Numerical Study of Tympanosclerosis Including Its Effect on Human Hearing
Abstract
:1. Introduction
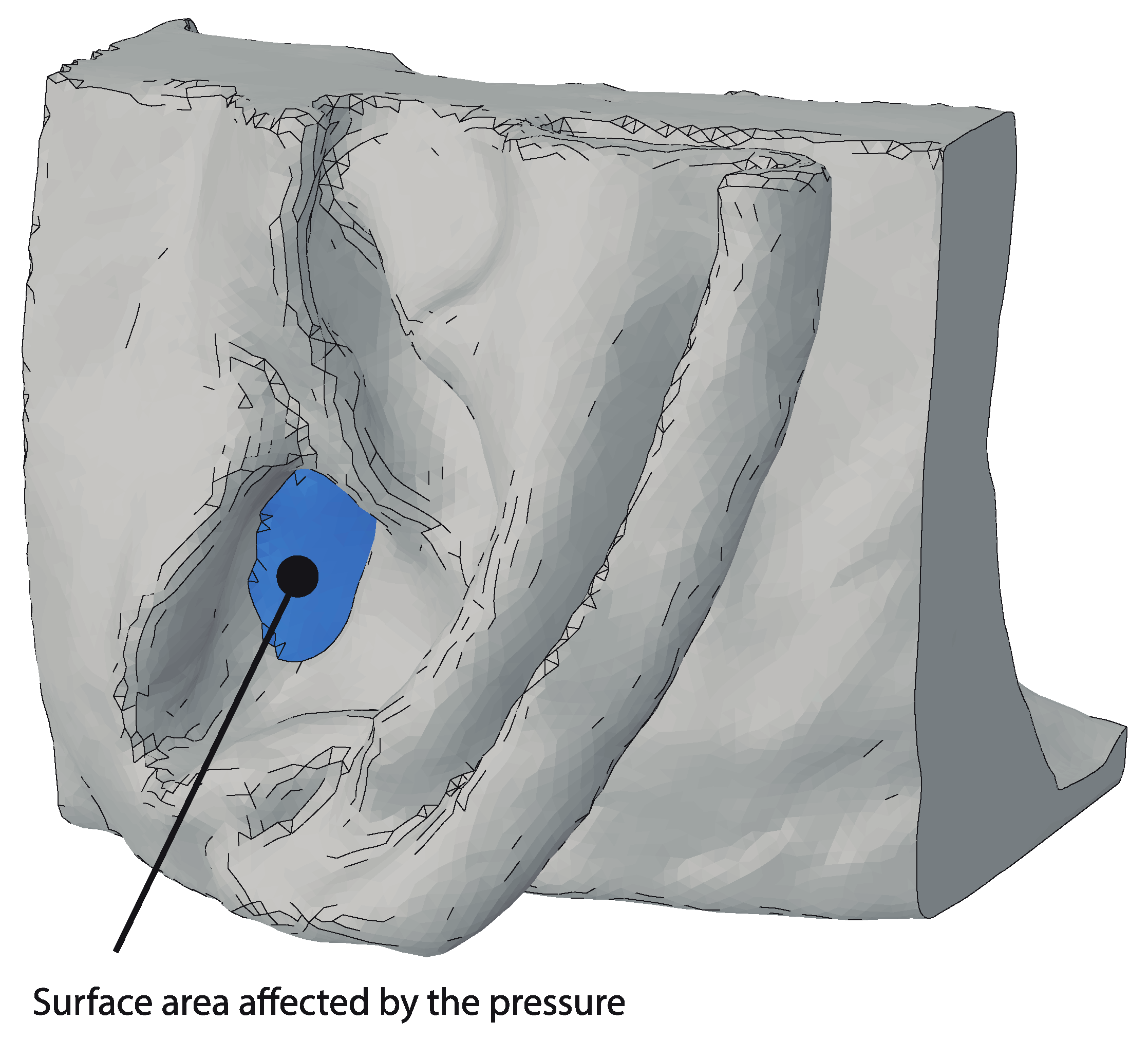
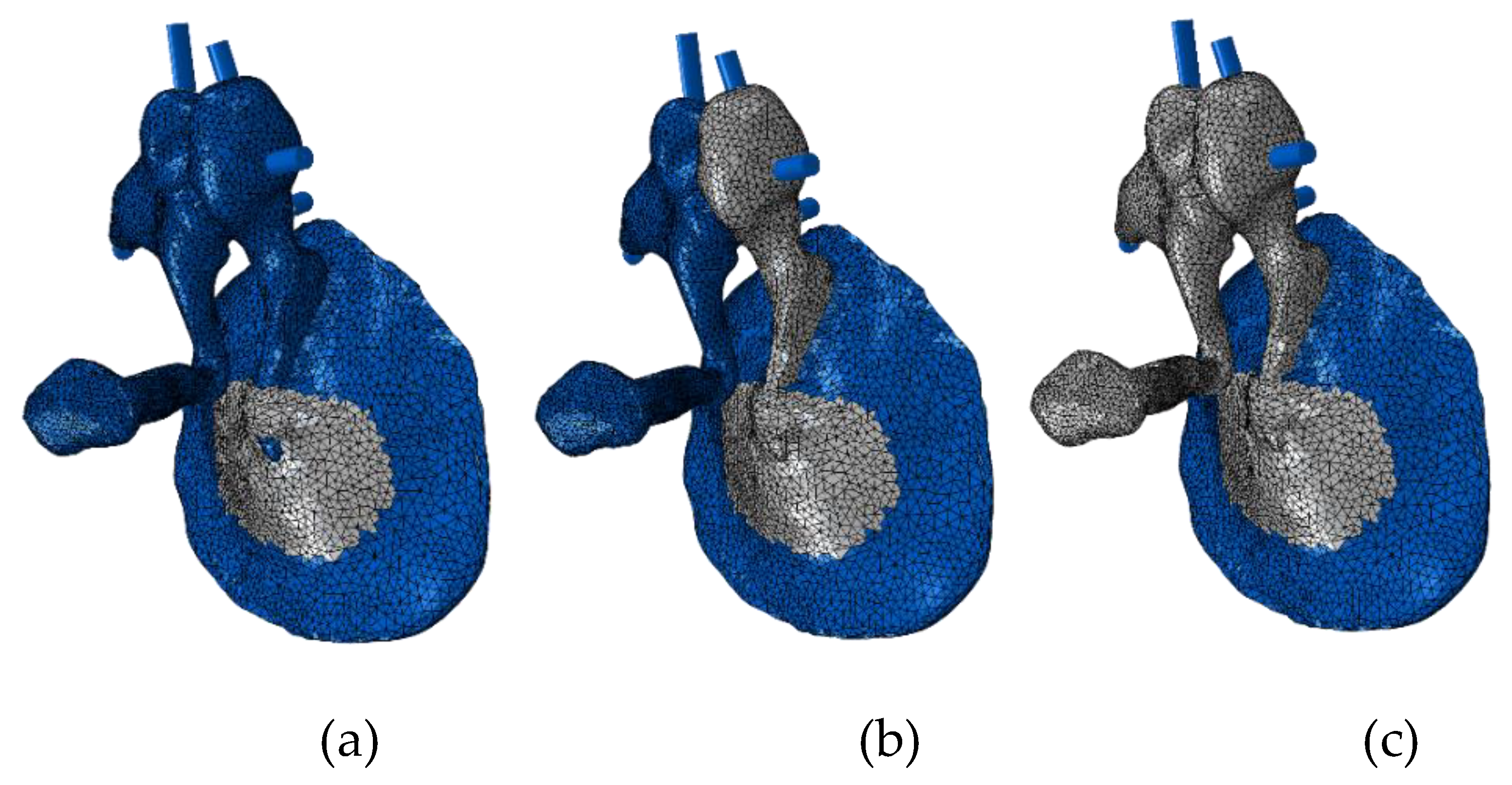
2. Methods
3. Results
3.1. Eardrum Behavior
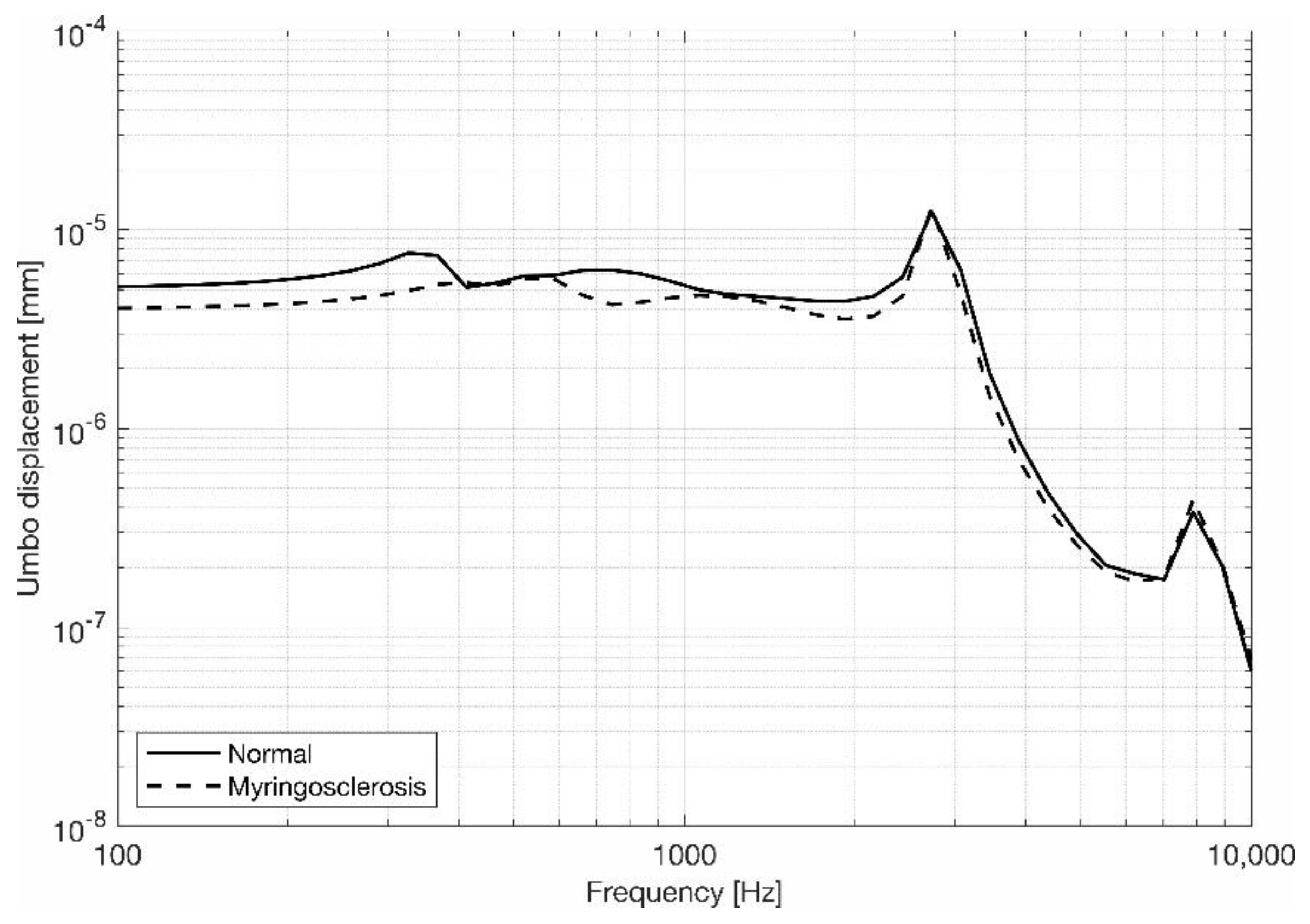
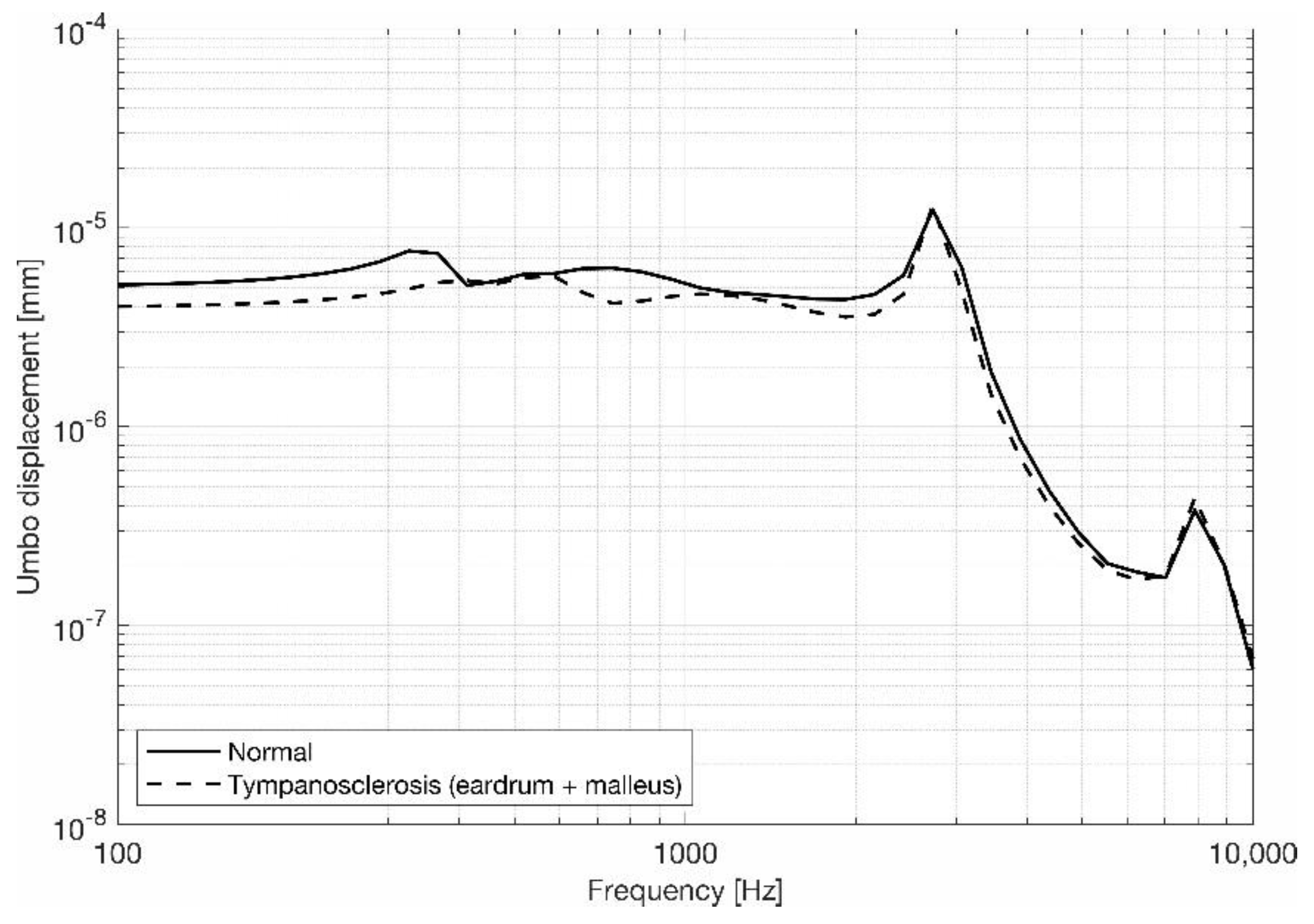
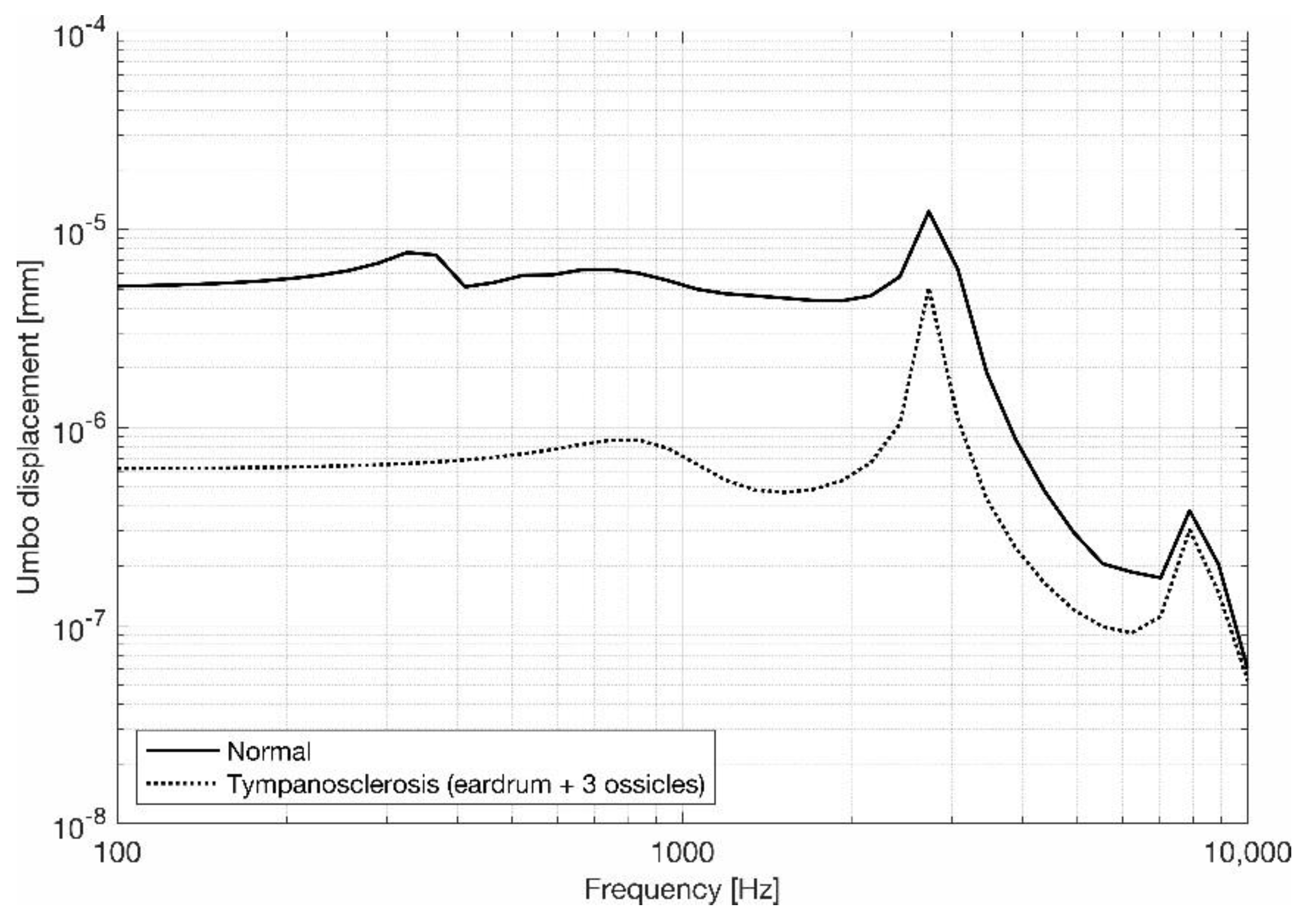
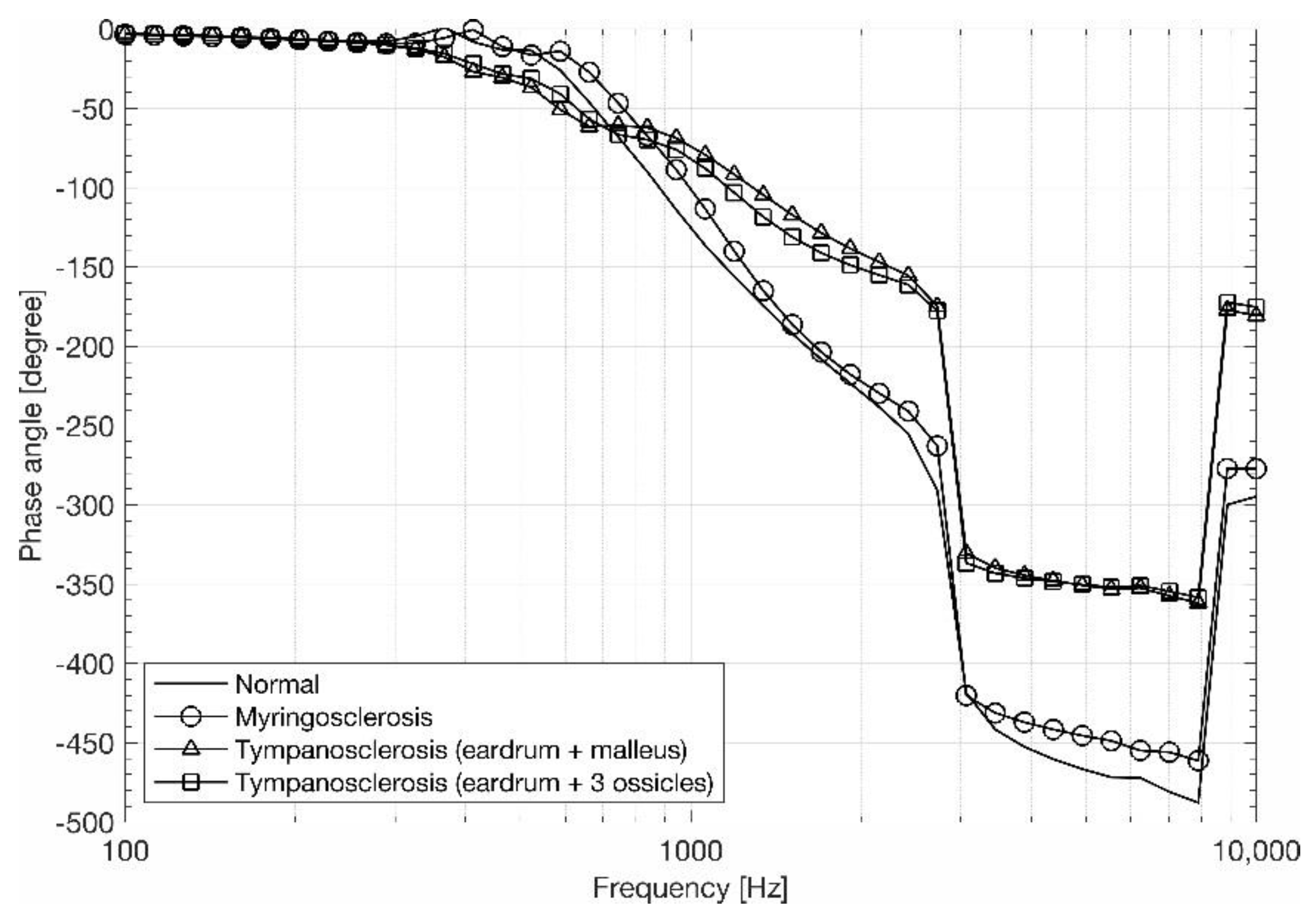
3.2. Stapes Behavior
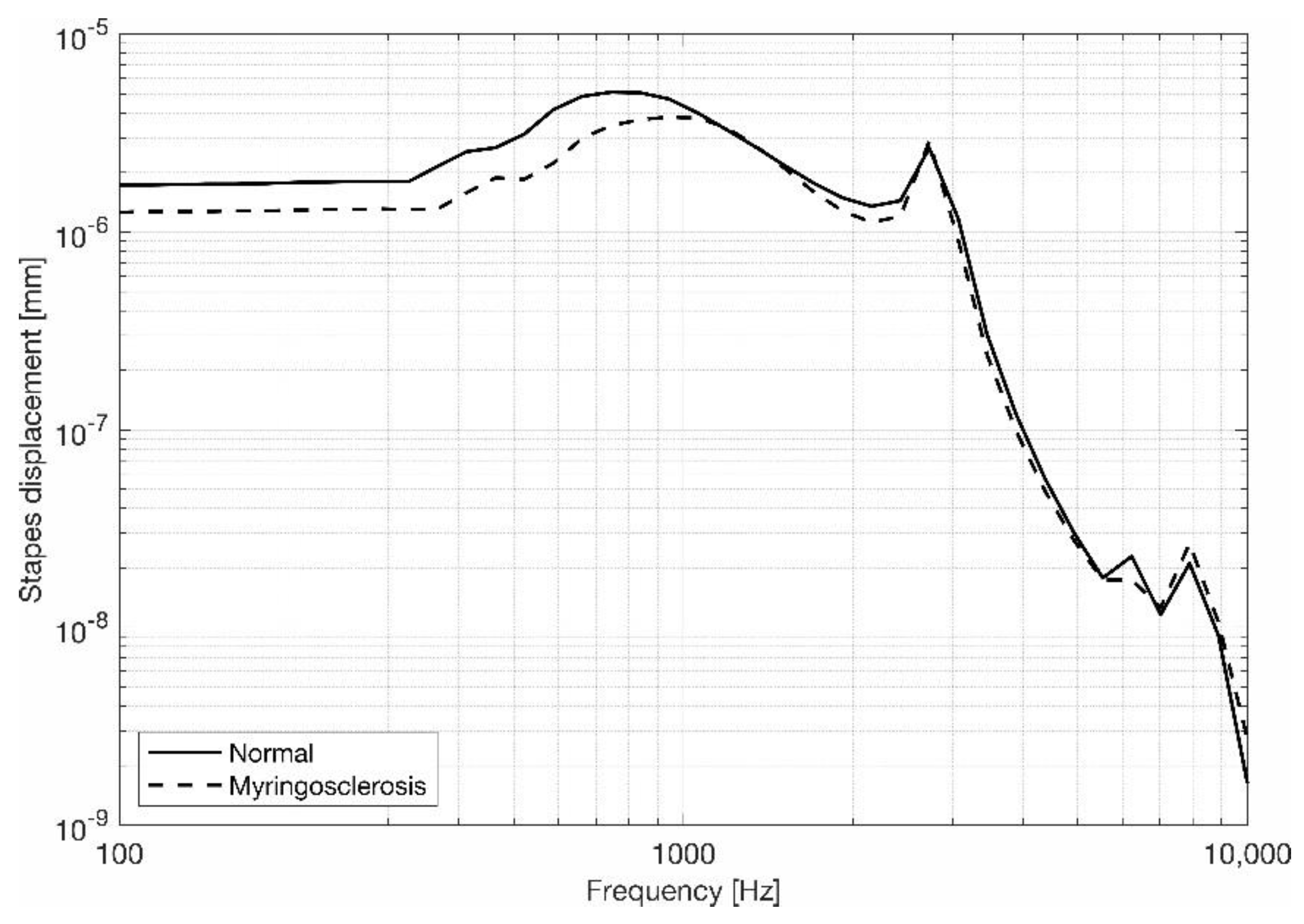
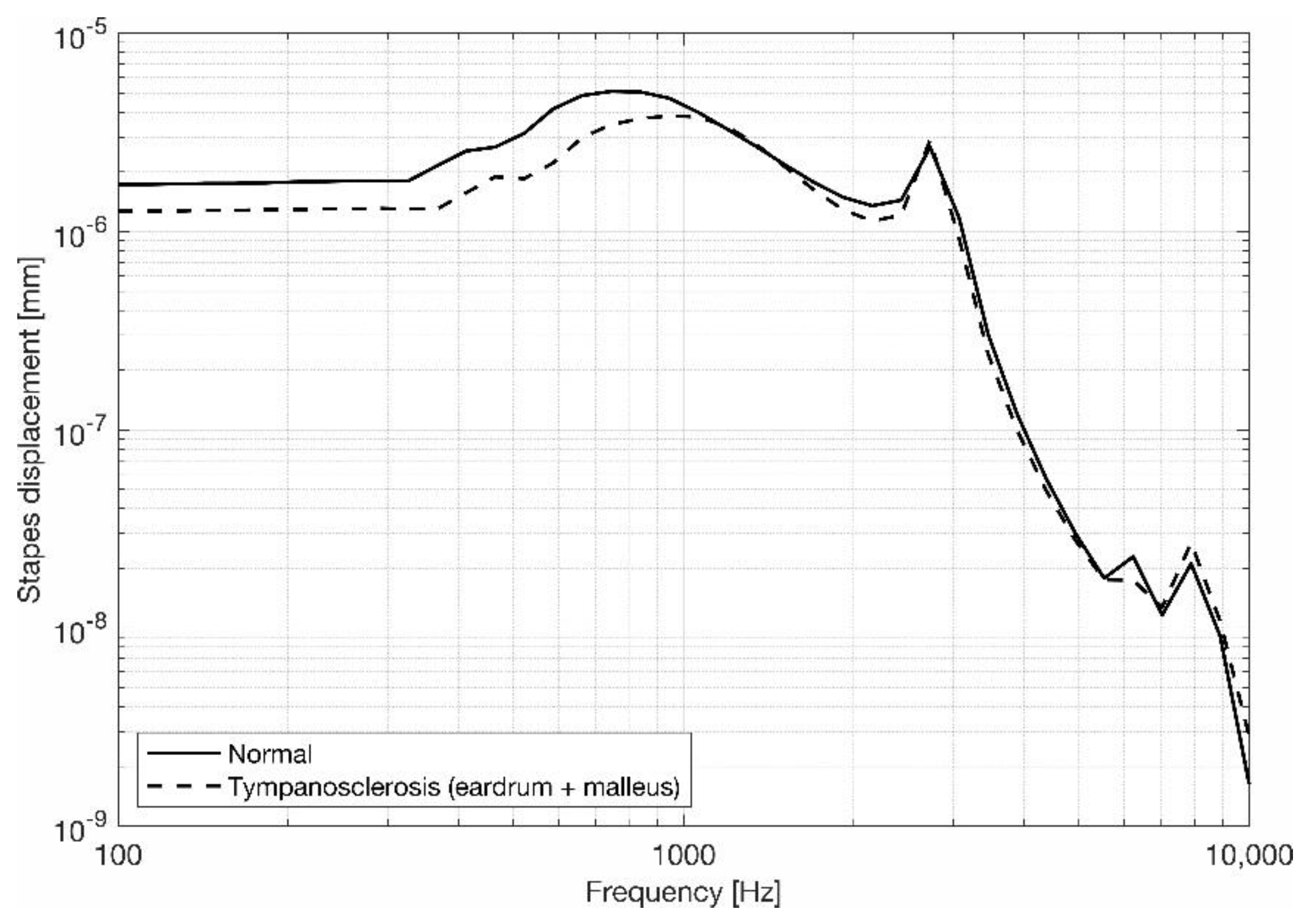
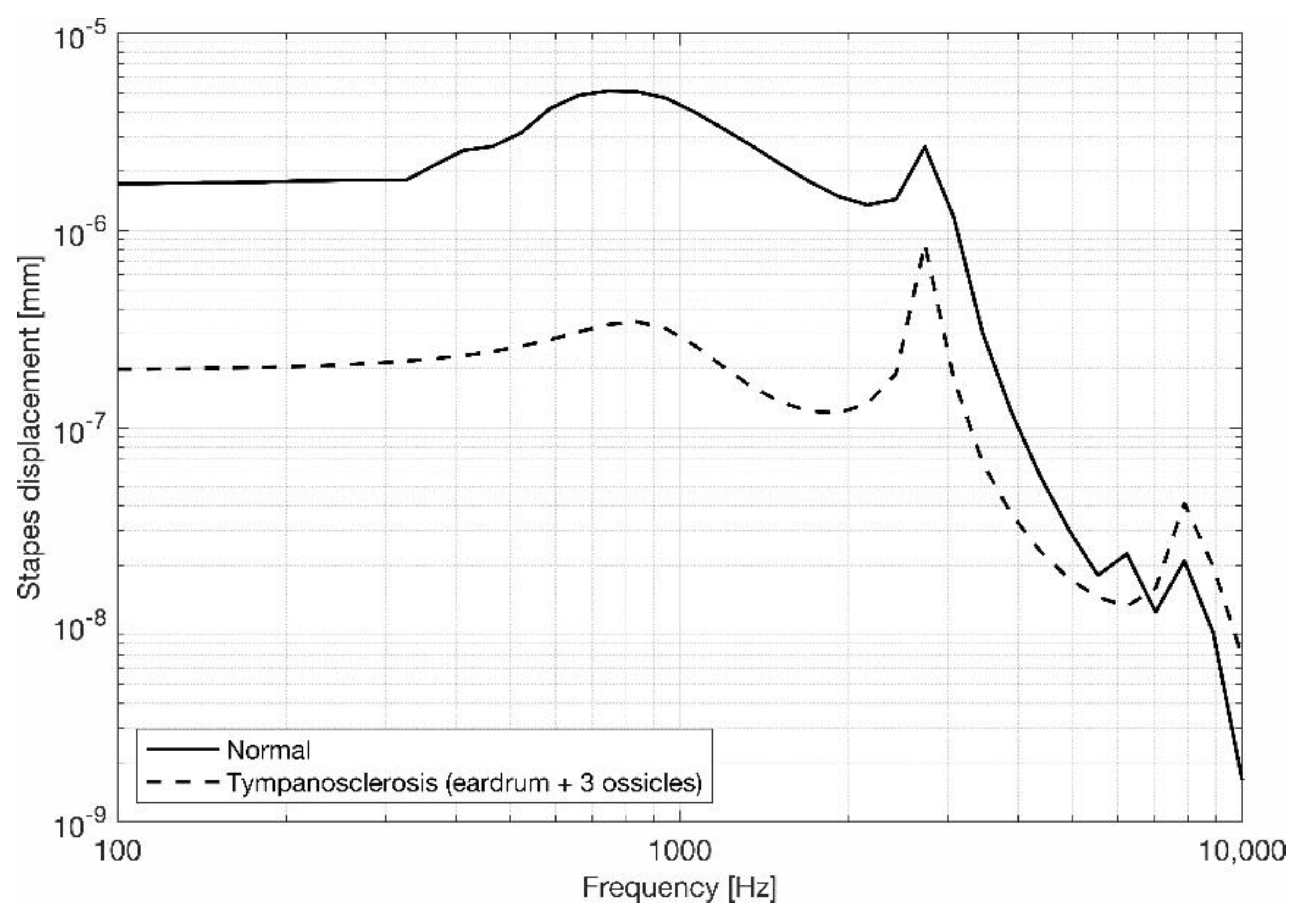
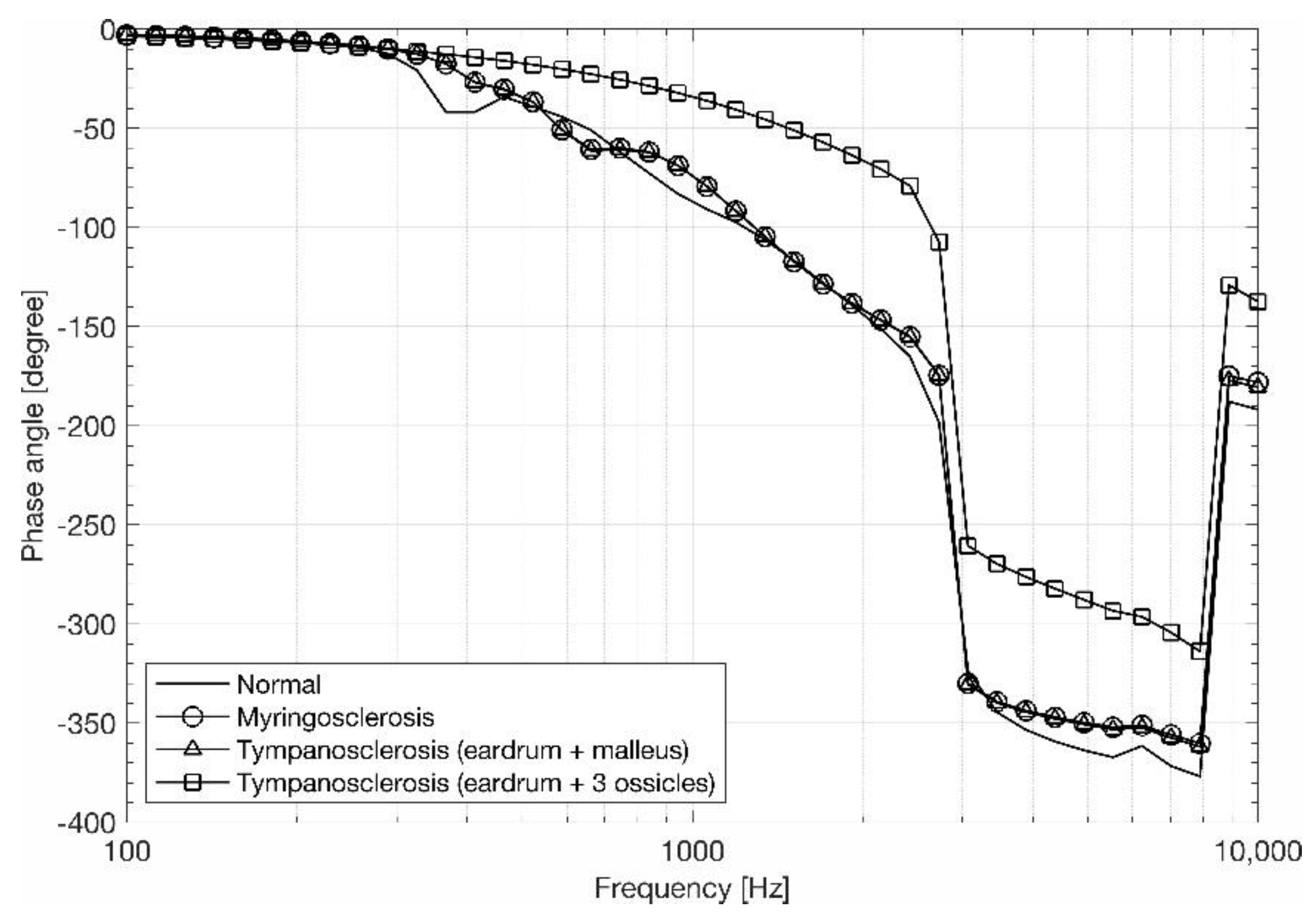
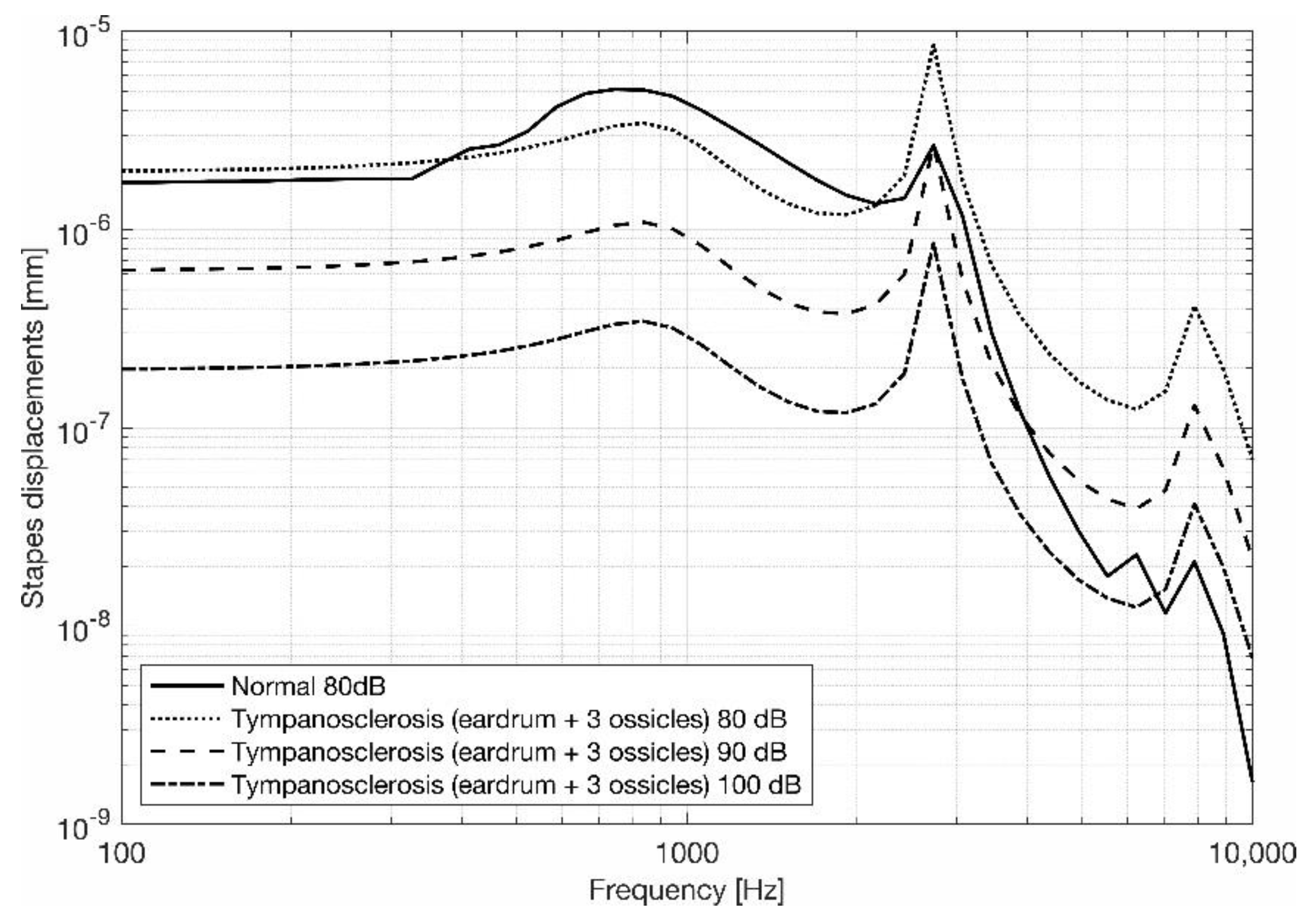
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinç, A.E.; Cömert, F.; Damar, M.; Sevik, E.S.; Erdem, D.; Isik, H. Role of Chlamydia pneumoniae and Helicobacteria pylori in the development of tympanosclerosis. Eur. Arch. Otorhinolaryngol. 2016, 273, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Flint, P.; Haughey, B.; Lund, V.; Niparko, J.; Robbins, K.; Thomas, J.R.; Lesperance, M. Cummings Otolaryngology–Head and Neck Surgery, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ferri, M.; Faggioli, G.L.; Ferri, G.G.; Pirodda, A. Is carotid stenosis correlated with tympanosclerosis. Int. Angiol. J. Int. Union Angiol. 2004, 23, 144–146. [Google Scholar]
