Fluctuating Asymmetry and Stress in Macaca fuscata: Does Captivity Affect Morphology?
Abstract
:1. Introduction
- Directional Asymmetry (DA) occurs when one side of a structure is consistently different (e.g., larger, smaller or differently shaped) than the other side [12]. DA in a biological structure might indicate that the deviating side serves a new specific function, and it is being consistently selected by nature over time (lateralised behaviour) [13].
- Bimodal Asymmetry (also called anti-symmetry) [14] occurs when both sides deviate from symmetry in equal proportions, creating a bimodal distribution. Biologically, this means that both left and right deviations from symmetry are favoured in equal proportions, with symmetric individuals being less frequent.
- Finally, Fluctuating Asymmetry (FA) indicates that both sides deviate from symmetry with no side preferred and with deviations that are random and non-directional [15].
2. Material and Methods
2.1. The Sample
2.2. The Analytical Approach
3. Results
4. Discussion
- (1)
- The stress associated with the captive conditions of the individuals analysed was not strong enough to trigger a change in FA. In this case, captivity may not impose sufficient environmental stress to trigger a response in the asymmetry pattern of the mandible.
- (2)
- FA is not a good indicator to detect environmental stress, at least in the conditions and for the species analysed.
- (3)
- The mandible is not an ideal region to identify developmental stress using FA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Costa, M.; Mateus, R.P.; Moura, M.O. Constant fluctuating asymmetry but not directional asymmetry along the geographic distribution of Drosophila antonietae (Diptera, Drosophilidae). Rev. Bras. Entomol. 2015, 59, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Hallgrímsson, B. Fluctuating Asymmetry in the Mammalian Skeleton. In Evolutionary Biology; Hecht, M.K., Macintyre, R.J., Clegg, M.T., Eds.; Springer: Boston, MA, USA, 1988. [Google Scholar]
- Hallgrímsson, B. Fluctuating asymmetry in Macaca fascicularis: A study of the etiology of developmental noise. Int. J. Primatol. 1993, 14, 421–443. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Developmental instability as a research tool: Using patterns of fluctuating asymmetry to infer the developmental origins of morphological integration. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, B.; Forbes, M.R.; Houle, D. Fluctuating asymmetry as a bioindicator of stress: Comparing efficacy of analyses involving multiple traits. Am. Nat. 2000, 155, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Oxilia, G.; Menghi Sartorio, J.C.; Bortolini, E.; Zampirolo, G.; Papini, A.; Boggioni, M.; Martini, S.; Marciani, F.; Arrighi, S.; Figus, C.; et al. Exploring directional and fluctuating asymmetry in the human palate during growth. Am. J. Phys. Anthropol. 2021, 175, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Crespi, B.J.; Bookstein, F.L. Fluctuating asymmetry in the honey bee, Apis mellifera: Effects of ploidy and hybridization. J. Evol. Biol. 1997, 10, 551–574. [Google Scholar] [CrossRef]
- Vervust, B.; Van Dongen, S.; Grbac, I.; Van Damme, R. Fluctuating asymmetry, physiological performance, and stress in island populations of the Italian Wall Lizard (Podarcis sicula). J. Herpetol. 2008, 42, 369–377. [Google Scholar] [CrossRef]
- Zakharov, V.M.; Shadrina, E.G.; Trofimov, I.E. Fluctuating Asymmetry, Developmental Noise and Developmental Stability: Future Prospects for the Population Developmental Biology Approach. Symmetry 2020, 12, 1376. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M. Directional asymmetry in the yellow-bellied slider turtles (Trachemys scripta scripta) (Schoepff 1792). Herpetol. Notes 2020, 13, 587–592. [Google Scholar]
- Abdel Fatah, E.E.; Shirley, N.R.; Mahfouz, M.R.; Auerbach, B.M. A three-dimensional analysis of bilateral directional asymmetry in the human clavicle. Am. J. Phys. Anthropol. 2012, 149, 547–559. [Google Scholar] [CrossRef]
- Ginot, S.; Agret, S.; Claude, J. Bite force performance, fluctuating asymmetry and antisymmetry in the mandible of inbred and outbred wild-derived strains of mice (Mus musculus domesticus). Evol. Biol. 2018, 45, 287–302. [Google Scholar] [CrossRef]
