, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Madsen, P.; Lebwohl, N. Stimulus-evoked EMG monitoring during transpedicular lumbosacral spine instrumentation. Initial clinical results. Spine (Philipa Pa 1976) 1994, 19, 2780–2786. [Google Scholar] [CrossRef] [PubMed]
- Lenke, L.G.; Padberg, A.M.; Russo, M.H.; Bridewell, K.H.; Gelb, D.E. Triggered electromyographic threshold for accuracy of pedicle screw placement. An animal model and clinical correlation. Spine (Philipa Pa 1976) 1995, 20, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Lenke, L.G.; Raynor, B.; Long, J.; Bridwell, K.H.; Padberg, A. Triggered electromyographic threshold for accuracy of thoracic pedicle screw placement in a porcine model. Spine (Philipa Pa 1976) 2001, 26, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Raynor, B.L.; Lenke, L.G.; Bridwell, K.H.; Taylor, B.A.; Padberg, A.M. Correlation between low triggered electromyographic thresholds and lumbar pedicle screw malposition: Analysis of 4857 screws. Spine (Philipa Pa 1976) 2007, 32, 2673–2678. [Google Scholar] [CrossRef] [Green Version]
- Raynor, B.L.; Lenke, L.G.; Kim, Y.; Hanson, D.S.; Wilson-Holden, T.J.; Bridwell, K.H.; Padberg, A.M. Can triggered electromyograph thresholds predict safe thoracic pedicle screw placement? Spine (Philipa Pa 1976) 2002, 27, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Choi, S.; Lee, S.; Na, H.; Seung, E.; Shin, S.; Lee, B. Application of attachable magnetic nerve stimulator in intraoperative facial nerve monitoring during ear surgery. Otolaryngol. Head Neck Surg. 2020, 162, 773–775. [Google Scholar] [CrossRef]
- Sung, E.S.; Lee, J.; Shin, S.; Chois, S.; Jung, D.; Lee, B. Development of a novel detachable magnetic nerve stimulator for intraoperative neuromonitoring. World J. Surg. 2018, 42, 137–142. [Google Scholar] [CrossRef]
- Maguire, J.; Wallace, S.; Madiga, R.; Leppanen, R.; Draper, V. Evaluation of intrapedicular screw position using intraoperative evoked electromyography. Spine (Philipa Pa 1976) 1995, 20, 1068–1074. [Google Scholar] [CrossRef]
- De Blas, G.; Barrios, C.; Regidor, I.; Montes, E.; Burgos, J.; Piza-Vallespir, G.; Hevia, E. Safe pedicle screw placement in thoracic scoliotic curves using t-EMG: Stimulation threshold variability at concavity and convexity in apex segments. Spine (Philipa Pa 1976) 2012, 37, E387–E395. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Kim, H.R.; Lee, C.; Kim, J.; Sala, F. Can triggered electromyography thresholds assure accurate pedicle screw placements? A systematic review and meta-analysis of diagnostic test accuracy. Clin. Neurophysiol. 2015, 126, 2019–2025. [Google Scholar] [CrossRef]
- Shaw, K.A.; Murphy, J.S.; Devito, D.P. Accuracy of robot-assisted pedicle screw insertion in adolescent idiopathic scoliosis: Is triggered electromyographic pedicle screw stimulation necessary? J. Spine Surg. 2018, 4, 187–194. [Google Scholar] [CrossRef]
- Sung, E.; Lee, J.; Kim, S.H.; Shin, S.; Jung, D.; Lee, B. Developmnet of an attachable endoscopic nerve stimulator for intraoperative neuromonitoring during endoscopic or robotic thyroidectomy. Otolaryngol. Head Neck Surg. 2018, 158, 465–468. [Google Scholar] [CrossRef] [PubMed]

| Group A (Normal) | Group B (Abnormal) | |||||
|---|---|---|---|---|---|---|
| Triggered Threshold (mA) | Conventional Stimulator | Magnetic Stimulator | Conventional Stimulator | Magnetic Stimulator | ||
| Pig 1 | L4 | Rt. | 16 | 17 | 3.5 | 3.5 |
| Lt. | 12 | 12 | 1.0 | 0.8 | ||
| L5 | Rt. | 11 | 12 | 0.7 | 0.7 | |
| Lt. | 10 | 10 | 3.0 | 3.5 | ||
| Pig 2 | L4 | Rt. | 13 | 14 | 3.5 | 3.5 |
| Lt. | 10 | 10 | 2.5 | 2.0 | ||
| L5 | Rt. | 8 | 8 | 2.0 | 2.0 | |
| Lt. | 9 | 10 | 1.5 | 1.5 | ||
| Pig 3 | L4 | Rt. | 10 | 10 | 0.8 | 1.0 |
| Lt. | 8 | 7 | 3.0 | 3.0 | ||
| L5 | Rt. | 9 | 9 | 2.5 | 2.5 | |
| Lt. | 12 | 12 | 3.0 | 2.5 | ||
| Pig 4 | L4 | Rt. | 15 | 16 | 1.0 | 1.5 |
| Lt. | 13 | 13 | 2.5 | 2.5 | ||
| L5 | Rt. | 12 | 13 | 0.7 | 1.0 | |
| Lt. | 15 | 15 | 1.5 | 1.5 | ||
| Pig 5 | L4 | Rt. | 11 | 11 | 2.0 | 2.5 |
| Lt. | 9 | 9 | 3.0 | 3.0 | ||
| L5 | Rt. | 11 | 12 | 3.0 | 3.5 | |
| Lt. | 10 | 10 | 3.0 | 3.0 | ||
| Mean ± SD | 11.2 ± 2.25 | 11.5 ± 2.56 | 2.19 ± 0.95 | 2.25 ± 0.94 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, T.S.; Shin, S.-C.; Kwon, H.-K.; Sung, E.-S.; Jun, S.B.; Lee, B.-J.; Lee, J.S. Application of a Novel Attachable Magnetic Nerve Stimulating Probe in Intraoperative Lumbar Pedicle Screw Placement: A Porcine Model Study. Appl. Sci. 2021, 11, 7801. https://doi.org/10.3390/app11177801
Goh TS, Shin S-C, Kwon H-K, Sung E-S, Jun SB, Lee B-J, Lee JS. Application of a Novel Attachable Magnetic Nerve Stimulating Probe in Intraoperative Lumbar Pedicle Screw Placement: A Porcine Model Study. Applied Sciences. 2021; 11(17):7801. https://doi.org/10.3390/app11177801
Chicago/Turabian StyleGoh, Tae Sik, Sung-Chan Shin, Hyun-Keun Kwon, Eui-Suk Sung, Se Bin Jun, Byung-Joo Lee, and Jung Sub Lee. 2021. "Application of a Novel Attachable Magnetic Nerve Stimulating Probe in Intraoperative Lumbar Pedicle Screw Placement: A Porcine Model Study" Applied Sciences 11, no. 17: 7801. https://doi.org/10.3390/app11177801