- Wallace, I.F.; Berkman, N.D.; Lohr, K.N.; Harrison, M.F.; Kimple, A.J.; Steiner, M.J. Surgical treatments for otitis media with effusion: A systematic review. Pediatrics 2014, 133, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.L.; Tsao, Y.H.; Cheng, H.M.; Lien, C.F.; Hsu, C.H.; Huang, C.Y.; Shiao, A.S. Grommets for otitis media with effusion in children with cleft palate: A systematic review. Pediatrics 2014, 134, 983–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onusko, E.M. Tympanometry. Am. Fam. Physician 2004, 70, 1713–1720. [Google Scholar] [PubMed]
- Wu, Y.; Yin, S.; Zhu, H.; Zhang, S. Tympanosclerosis incidence among patients with chronic suppurative otitis media. J. Clin. Otorhinolaryngol. 2006, 20, 1016–1017. [Google Scholar]
- Doner, F.; Yariktas, M.; Dogru, H.; Uzun, H.; Aydin, S.; Delibas, N. The biochemical analysis of tympanosclerotic plaques. Otolaryngol. Head Neck Surg. 2003, 128, 742–745. [Google Scholar] [CrossRef]
- Gibb, A.G.; Pang, Y.T. Current considerations in the etiology and diagnosis of tympanosclerosis. Eur. Arch. Oto Rhino L. 1994, 251, 439–451. [Google Scholar] [CrossRef]
- Selcuk, A.; Ensarı, S.; Sargın, A.K.; Can, B.; Dere, H. Histopathological classification of tympanosclerotic plaques. Eur. Arch. Oto Rhino L. 2008, 265, 409–413. [Google Scholar] [CrossRef]