- Dongen, S.V. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Canché-Delgado, A.; Maldonado-López, Y.; Fernandes, G.W.; Oyama, K.; González-Rodríguez, A. Patterns of herbivory and leaf morphology in two Mexican hybrid oak complexes: Importance of fluctuating asymmetry as indicator of environmental stress in hybrid plants. Ecol. Indic. 2018, 90, 164–170. [Google Scholar] [CrossRef]
- DeLeon, V.B. Fluctuating asymmetry and stress in a medieval Nubian population. Am. J. Phys. Anthropol. 2007, 132, 520–534. [Google Scholar] [CrossRef]
- Graham, J.H.; Özener, B. Fluctuating asymmetry of human populations: A review. Symmetry 2016, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Hallgrímsson, B.; Miyake, T.; Wilmore, K.; Hall, B.K. Embryological origins of developmental stability: Size, shape and fluctuating asymmetry in prenatal random bred mice. J. Exp. Zool. 2003, 296, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Parsons, P.A. Fluctuating asymmetry: An epigenetic measure of stress. Biol. Rev. 1990, 65, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Willmore, K.E.; Klingenberg, C.P.; Hallgrímsson, B. The relationship between fluctuating asymmetry and environmental variance in rhesus macaque skulls. Evolution 2005, 59, 898–909. [Google Scholar] [CrossRef]
- Aparicio, J.M. Patterns of growth and fluctuating asymmetry: The effects of asymmetrical investment in traits with determinate growth. Behav. Ecol. Sociobiol. 2001, 49, 273–282. [Google Scholar] [CrossRef]
- Siegel, M.I.; Doyle, W.J. Stress and fluctuating limb asymmetry in various species of rodents. Growth 1975, 39, 363–369. [Google Scholar]
- Badyaev, A.V.; Foresman, K.R.; Fernandes, M.V. Stress and developmental stability: Vegetation removal causes increased fluctuating asymmetry in shrews. Ecology 2000, 81, 336–345. [Google Scholar] [CrossRef]
- Joyce, B.J.; Brown, G.E. Short-term captivity drives hypothalamic plasticity and asymmetry in wild-caught northern red bellied dace (Chrosomus eos). J. Fish Biol. 2020, 97, 577–582. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G. Captivity effects on wide-ranging carnivores. Nature 2003, 425, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Knierim, U.; Van Dongen, S.; Forkman, B.; Tuyttens, F.A.M.; Špinka, M.; Campo, J.L.; Weissengruber, G.E. Fluctuating asymmetry as an animal welfare indicator—A review of methodology and validity. Physiol. Behav. 2007, 92, 398–421. [Google Scholar] [CrossRef] [PubMed]
- Siciliano-Martina, L.; Light, J.E.; Lawing, A.M. Cranial morphology of captive mammals: A meta-analysis. Front. Zool. 2021, 18, 4. [Google Scholar] [CrossRef]
- Almeida, D.; Almodóvar, A.; Nicola, G.G.; Elvira, B. Fluctuating asymmetry, abnormalities and parasitism as indicators of environmental stress in cultured stocks of goldfish and carp. Aquaculture 2008, 279, 120–125. [Google Scholar] [CrossRef]
- Lens, L.U.C.; Van Dongen, S.; Kark, S.; Matthysen, E. Fluctuating asymmetry as an indicator of fitness: Can we bridge the gap between studies? Biol. Rev. 2002, 77, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaluddin, S.N.; Tanaka, M.; Wakamori, H.; Nishimura, T.; Ito, T. Phenotypic plasticity in the mandibular morphology of Japanese macaques: Captive–wild comparison. R. Soc. Open Sci. 2019, 6, 181382. [Google Scholar] [CrossRef] [Green Version]
- Leamy, L. Morphological integration of fluctuating asymmetry in the mouse mandible. Genetica 1993, 89, 139. [Google Scholar] [CrossRef]
- Neubauer, S.; Gunz, P.; Scott, N.A.; Hublin, J.J.; Mitteroecker, P. Evolution of brain lateralization: A shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci. Adv. 2020, 6, eaax9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: New York, NY, USA, 1997. [Google Scholar]
- Mardia, K.V.; Bookstein, F.L.; Moreton, I.J. Statistical assessment of bilateral symmetry of shapes. Biometrika 2000, 87, 285–300. [Google Scholar] [CrossRef]
- Bookstein, F.L.; Green, W.D. Thin-plate spline for deformations with specified derivatives. In Proceedings of the Mathematical Methods in Medical Imaging II Symposium, San Diego, CA, USA, 11–16 July 1993; International Society for Optics and Photonics: Bellingham, WA, USA, 1993; Volume 2035, pp. 14–28. [Google Scholar]
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating asymmetry: Methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/(version4.1.1) (accessed on 5 May 2021).