- Sennaroglu, L.; Gungor, V.; Atay, G.; Ozer, S. Manubrio-stapedioplasty: New surgical technique for malleus and incus fixation due to tympanosclerosis. J. Laryngol. Otol. 2015, 129, 587–590. [Google Scholar] [CrossRef]
- Sakalli, E.; Celikyurt, C.; Guler, B.; Bişkin, S.; Tansuker, H.D.; Erdurak, S.C. Surgery of isolated malleus fixation due to tympanosclerosis. Eur. Arch. Oto Rhino Laryngol. 2014, 272, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Berdich, K.; Gentil, F.; Parente, M.; Garbe, C.; Santos, C.; Paço, J.; Jorge, R.N.; Martins, P.; Faur, N. Finite element analysis of the transfer of sound in the myringosclerotic ear. Comput. Methods Biomech. Biomed. Eng. 2015, 19, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Marin, K.C.; Berdich-Kun, K.N.; Gentil, F.; Parente, M.; Natal, R.J.; Marin, H.A.; Poenaru, M.; Popa, D.R. Application of a finite element model in the diagnosis process of middle ear pathologies. Rom. J. Morphol. Embryol. 2014, 55, 1511–1514. [Google Scholar] [PubMed]
- Gentil, F.; Garbe, C.; Parente, M.; Martins, P.; Ferreira, A.; Natal, R.; Santos, C.; Paço, J. Analysis of eardrum pathologies using the finite element method. J. Mech. Med. Biol. 2014, 14, 1450034. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Mikkelsen, J.; Nissum, K.; Vesterbæk, J.B. The Visible Ear, Project Group CVG8-820 in Faculty of Engineering and Science Aalborg University; Institute of Electronic Systems: Aalborg, Denmark, 2005. [Google Scholar]
- Areias, B.; Parente, M.P.L.; Santos, C.; Gentil, F.; Jorge, R.M.N. The human otitis media with effusion: A numerical-based study. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Hibbit, D.K.B.; Sorenson, P. ABAQUS Analysis User’s Manual Version 6.14-1; Dassault Systèmes: Vélizy-Villacoublay, France, 2014. [Google Scholar]
- Gentil, F.; Parente, M.; Martins, P.; Garbe, C.; Paço, J.; Ferreira, A.; Tavares, J.M.R.S.; Jorge, R.N. The influence of muscles activation on the dynamical behavior of the tympano-ossicular system of the middle ear. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Gan, R.Z.; Chang, K.H.; Dormer, K.J. Computer-integrated finite element modeling of human middle ear. Biomech. Model. Mechanobiol. 2002, 1, 109–122. [Google Scholar] [CrossRef]
- Prendergast, P.J.; Ferris, P.; Rice, H.J.; Blayney, A.W. Vibro-acoustic modelling of the outer and middle ear using the finite-element method. Audiol. Neurootol. 