- Profico, A.; Costantino, B.; Silvia, C.; Marina, M.; Paolo, P.; Alessio, V.; Pasquale, R. Arothron: An R package for geometric morphometric methods and virtual anthropology applications. Am. J. Phys. Anthropol. 2021, 176, 144–151. [Google Scholar]
- Schlager, S. Morpho and Rvcg—Shape Analysis in R. In Statistical Shape and Deformation Analysis; Zheng, G., Li, S., Szekely, G., Eds.; Academic Press: London, UK, 2017; pp. 217–256. [Google Scholar]
- Adams, D.C. Otárola-Castillo, E. geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Hill, A.K.; Cárdenas, R.A.; Wheatley, J.R.; Welling, L.L.; Burriss, R.P.; Claes, P.; Apicella, C.L.; McDaniel, M.A.; Little, A.C.; Shriver, M.D.; et al. Are there vocal cues to human developmental stability? Relationships between facial fluctuating asymmetry and voice attractiveness. Evol. Hum. Behav. 2017, 38, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Schlager, S.; Rüdell, A. Analysis of the human osseous nasal shape—Population differences and sexual dimorphism. Am. J. Phys. Anthropol. 2015, 157, 571–581. [Google Scholar] [CrossRef]
- Cattaneo, P.M.; Kofod, T.; Dalstra, M.; Melsen, B. Using the finite element method to model the biomechanics of the asymmetric mandible before, during and after skeletal correction by distraction osteogenesis. Comput. Methods Biomech. Biomed. Eng. 2005, 8, 157–165. [Google Scholar] [CrossRef] [PubMed]
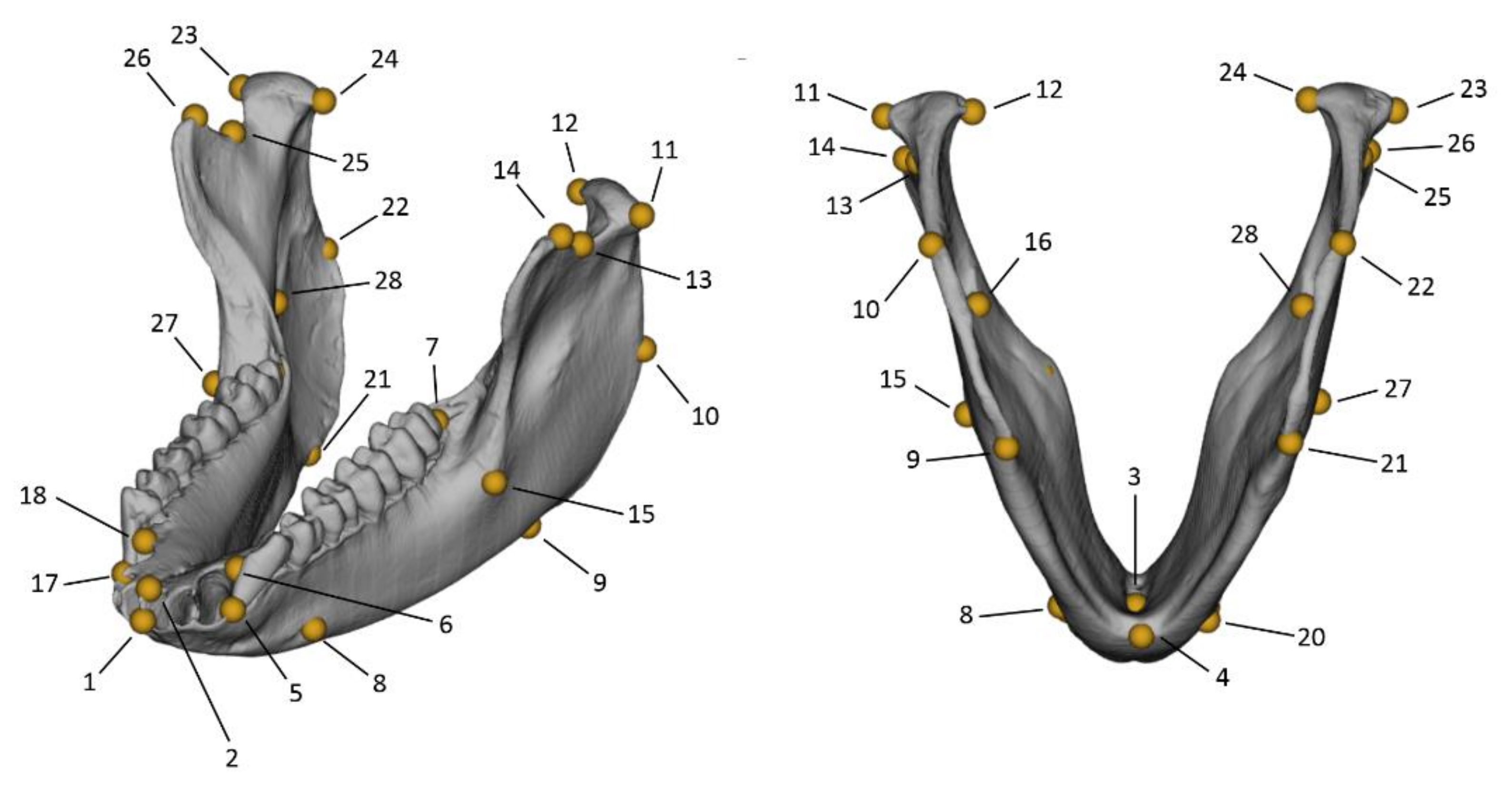

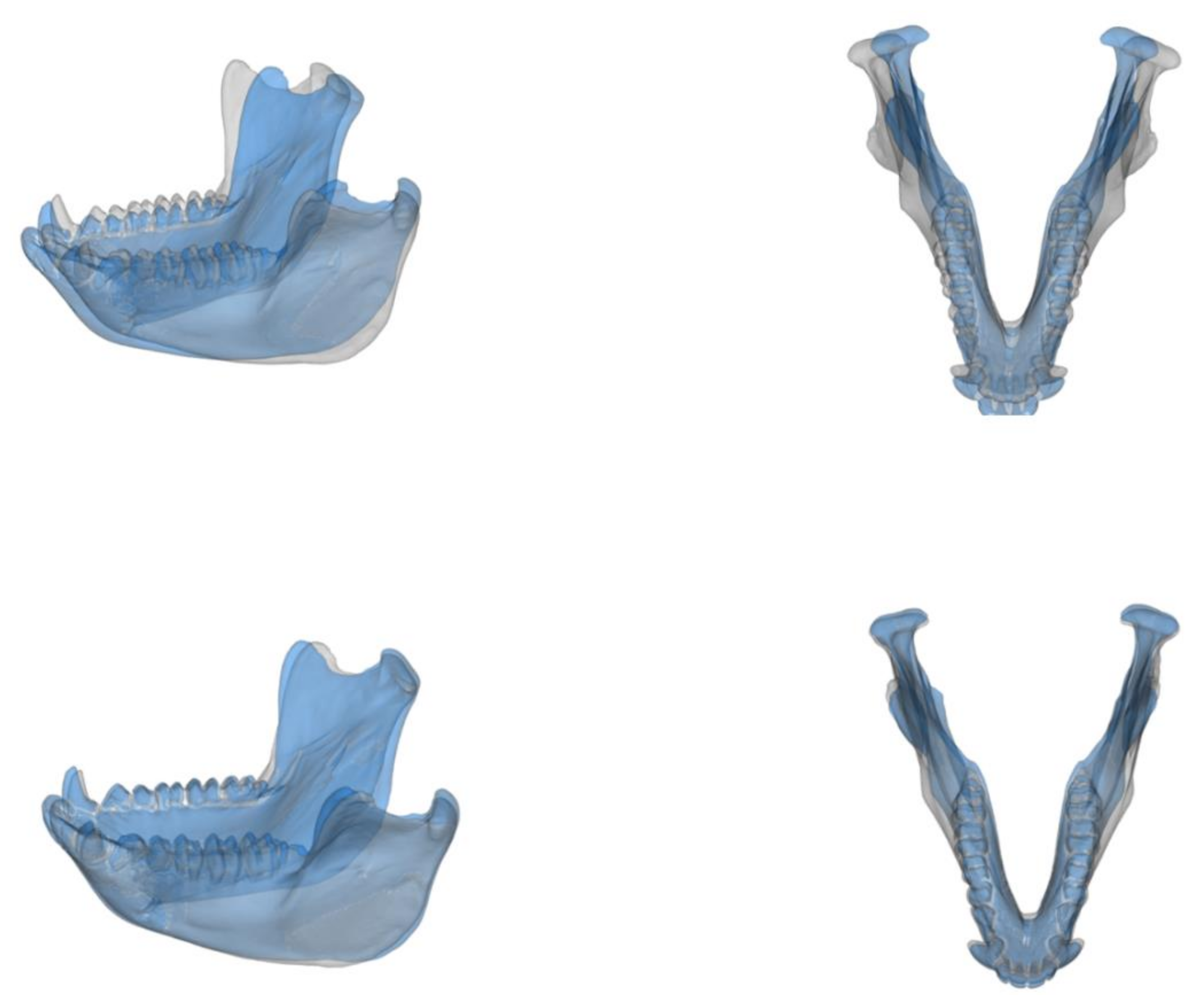
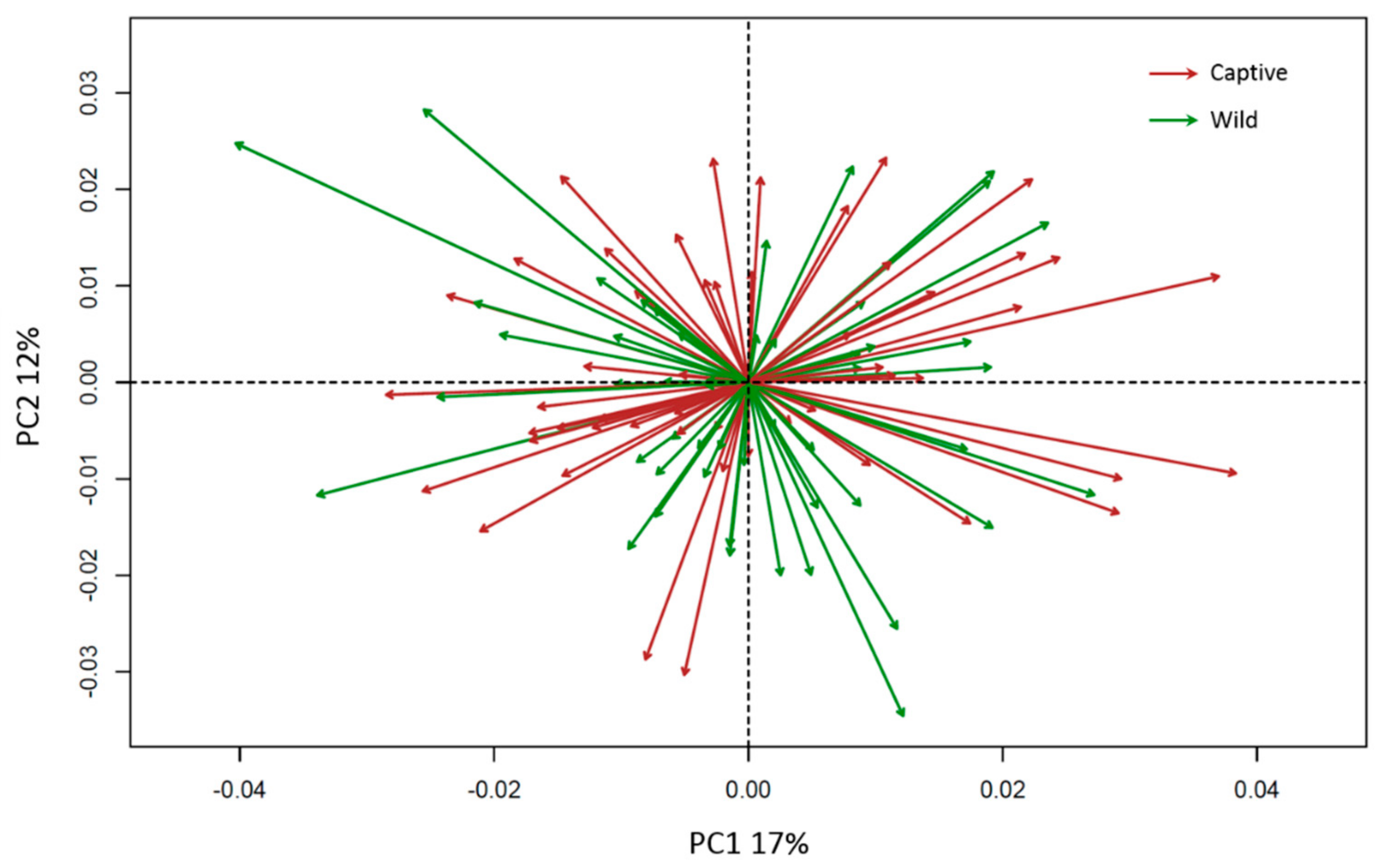

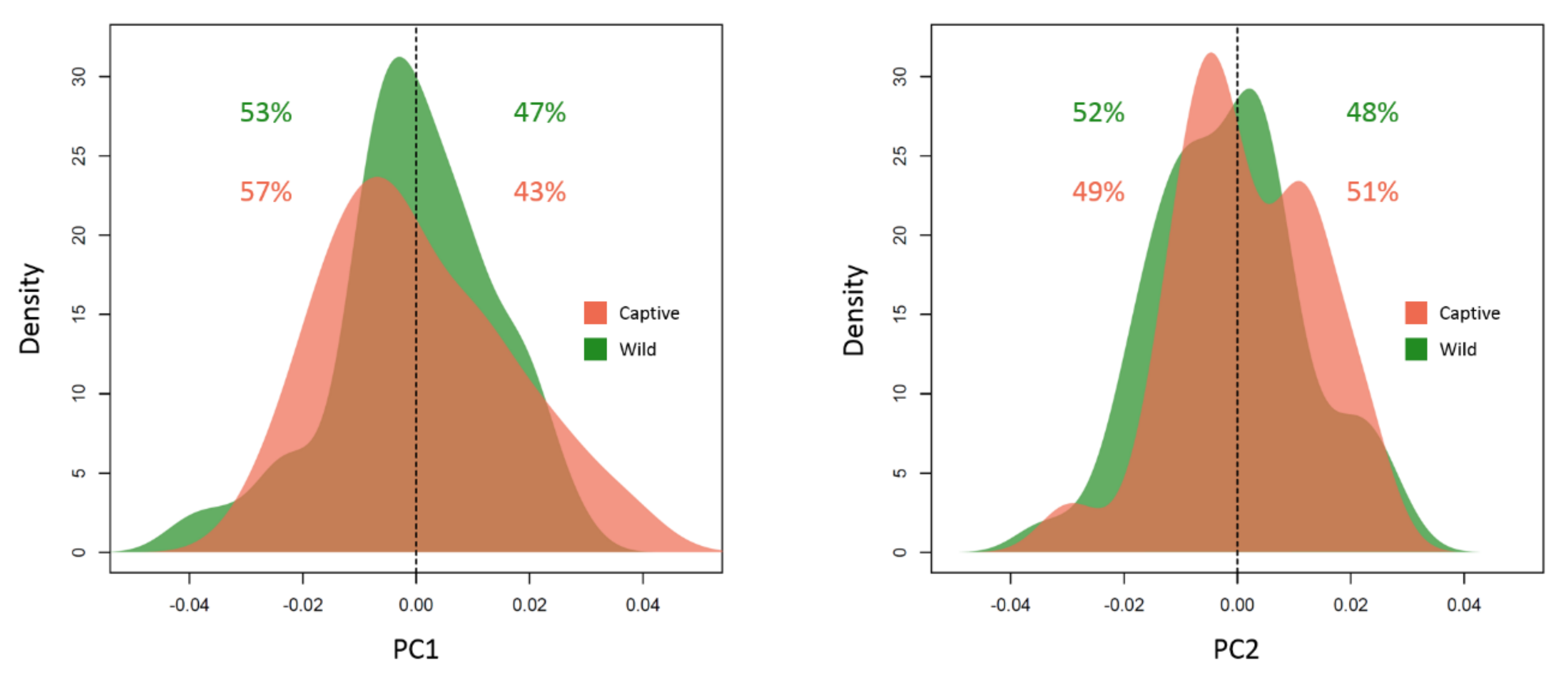
| Landmark Number | Landmark Definition | Type |
|---|---|---|
| 1 | The buccal point at the superior tip of the septum, between the mandibular central incisors. | I |
| 2 | The lingual point at the superior tip of the septum between the mandibular central incisors. | I |
| 3 | The lowermost point of the median lingual foramen. | I |
| 4 | On the mid-sagittal plane, the most inferior and posterior point of the mandibular symphysis. | II |