1999, 4, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gentil, F.; Parente, M.; Martins, P.; Garbe, C.; Jorge, R.N.; Ferreira, A.; Tavares, J.M.R.S. The influence of mechanical behaviour of the middle ear ligaments: A finite element analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 68–76. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chawla, A.; Karthikeyan, B. A review of the mechanical properties of human body soft tissues in the head, neck and spine. J. Inst. Eng. 2006, 87, 10–24. [Google Scholar]
- Orlovskii, V.P.; Komlev, V.S.; Barinov, S.M. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg Mater. 2002, 38, 973–984. [Google Scholar] [CrossRef]
- Nishihara, S.; Goode, R. Measurement of Tympanic Membrane Vibration in 99 Human Ears. In Middle Ear Mechanics in Research and Otosurgery; Huttenbrink, K.B., Ed.; Department of Oto-Rhino-Laryngology, Dresden University of Technology: Dresden, Germany, 1996; pp. 91–93. [Google Scholar]
- Gan, R.Z.; Feng, B.; Sun, Q. Three-dimensional finite element modeling of human ear for sound transmission. Ann. Biomed. Eng. 2004, 32, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Ball, G.; Asai, M.; Goode, R.L. The Vibration Pattern of the Tympanic Membrane after Placement of a Total Ossicular Replacement Prosthesis. In Proceeding of the International Workshop on Middle Ear Mechanics in Research and Otosurgery, Dresden, Germany, 19–22 September 1996; Department of Oto-Rhino-Laryngology, Univ. Hospital Carl Gustav Carus, University of Technology: Dresden, Germany, 1997; pp. 219–222. [Google Scholar]
- Lee, C.F.; Chen, P.-R.; Lee, W.-J.; Chen, J.H.; Liu, T.-C. Computer aided three-dimensional reconstruction and modeling of middle ear biomechanics by high-resolution computed tomography and finite element analysis. Biomed. Eng. Appl. Basis Commun. 2006, 18, 214–221. [Google Scholar] [CrossRef]
- Areias, B.; Santos, C.; Jorge, R.M.N.; Gentil, F.; Parente, M.P. Finite element modelling of sound transmission from outer to inner ear. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Asiri, S.; Hasham, A.; Anazy, F.; Zakzouk, S.; Banjar, A. Tympanosclerosis: Review of literature and incidence among patients with middle-ear infection. J Laryngol Otol. 1999, 113, 1076–1080. [Google Scholar] [CrossRef]

| Ear Components | Density ρ (kg/m3) | Young’s Modulus E (Pa) | |
|---|---|---|---|
| Eardrum | Pars tensa | 1.2 × 103 | 3.2 × 107 (radial) |
| 2.0 × 107 (circumferential) | |||
| Pars flaccida | 1.0 × 107 | ||
| Malleus | 2.55 × 103 (Head) | 1.41 × 1010 | |
| 4.53 × 103 (Neck) | |||
| 3.70 × 103 (Handle) | |||
| Incus | 2.36 × 103 (Body) | ||
| 2.26 × 103 (Short process) | |||
| 5.08 × 103 (Long process) | |||
| Stapes | 2.2 × 103 | ||