| 5–17 | The buccal point at the superior tip of the septum distally to the canine. | I |
| 6–18 | The lingual point at the superior tip of the septum distally to the canine. | I |
| 7–19 | The most posterior point of the tooth row, distally to the third molar. | II |
| 8–20 | The most posterior point on the rim of the mental foramen—if multiple foramina present, consider the most anterior. | I |
| 9–21 | The most inferior point of the gonial region, at the inferior margin of the masseteric fossa. | II |
| 10–22 | The most superior point of the gonial region, at the most posterior margin of the masseteric fossa. | II |
| 11–23 | The most lateral point of the mandibular condyle. | II |
| 12–24 | The medial point of the mandibular condyle. | II |
| 13–25 | The lowermost point on the mandibular notch. | II |
| 14–26 | The anterior end point of the mandibular notch. | II |
| 15–27 | The most lateral point along the linea obliqua. | II |
| 16–28 | The most infero-anterior point of the rim of the mandibular foramen. | I |
| Overall Shape | |||
|---|---|---|---|
| F-Statistic | R2 | p-Value | |
| Replica | 0.52 | 0.002 | 0.94 |
| Individual | 5.26 | 0.03 | 0.001 *** |
| Replica × Individual | 0.08 | 0.0004 | 1.00 |
| Symmetric Shape | |||
|---|---|---|---|
| F-Statistic | R2 | p-Value | |
| Group | 17.50 | 0.14 | 0.001 *** |
| Sex | 9.35 | 0.07 | 0.001 *** |
| Group × Sex | 0.83 | 0.007 | 0.64 |
| Asymmetric Shape | |||
|---|---|---|---|
| F-Statistic | R2 | p-Value | |
| Group | 0.65 | 0.006 | 0.86 |
| Sex | 0.88 | 0.008 | 0.60 |
| Group × Sex | 1.03 | 0.01 | 0.41 |
| Overall Shape | F-Statistic | R2 | p-Value | Interpretation |
|---|---|---|---|---|
| Sex | 34.41 | 0.13 | 0.001 *** | Sexual dimorphism |
| Group | 17.37 | 0.065 | 0.001 *** | Environmental condition |
| Reflection | 0.75 | 0.002 | 0.72 | Directional asymmetry |
| Size | 13.38 | 0.05 | 0.001 *** | Allometry |
| Individual × Reflection | 0.14 | 0.0005 | 1.00 | Fluctuating asymmetry |
| Group × Reflection | 0.21 | 0.0008 | 1.00 | Group asymmetry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, F.; Alfieri, F.; Towle, I.; Profico, A.; Veneziano, A. Fluctuating Asymmetry and Stress in Macaca fuscata: Does Captivity Affect Morphology? Appl. Sci. 2021, 11, 7879. https://doi.org/10.3390/app11177879
Landi F, Alfieri F, Towle I, Profico A, Veneziano A. Fluctuating Asymmetry and Stress in Macaca fuscata: Does Captivity Affect Morphology? Applied Sciences. 2021; 11(17):7879. https://doi.org/10.3390/app11177879
Chicago/Turabian StyleLandi, Federica, Fabio Alfieri, Ian Towle, Antonio Profico, and Alessio Veneziano. 2021. "Fluctuating Asymmetry and Stress in Macaca fuscata: Does Captivity Affect Morphology?" Applied Sciences 11, no. 17: 7879. https://doi.org/10.3390/app11177879
APA StyleLandi, F., Alfieri, F., Towle, I., Profico, A., & Veneziano, A. (2021). Fluctuating Asymmetry and Stress in Macaca fuscata: Does Captivity Affect Morphology? Applied Sciences, 11(17), 7879. https://doi.org/10.3390/app11177879