| Incudomalleolar joint | 3.2 × 103 | ||
| Incudostapedial joint | 1.2 × 103 | 6.0 × 105 | |
| Ear Components | Density ρ (kg/m3) | Young’s Modulus E (Pa) | Bulk Modulus B (Pa) |
|---|---|---|---|
| Superior malleolar ligament | 2.5 × 103 | 4.9 × 104 | |
| Lateral malleolar ligament | 6.7 × 104 | ||
| Anterior malleolar ligament | 2.1 × 106 | ||
| Superior incudal ligament | 4.9 × 104 | ||
| Posterior incudal ligament | 6.5 × 105 | ||
| Tensor tympani tendon | 2.6 × 106 | ||
| Stapedius tendon | 5.2 × 105 | ||
| Stapedius annular ligament | 2.0 × 104 | ||
| Tympanic annulus | 1.2 × 103 | 6.0 × 104 | |
| Tympanic cavity air | 1.164 | 1.01 × 105 | |
| Tympanic cavity fluid | 1000 | - | 2.2 × 109 |
| Cochlear fluid |
| Young’s Modulus | Shear’s Modulus | Poisson’s Ratio | Density |
|---|---|---|---|
| (N/m2) | (N/m2) | - | Kg/m3 |
| 120.6 × 109 | 47.77 × 109 | 0.262 | 3160 |
| Nodes | Elements | Element Type | |
|---|---|---|---|
| Eardrum | C3D4 | ||
| Tympanic annulus | C3D4 | ||
| Malleus | C3D4 | ||
| Incudomalleolar joint | C3D4 | ||
| Incus | C3D4 | ||
| Incudostapedial joint | C3D4 | ||
| Stapes | C3D4 | ||
| Incudostapedial joint | C3D4 | ||
| Stapedius annular ligament | C3D4 | ||
| Cochlear fluid | AC3D4 | ||
| Bone | C3D4 | ||
| Jaw | C3D4 | ||
| Soft tissues | C3D4 | ||
| Ear cartilage | C3D4 | ||
| External auditory canal | AC3D4 | ||
| Tympanic cavity | AC3D4 | ||
| Superior malleolar ligament | T3D2 | ||
| Lateral malleolar ligament | T3D2 | ||
| Anterior malleolar ligament | T3D2 | ||
| Superior incudal ligament | T3D2 | ||
| Posterior incudal ligament | T3D2 | ||
| Tensor tympani tendon | T3D2 | ||
| Stapedius tendon | T3D2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentil, F.; Parente, M.; Santos, C.; Areias, B.; Jorge, R.N. Numerical Study of Tympanosclerosis Including Its Effect on Human Hearing. Appl. Sci. 2023, 13, 1665. https://doi.org/10.3390/app13031665
Gentil F, Parente M, Santos C, Areias B, Jorge RN. Numerical Study of Tympanosclerosis Including Its Effect on Human Hearing. Applied Sciences. 2023; 13(3):1665. https://doi.org/10.3390/app13031665
Chicago/Turabian StyleGentil, Fernanda, Marco Parente, Carla Santos, Bruno Areias, and Renato Natal Jorge. 2023. "Numerical Study of Tympanosclerosis Including Its Effect on Human Hearing" Applied Sciences 13, no. 3: 1665. https://doi.org/10.3390/app13031665
APA StyleGentil, F., Parente, M., Santos, C., Areias, B., & Jorge, R. N. (2023). Numerical Study of Tympanosclerosis Including Its Effect on Human Hearing. Applied Sciences, 13(3), 1665. https://doi.org/10.3390/app13031665






